Research Article Open Access
Comparative Study on the Influence of Epigallocatechin-3-gallat e and/ or Coenzyme Q10 against Alzheimer's disease Induced by Aluminium in Normally-Fed and Protein Malnourished Rats
Azza A Ali1*, Hebatalla I Ahmed1, Mona G Khalil2, Asmaa I Alwakeel1 and Karema Abu-Elfotuh1
1Department of Pharmacology and Toxicology, Faculty of Pharmacy, Al-Azhar University, Cairo, Egypt
2Department of Pharmacology and Toxicology, Faculty of Pharmacy, Modern University for Technology and Information, Cairo, Egypt
- *Corresponding Author:
- Azza A Ali
Prof and Head of Pharmacology and Toxicology Department
Faculty of Pharmacy, Al-Azhar University, Cairo, Egypt
Tel: +20 01061905439
E-mail: azzamoro@gmail.com
Received date: May 19, 2016; Accepted date: June 08, 2016; Published date: June 15, 2016
Citation: Ali AA, Ahmed HI, Khalil MG, Alwakeel AI, Elfotuh KA (2016) Comparative Study on the Influence of Epigallocatechin-3-gallate and/or Coenzyme Q10 against Alzheimer’s disease Induced by Aluminium in Normally-Fed and Protein Malnourished Rats. J Alzheimers Dis Parkinsonism 6:240. doi:10.4172/2161-0460.1000240
Copyright: © 2016 Ali AA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Alzheimers Disease & Parkinsonism
Abstract
Background: Alzheimer’s disease (AD) is a neurodegenerative disorder greatly influenced by oxidative stress and mitochondrial dysfunction which may lead to deposition of β-amyloid (Aβ) peptides. Protein malnutrition increases oxidative damage in cortex, hippocampus and cerebellum. Epigallocatechin-3-gallate (EGCG) has health-promoting effects in CNS, while Coenzyme Q10 (CoQ10) is intracellular antioxidant and mitochondrial membrane stabilizer.
Objective: To investigate the possible protective effect of EGCG and/or CoQ10 against aluminium-induced neurotoxicity presenting symptoms that mimic AD in both normally-fed (NF) and protein malnourished (PM) rats.
Methods: Ten groups of rats were used; five for NF (20% casein) and the same for PM (10% casein). Both NF and PM groups received daily for four weeks; either saline for control or AlCl3 (70 mg/kg, I.P) for AD induction groups, treated groups received together with AlCl3 either EGCG (10 mg/kg, I.P), CoQ10 (200 mg/kg, P.O) or combination of both. Histopathological changes in the brain and biochemical changes in Aβ, Acetyl cholinesterase (ACHE) as well as oxidative parameters (MDA, SOD, TAC) were evaluated for all groups.
Results: The study revealed that, brain neurological damage characterizing induction of AD as indicated by histopathological changes in the brain and the increase in Aβ, ACHE and MDA as well as the decrease in SOD and TAC was more pronounced in PM rats. Administration of EGCG and/or CoQ10 during induction of AD showed protective effect in both NF and PM rats as indicated by the decreased Aβ, ACHE, MDA together with the increased SOD, TAC and confirmed by histo pathological examinations in different brain regions. However, the effect of combined treatment was more pronounced in both NF and PM rats.
Conclusion: PM is a risk factor in induction of AD, EGCG and CoQ10 combined therapy has marked protective effects during induction of AD in both NF and PM rats rather than each individual treatment.
Keywords
Alzheimer’s disease; AlCl3; Protein malnutrition; Oxidative stress; Epigallocatechin-3-gallate; Coenzyme Q10; Rats
Introduction
Alzheimer’s disease (AD) is a neurodegenerative disorder that leads to memory loss and nerve cell death throughout the brain and represents the major cause of dementia in the world [1]. The number of AD patients is expected to reach 106.8 million worldwide by the year 2050; therefore, the disease is considered a growing public health concern with major socioeconomic burden [2]. AD risk factors and disease-modifying factors have gained much attention [3]. A number of factors may increase the chances of developing the disease, some risk factors can be changed or controlled while others cannot [3,4]. Stress, diabetes and depression have been associated with increased risk of AD, while cognitive engagement and physical activities have been linked to decreased risk of AD [4].
Recently, there have been growing evidences supporting the role of nutrition in AD [5]. A number of dietary components such as antioxidants, vitamins and polyphenols have been reported to decrease the risk of AD incidence, while saturated fatty acids, high-calorie intake, heavy smoking and excess alcohol consumption were identified as risk factors [6]. Dietary patterns have emerged to explore the relationship between diet and AD [7]. Patients with AD have a worse nutritional status compared to others and usually suffer from malnutrition and weight loss [8,9]. In addition, weight loss was reported to predict rapid cognitive decline in AD patients [10,11]. Consequently, treatment of weight loss and malnutrition may be important to decrease the risk of AD. Although the mechanisms of many nutrients such as antioxidants and vitamins to decrease the risk of AD are not clear, but reducing oxidative stress, inflammatory mediators together with both Aβ and tau pathologies can attenuate cognitive deterioration associated AD [12,13].
Aluminum (Al) is considered the third most abundant element and the most common metal in the earth’s crust [14]. Al is increasingly taken into our bodies through food, air, water, and even drugs as a result of high rate global industrialization and consequent pollution [15]. It is found in many manufactured foods and is added as alum for treating drinking water for purification purposes [16]. Excessive Al intake can lead to deposition of Aβ in central nerve cells and over expression of β-amyloid precursor protein (APP), so it can be considered as a potential etiological factor in AD [17,18]. It is known that the neurotoxicity of Aβ is associated with oxidative stress; generation of reactive oxygen species and it can lead to the damage of neuronal membrane, lipids, proteins, and nucleic acids [19,20]. Consequently, oxidative stress is thought to play an effective role in pathogenesis of AD [21]. Oxygenate radicals can interrupt the integrity and performance of the cell [22]. The brain tissue also contains a lot of unsaturated fatty acids which are especially vulnerable for free radical attacks [23]. Therefore, antioxidant substances can play an important role in prevention and cure of AD [24]. Acetylcholinesterase (ACHE) has been also found to co localize with Aβ deposits and promotes the assembly of Aβ into amyloid fibrils forming Aβ-ACHE complex that is more toxic than amyloid fibrils [20].
Coenzyme Q10 (CoQ10) or ubiquinone, is a fat-soluble, pseudo-vitamin substance that is mainly present in the mitochondria and has an important role in generating ATP as a component of the electron transport chain [25]. It has been documented that CoQ10 acts as a powerful antioxidant and radical scavenger [26,27]. It can also help to regenerate other antioxidants, so it has been used as anti-aging and is effective in the improvement of cognitive disorders [28]. In the body, cells synthesize CoQ10 from the amino acid tyrosine, requiring adequate levels of vitamins such as folic acid [29]. Consequently, folic Acid is considered an essential cofactor for the endogenous synthesis of CoQ10 and any deficiency in folic acid would result in a deficiency in CoQ10 [30]. The efficacy of CoQ10 appears to be promising for alleviating neurodegenerative disorders [31]. It is worthy to note that CoQ10 is reduced in AD patients [32,33].
Epigallocatchin-3-gallate (EGCG) is the most abundant and active compound responsible for most of green tea’s role in promoting good health. It acts through different pathways; as antioxidant, anti-inflammatory, anti-atherogenic and also showing gene expression content, functioning through growth factor-mediated pathways [34]. EGCG as antioxidant is highly effective in protecting cells; it also has COX-2 inhibiting property [34,35]. EGCG can attenuate peroxide production in glial cells by either inhibiting the deamination of monoamines or acting as a free radical scavenger [36].
Therefore, the aim of this study was to investigate the effect of protein malnutrition on induction of AD and the possible protective effect of EGCG and/or CoQ10 against aluminium-induced neurotoxicity presenting symptoms that mimic AD in both NF and PM rats.
Materials and Methods
Animals
Eighty male Sprague Dawley rats were used. Rats weighing 180- 200 g were obtained from Nile Co. for Pharmaceuticals and Chemical Industries, Cairo, Egypt. They were housed in stainless-steel cages (four per cage); under the same adequate conditions, with alternatively 12 H light and dark cycles, at a temperature of 25 ± 1°C and water was given ad-libitum. Two weeks before starting the experimental nutrition [standard protein diet for NF rats and low protein diet for PM rats], all rats were daily provided with the dietary requirements of diet pellets (standard); contained protein (20%), fiber (5%), fat (3.5%), ash (6.5%) as well as vitamin mixture (El-Nasr, Abu Zaabal, Cairo, Egypt). On the other hand, each 100 g of the standard protein diet [20% casein diet] contained casein (20 g), sucrose (70 g), salt mixture (4 g), oil and oil-soluble vitamins (5 g) and (0.6 g) vitamin mixture in starch [37]. Low protein diet [10% casein diet, chosen for this study] has the same composition as standard protein diet except that casein was reduced to 10 g instead of 20 g per each 100 g of the diet. The remained 10 g was replaced by sucrose-starch mixture. In accordance with ethical guidelines of Al-Azhar University (Faculty of Pharmacy) Egypt, the study was conducted.
Drugs and chemicals
From Sigma Chemical Co. (St. Louis, MO, USA); EGCG, CoQ10 and Aluminum chloride - hydrated (AlCl3.6H2O) were purchased. EGCG and AlCl3 were freshly dissolved in distilled water while CoQ10 was suspended in 1% aqueous solution of Tween 80; suspensions were freshly prepared every day. All other solvents and chemicals were of the highest grade-commercially available.
Experimental design
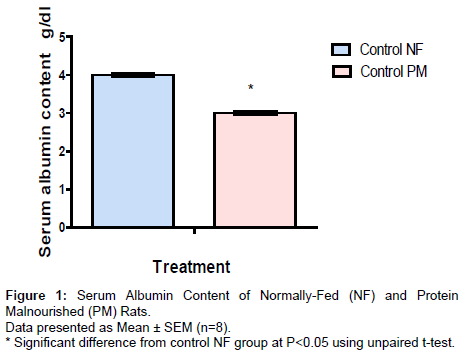
Animals were randomly assigned to ten groups (8 rats/group); five groups were maintained feeding on 20% casein diet (NF rats) and five groups on 10% casein diet (PM rats) for four weeks. During these four weeks of experimental nutrition, both groups of NF and PM were treated as follows; group served as control and was given saline daily, the other four groups were injected daily with AlCl3.6H2O (70 mg/kg I.P) [38]. One of these four groups served as AD model group, while the others three groups were treated with EGCG (10 mg/kg, I.P every other day) [39], CoQ10 (200 mg/kg, P.O daily) [40] and with their combination together with AlCl3 during the four weeks of AD induction. The dose volume for all administered drugs was not exceeding 0.5 ml/200 g body weight. At the end of the four weeks, blood samples were withdrawn from the retro-orbital venous plexus of control NF and PM groups to ensure the nutrition status by determination of serum albumin content (Figure 1). Serum samples were obtained by centrifugation at 3000 rpm for 10 min. After rats were sacrificed, the brain tissues were dissected. All brain tissues from all groups were subjected immediately for analysis or they were kept frozen until the time of analysis (at -80°C) after washing with ice-cold saline. The homogenates of the brain tissues in saline were used to assess the oxidative stress markers as superoxide dismutase (SOD) and total antioxidant capacity (TAC) as well as lipid peroxides which were expressed as malondialdehyde (MDA). Acetylcholine esterase (ACHE) content and Aβ content were also determined. For histopathological examinations, specimens from all brain areas were taken from different treated groups.
Biochemical parameters
Protein estimation: The protein content was measured in the brain homogenates using bovine serum albumin as a standard and according to Bradford method [41].
Determination of serum albumin content: Serum albumin content was assessed by using ready-made kits obtained from Stanbio Laboratory Inc. (San Antonio, USA).
Assessment of oxidative stress biomarkers: In the brain homogenate for each rat, MDA and SOD as well as TAC were measured. By estimating the level of thiobarbituric acid reactive substances (TBARS), lipid peroxidation can be determined as MDA [42]. Relying on the ability of the enzyme to inhibit the phenazine methosulphate mediated reduction of nitroblue tetrazolium dye, SOD content was assessed where the increase in absorbance at 560 nm for 5 min is measured [43]. On the other hand, determination of TAC is performed through the reaction with a defined amount of exogenously provide H2O2. The residual H2O2 is colorimetrically determined by the enzymatic reaction that involves the conversion of 3, 5-dichloro-2- hydroxybenzene sulphonate to a colored product [44].
Determination of ACHE content: In the brain tissue homogenate, ACHE content was assessed according to the manufacturer’s instructions by using ELISA Kits from Ray Biotech, Inc. (USA).
Determination of Aβ content: Determination of Aβ was assessed in brain tissue homogenate according to the manufacturer’s instructions by using ELISA Kits (USCN Life Science, Inc., Product Number MBS702915).
Histopathological examination of the brain
In 10% formalin for 24 h, brain specimens were fixed then they were washed with tap water, they were prepared and stained for light microscopy [45]. For dehydration; serial dilutions of alcohol were used (methyl, ethyl and absolute ethyl). In hot air oven at 56ºC for 24 h, specimens were cleared in xylene embedded in paraffin. By using microtome at 4 microns thickness, paraffin bees wax tissue blocks were sectioned. Then, sections were collected on glass slides and deparaffinized. They were stained for routine histological examination using Hematoxylin and Eosin stain.
Statistical Analysis
Data are expressed as mean ± SEM. Multiple comparisons were performed using one-way ANOVA followed by Tukey Kramer as a post hoc test. Unpaired t-test was used to compare two different treatments. As a criterion for significance, 0.05 level of probability was used. By using Instat (version 3) software package, all statistical analyses were performed and by using GraphPad Prism (ISI®, USA) software (version 5), graphs were sketched.
Results
Serum albumin content of NF and PM rats
Protein malnutrition induced by feeding rats with low protein diet (10% casein) for 4 weeks produced marked decrease in serum albumin amounted to 75% from the corresponding control NF group.
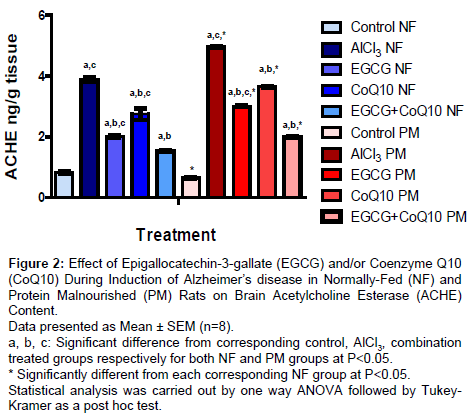
Brain acetylcholine esterase (ACHE) content
Results are shown in (Figure 2); administration EGCG, CoQ10 and their combination during induction of AD in both NF and PM produced significant decrease in ACHE content amounted to 47.68%, 29.12% and 60.31% respectively in NF groups and to 39.29%, 26.41% and 59.88% respectively in PM groups as compared to corresponding AD model group. However, PM itself markedly increased ACHE content in all AD model groups either untreated (AlCl3) or treated (AlCl3+EGCG, AlCl3+CoQ10 and AlCl3+EGCG+CoQ10) than their corresponding NF reaching to 127.8%, 148.3%, 140% and 129.22% respectively. It is worthy to note that the base line control level of ACHE content was lower in PM rats than the corresponding control NF group amounted to 77.5%.
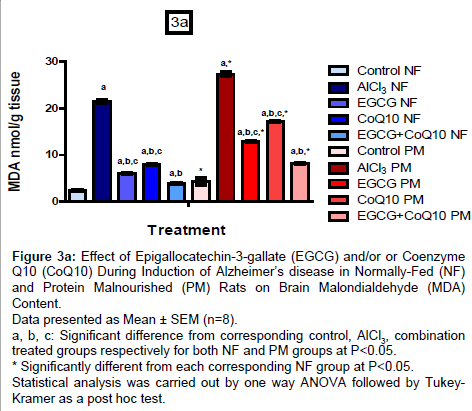
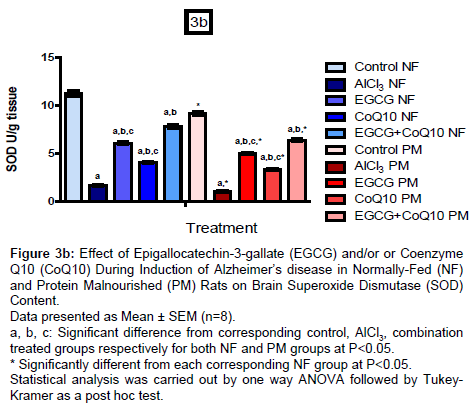
Brain oxidative stress biomarkers (MDA, SOD and TAC)
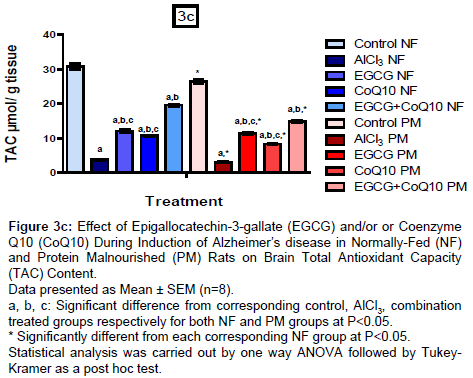
Results are shown in (Figures 3a-3c), administration of EGCG or CoQ10 alone or in combination during induction of AD in NF and PM rats showed marked decrease in MDA content with respect to un-treated AD model by 71.96%, 62.71% and 81.68% respectively in NF groups and by 52.59%, 36.83% and 69.96% respectively in PM groups. PM itself induced marked increase in MDA content in all groups (control, un-treated and treated AD model). MDA content reached to 177.23%, 129.56%, 215.16%, 215.54% and 208.62% respectively as compared to their corresponding NF values.
On the other hand, administration of EGCG or CoQ10 alone or in combination during induction of AD in NF and PM rats resulted in marked increase in SOD content with respect to their corresponding AD model group (un-treated) reached to 267.47%, 147.59% and 371.69% respectively in NF rats, while reached to 314.05%, 175.21% and 427.27% respectively in PM rats. In addition, TAC content also increased in response to treatment by 218.89%, 182.41% and 414.44% respectively in NF groups and by 273.77%, 174.09% and 388.85% respectively in PM groups as compared to the un-treated AD model groups. Content of SOD and TAC in all PM groups have been decreased than the corresponding NF groups (control, un-treated and treated AD model) amounted to 81.56%, 61.50%, 82.13%, 81.02% and 81.48% respectively for SOD and to 85.91%, 80.05%, 93.82%, 77.69% and 76.07% respectively for TAC.
Brain β-amyloid (Aβ) content
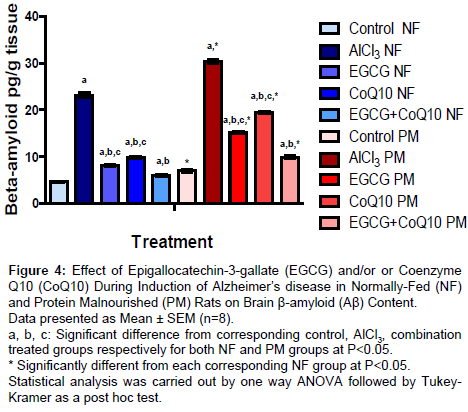
Rats treated with EGCG, CoQ10 or their combination during induction of AD in both NF and PM rats showed significant decrease in Aβ content by 64.94%, 57.05% and 74.12% respectively in NF rats, while in PM rats Aβ content decreased by 50.15%, 36.07 and 67.22 respectively with respect to the corresponding un-treated AD model group. In all groups (control, un-treated and treated AD model), PM produced a significant increase in Aβ content. It reached to 151.82%, 130.09%, 186.1%, 194.78% and 165.83% respectively compared to corresponding NF groups (Figure 4).
Histopathological alterations in the brain
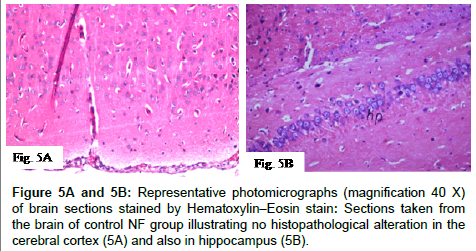
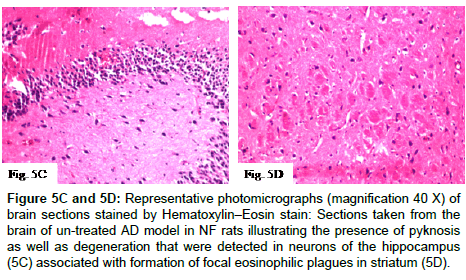
Histopathological alterations in brain specimens from different groups of NF rats are shown in (Figures 5A-5F and 5H) and (Table 1) that illustrates the severity or the intensity of the alterations in the brain of the NF rats. Brain specimens from control NF rats illustrated that the histological structure of the cerebral cortex and the hippocampus was normal while, brain specimens of NF rats injected with AlCl3 for four weeks (model that mimics AD) showed degeneration and pyknosis in hippocampus neurons associated with formation of focal eosinophilic plagues in striatum. Treatment of NF rats with EGCG markedly ameliorated the pathological changes induced by AlCl3, where the hippocampus was histological intact, while treatment of NF rats with CoQ10 illustrated normal histological structure in both hippocampus and cerebral cortex. Additionally, no histopathological alterations in hippocampus were detected with combined administration of EGCG and CoQ10 to NF rats during the four weeks of AD induction.
Figure 5C and 5D: Representative photomicrographs (magnification 40X) of brain sections stained by Hematoxylin–Eosin stain:Sections taken from the brain of un-treated AD model in NF rats illustrating the presence of pyknosis as well as degeneration that were detected in neurons of the hippocampus (5C) associated with formation of focal eosinophilic plagues in striatum(5D).
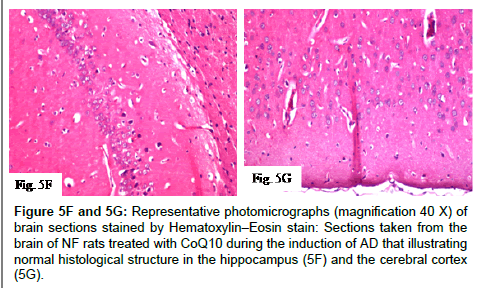
Figure 5F and 5G: Representative photomicrographs (magnification 40X) of brain sections stained by Hematoxylin–Eosin stain: Sections taken from the brain of NF rats treated with CoQ10 during the induction of AD that illustrating normal histological structure in the hippocampus (5F) and the cerebral cortex (5G).
| Histopathological alterations | Control NF | AlCl3 70mg/kg NF | AlCl3 +EGCG NF | AlCl3 +CoQ10 NF | AlCl3 +CoQ10+EGCG NF |
|---|---|---|---|---|---|
| Degeneration and pyknosis in the neurons of hippocampus | - | +++ | - | - | - |
| Formation of eosinophillic plaques in striatum | - | +++ | - | - | - |
+++ Severe - Nil
Table 1: Effect of epigallocatechin-3-gallate (EGCG) and/or coenzyme Q10 (CoQ10) during AD induction against the severity of histopathological alterations in the brain of NF rats.
Histopathological alterations in brain specimens of PM groups are shown in (Figures 5I, 5K-5O and 5R) and (Table 2) that illustrated the severity or the intensity of these alterations in the brain of the PM rats. Brain specimens of control PM rats showed normal histological structure, while brain specimens of PM rats injected with AlCl3 for four weeks (model that mimics AD) showed very severe pyknosis and degeneration in hippocampus neurons associated with focal eosinophilic plagues formation in striatum. On the other hand, treatment of PM rats with EGCG showed pyknosis in the neurons of the hippocampus associated with plagues formation in the striatum, while treatment with CoQ10 showed normal histological structure in the hippocampus. Moreover, combined treatment of PM rats with EGCG and CoQ10 during the four weeks of AD induction illustrated that there was no histopathological alteration in hippocampus and cerebral cortex.
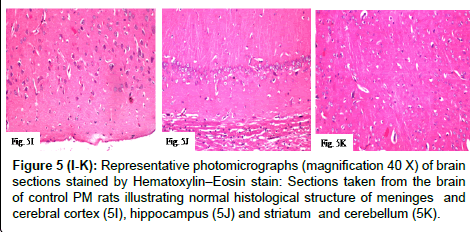
Figure 5(I-K): Representative photomicrographs (magnification 40X) of brain sections stained by Hematoxylin–Eosin stain: Sections taken from the brain of control PM rats illustrating normal histological structure of meninges and cerebral cortex (5I), hippocampus (5J) and striatum and cerebellum (5K).
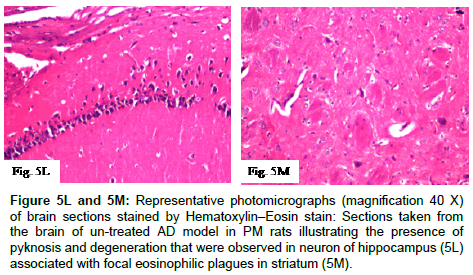
Figure 5L and 5M: Representative photomicrographs (magnification 40X) of brain sections stained by Hematoxylin–Eosin stain:Sections taken from the brain of un-treated AD model in PM rats illustrating the presence of pyknosis and degeneration that were observed in neuron of hippocampus (5L) associated with focal eosinophilic plagues in striatum (5M).
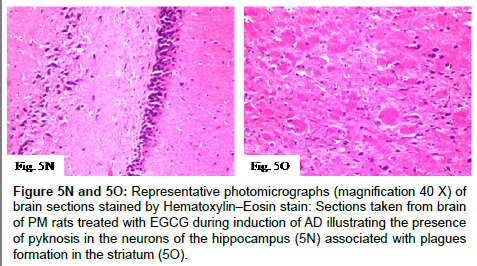
Figure 5N and 5O: Representative photomicrographs (magnification 40 X) of brain sections stained by Hematoxylin–Eosin stain:Sections taken from brain of PM rats treated with EGCG during induction of AD illustrating the presence of pyknosis in the neurons of the hippocampus (5N) associated with plagues formation in the striatum (5O).
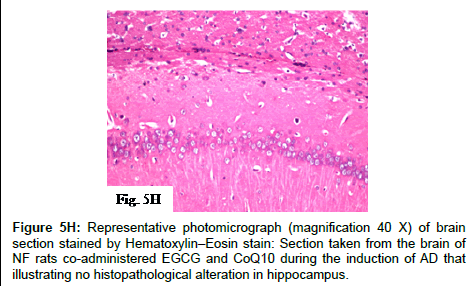
Figure 5Q and 5R: Representative photomicrographs (magnification 40X) of brain sections stained by Hematoxylin–Eosin stain: Sections taken from the brain of PM rats co-administered EGCG and CoQ10 during the induction of AD illustrating no histopathological alteration in hippocampus (5Q) and cerebral cortex (5R).
| Histopathological alterations | Control PM | AlCl3 70mg/kg PM | AlCl3 +EGCG PM | AlCl3 +CoQ10 PM | AlCl3 +CoQ10+EGCG PM |
|---|---|---|---|---|---|
| Degeneration and pyknosis in the neurons of hippocampus | - | ++++ | + | - | - |
| Formation of eosinophillic plaques in striatum | - | ++++ | + | - | - |
++++ Very Severe + Mild - Ni
Table 2: Effect of epigallocatechin-3-gallate (EGCG) and/or coenzyme Q10 (CoQ10) during AD induction against the severity of histopathological alterations in the brain of PM rats.
According to the mentioned results, it is clear that the severity of the brain neurological damage induced by AlCl3 was more pronounced in PM rats than that in NF rats as indicated by the histopathological alterations in different brain regions as well as the biochemical changes in Aβ, Acetyl cholinesterase (ACHE) and oxidative stress markers. On the other hand, the improvement induced by EGCG was more pronounced in NF rats than in PM rats, while there were marked improvements induced by CoQ10 either alone or in combination with EGCG in both NF and PM rats as indicated by the histopathological alterations in different brain regions.
Discussion
In the present investigation, induction of AD-like behavior in rats was done by injection of AlCl3 (70 mg/kg, I.P) daily for consecutive four weeks to rats feeding with either standard protein diet containing 20% casein (NF rats) or low protein diet containing 10% casein (PM rats). Al has been suggested as a causal factor in AD, in part because of reports showing the toxicity of Al, the elevation of Al concentrations in the brains of patients with AD, and an association between Al concentrations in water and the prevalence of AD [46]. Also Al is a notable neurotoxicant that causes acceleration of oxidative damage to biomolecules. Furthermore, Al salts have been reported to cause cell depletion in the hippocampus [47] and degeneration of cholinergic terminals in the cortical areas. It accumulates in the cingulated bundle and thereby induces learning deficits [48].
Results of the present study showed that administration of AlCl3 to both NF and PM rats significantly increased ACHE content; a marker of loss of cholinergic neurons in the brain, but PM rats showed significant increase in the ACHE content more than NF rats. The present result might be attributed to the ability of Al to alter the blood brain barrier and produce marked changes in the cholinergic neurotransmission [49]. Al is well known as a potent brain cholinotoxin with slow accumulation rate [50,51]. The present results are also in parallel with those previously recorded a significant increase in ACHE content in rats treated with AlCl3 (50 mg/kg) daily for three months [52]. It is also reported that ACHE content in different brain regions increased after administration of AlCl3 [53]. The elevation in ACHE content may be attributed to the direct neurotoxic effect of Al or to the disarrangement of the cell membrane phospholipids caused by the associated increase in lipid peroxidation [54].
The data obtained from this investigation showed that administration of AlCl3 significantly increased MDA and decreased SOD and TAC activities in both NF and PM rats. However, PM rats showed a significant increase in MDA and decrease in SOD and TAC activities as compared to NF rats. As previously mentioned, Al is a potent pro-oxidant known to enhance lipid peroxides in the cortex and hippocampus [55]. It has been also reported that, Al induces lipid peroxidation and alter physiological and biochemical characteristics of biological systems [55]. Moreover, as oxidative damage is mediated by free radicals, it was requisite to examine the status of endogenous antioxidant enzymes which are the first line of defence against free radical damage under oxidative stress conditions as in cases of malnutrition [6,8]. In this study, administration of AlCl3 resulted in marked elevation in oxidative stress as indicated by increases in lipid peroxidation (measured as MDA level) and decreases in, SOD and TAC. Al can also reduce axonal mitochondria turnover, disrupt of the Golgi or reduce the synaptic vesicles as a result of the oxidative stress status [56].
Lipid peroxidation is considered one of the main manifestations of oxidative damage and plays an important role in toxicity [57]. Concerning the data obtained in the present work, the elevation of lipid peroxidation in brain of Al-treated rats was evidenced by increased production of MDA. Similar results stated that daily AlCl3 via drinking water for six weeks induces significant increase in MDA concentration in hippocampus and frontal cortex of rats [58]. Nearly similar findings regarding administration of AlCl3 (50 mg/kg/day) in drinking water for a month induced oxidative damage with accumulation of lipid peroxidation [59]. It is also reported that injection of AlCl3 (I.P) for 60 days at different doses can accelerate lipid peroxidation in rat’s brain which may represent one of the most important intoxication mechanisms of Al [60]. The elevation of MDA could be also attributed to the ability of Al itself to accelerate oxidative damage to biomolecules like lipids, protein and nucleic acids [61]. The obtained data revealed also a significant inhibition in the activities of SOD and TAC in brain tissue of AlCl3 treated NF and PM rats. The present findings were consistent with the results of several investigators who revealed marked decrease in endogenous antioxidant after administration of different salts of Al [62]. It is also reported that administration of AlCl3 decreases the content of glutamate-s-transferase [63] and GST as well as the level of sulfhydryl group (SH) in the brain [64] which may be also included in the declining effect of Al on the expression of mRNA of the endogenous antioxidants [65]. Moreover and as a direct effect, intra-hippocampal injections of AlCl3 in rats induce significant increase in MDA concentration [66].
Results of the current study also showed that injection AlCl3 significantly increased Aβ content in the brain of NF and PM rats; PM rats showed significant increase in Aβ content than NF rats. Al is known as a cholinotoxin agent and its neurotoxic effect could be exerted by additional mechanisms such as: induction of oxidative stress, increased production of the ACHE that may be due to a direct action of Aβ which binds to nicotinic receptors or due to over expression of APP. Aβ induced by Al results in increased content of ACHE within and around Aβ plaques [67]. Al is also bound by the Aβ and was found co-localized with it in the AD brain [68]. Amyloid fibrils formed in the presence of Al were slightly thicker, significantly longer and spirally wound around each other. Subsequent studies have been conducted to confirm that amyloid beta sheets will bind up to 4 Al atoms and that binding increased the β- sheet content of the peptide [69]. The neurotoxicity of Aβ in whatever form may involve the formation of reactive oxygen species. Al is a pro-oxidant and is known to promote the oxidation content of Aβ in the presence of iron. It has also been linked to Aβ production through the immune response. It is also linked to activate complement which in turn has been linked to the enhanced aggregation of Aβ [68]. Moreover, self-aggregation of Aβ due to Al administration may lead to generation of hydrogen peroxide and hydroxyl radical via certain chemical reactions and may cause induction of membrane lipid peroxidation [70].
The next step was to confirm the previously mentioned results by the histological examination of different brain regions which showed that administration of AlCl3 (70 mg/kg) for 4 weeks to both NF and PM caused pyknosis and degenerations which were observed in the neuron of the hippocampus and also associated with focal eosinophilic plagues formation in the striatum. Severity of the brain neurological damage induced by Al was more pronounced in PM rats than in NF rats. As in addition to Al neurological damage; protein malnutrition induces deterioration in brain which may be due to increased oxidative stress. Dietary protein is a very important source of essential amino acids that can be used as intracellular antioxidants; therefore its restriction may lead to an increase in oxidative damage by diminishing antioxidant defences of the tissue [71].
On the other hand, cognitive dysfunction and oxidative stress are strongly correlated; agents that modulate reactive oxygen species may be potentially useful as anti-dementia. Further documents demonstrated that, the nutrients related to CNS development and functions are also those that modify individual differences in cognitive development and cognitive performance [72]. Cognition and behaviors such as learning, memory, anxiety and risk assessment depend on the proper development of the limbic system and associated neural areas [73]. Impaired development of limbic system neurons, which play an important role in normal affect and cognition, occurs when maternal protein intake is reduced with resulting brain abnormalities [74]. In recent years, there has been increasing evidence supporting the role of nutrition in AD [5]. A number of dietary factors such as antioxidants, vitamins and polyphenols have been reported to decrease the risk of AD [6].
Results of the present study also showed that administration of EGCG to NF and PM rats during induction of AD using AlCl3 (70 mg/kg) model significantly decreased Aβ content, ACHE content and MDA level, while significantly increased SOD and TAC content. The present results are in accordance with the results in which EGCG increased SOD content and protected against glycation end products induced neurotoxicity by decreasing ROS and MDA [75]. Results are also in agreement with the results showed that EGCG treatment led to increase in SOD content and decrease in MDA in the hippocampus [76]. In addition, EGCG is able to bind Aβ; it may act as an antioxidant and anti-inflammatory against Aβ aggregation in hippocampus and thus have a neuroprotective effect. Actually, Aβ neurotoxicity has been reported to be mediated by free radicals and attenuated by antioxidants and free radical scavengers [77]. Moreover, EGCG has been shown to prevent Aβ induced hippocampal neuronal cell death in cultured hippocampal neurons through its antioxidant properties [78]. In the present work, histological examinations in different brain regions confirmed other findings, where administration of EGCG during induction of AD in rats resulted in: no histopathological alteration in the hippocampus in NF group while, there was mild degeneration and pyknosis in the neurons of the hippocampus associated with plagues formation in the striatum observed in PM group. This result may refer to the marked deterioration caused by protein malnutrition. So, it could be concluded that EGCG has marked protection in NF rats, while in PM rats the protection was limited.
The present results also showed that administration of CoQ10 to NF and PM rats during induction of AD using AlCl3 (70 mg/kg) model significantly decreased Aβ content, ACHE content and MDA level, while significantly increased SOD and TAC content. CoQ10 is a member of the mitochondrial electron transport chain, which is capable of accepting either 1 or 2 electrons. It acts as a potent natural antioxidant, oxygen-derived free radical scavenger and as membrane stabilizer [79]. CoQ10 can stimulate ATPase content and participates in ATP production [80]. In addition, it is able to inhibit mitochondrial ROS generation and inner mitochondrial depolarization [81]. Moreover, plasma membrane protection against oxidative stress is increased due to CoQ10 supplementation [82]. Based on the link between the rate of Aβ production and oxidative stress, CoQ10 exerts its neuroprotective effect via reduction of oxidative stress [83]. The above mentioned functions of CoQ10 permit this coenzyme to exhibit an improvement in each of the biochemical markers as investigated in the present study. It can also protect from severe cholinergic neurons damage in the brain of rats [84]. In the light of what was mentioned; oxidative stress plays a pivotal role in AD [85], damages the neuronal membranes integrity through generation of free radicals [86], reduces the number of nerve cells in aging brain [87] while CoQ10 is a powerful antioxidant and has free radical scavenging property, consequently CoQ10 can reverse the neurodegenerative damages which characterized AD [88]. It is worthy to note that CoQ10 is reduced in AD patients [32,33].
On the other hand, it is well noticed that CoQ10 which is an essential cofactor in the mitochondrial electron transport pathway and has a powerful antioxidant capacity, thus can reduce the oxidative stress, counteract the oxidative damage and protect from brain neuro degeneration caused by PM together with AlCl3 as investigated in the present results. Moreover, the present results may be also explained based on the potential role of CoQ10 in the treatment of neurodegenerative damages associated AD [89]. It is also reported that pre-treatment with CoQ10 prevents Aβ accumulation; it can reduce the influx of extracellular Ca2+, and Ca2+ release from mitochondria due to opening the mitochondrial transition pore after β-amyloid uptake [90]. CoQ10 treatment can also decrease plaque area and number in the hippocampus as well as in the cortex [91] which is in agreement with the results of the histopathological examinations obtained in the current study. It is of great importance to note that, histological examinations of brains of NF and PM groups showed no histo pathological alteration in the hippocampus as well as in the cortex. These findings provide a great evidence of the marked improvement achieved with CoQ10 in NF and PM rats exposed to AlCl3.
Results of the present investigation also showed that combination treatment with EGCG and CoQ10 during induction of AD in both NF and PM rats using AlCl3 (70 mg/kg) model showed marked decrease in ACHE and MDA content as well as in Aβ content together with marked increase in SOD and TAC content. The improvement effect of the combination treatment was more pronounced than that shown with either EGCG or CoQ10 alone. Histological examinations of the different brain regions in both NF and PM groups confirmed these improvement effects.
Therefore, the present study highlights that combination of EGCG and CoQ10 improved all biochemical and histological changes induced by AlCl3 and provided neuro protective in both NF and PM rats, an effect that could be partially correlated with their antioxidant and/or anti-inflammatory properties. However, there is no published data regarding the effect of the combined treatments of EGCG with CoQ10 on AD either in NF or PM animals’ models. There is no also published data concerning the effect of either EGCG or CoQ10 alone on AD in PM animals’ models.
Consequently, it is clear that the severity of the brain neurological damage induced by AlCl3 was more pronounced in PM rats. It is worthy to note also that the base line level of ACHE was lower in PM rats than NF rats indicating their presence at high-risk with increased chances of developing AD. However, the improvement induced by EGCG (as indicated by the histopathological alterations in different brain regions) was more marked in NF rats than in PM rats, while similar improvements were obtained by CoQ10 or its combination with EGCG in both NF and PM rats.
Conclusion
Protein malnutrition is a risk factor in developing AD. It increases the susceptibility and the severity of the brain neurological degeneration associated the pathogenesis of AD. EGCG and/or CoQ10 are effective in minimizing the hazards of aluminum- induced AD. The combination treatment has more pronounced effect in minimizing the hazards of aluminum- induced AD than either EGCG or CoQ10 alone in both NF and PM rats. It is worthy to mention that, marked improvement is obtained by CoQ10 more than EGCG concerning the brain histopathological degenerations induced by aluminum in PM rats. However, further researches are needed to improve the quality of evidence relating to the association of AD with PM.
References
- Jicha GA, Carr SA (2010) Conceptual evolution in Alzheimer's disease: implications for understanding the clinical phenotype of progressive neurodegenerative disease. J Alzheimers Dis 19: 253-272.
- Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM (2007) Forecasting the global burden of Alzheimer's disease. Alzheimers Dement 3: 186-191.
- Jiang T, Yu JT, Tan L (2012) Novel disease-modifying therapies for Alzheimer's disease. J Alzheimers Dis 31: 475-492.
- Williams JW, Plassman BL, Burke J, Benjamin S (2010) Preventing Alzheimer's disease and cognitive decline. Evid Rep Technol Assess (Full Rep) 1-727.
- Shah R1 (2013) The role of nutrition and diet in Alzheimer disease: a systematic review. J Am Med Dir Assoc 14: 398-402.
- Ramassamy C, Belkacémi A (2011) Nutrition and Alzheimer's disease: is there any connection? Curr Alzheimer Res 8: 443-444.
- Hu FB (2002) Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol 13: 3-9.
- Guigoz Y, Lauque S, Vellas BJ (2002) Identifying the elderly at risk for malnutrition. The Mini Nutritional Assessment. Clin Geriatr Med 18: 737-757.
- Saragat B, Buffa R, Mereu E, Succa V, Cabras S, et al. (2012) Nutritional and psycho-functional status in elderly patients with Alzheimer's disease. J Nutr Health Aging 16: 231-236.
- Ousset PJ, Nourhashemi F, Reynish E, Vellas B (2008) Nutritional status is associated with disease progression in very mild Alzheimer disease. Alzheimer Dis Assoc Disord 22: 66-71.
- Soto ME, Secher M, Gillette-Guyonnet S, Abellan van Kan G, Andrieu S, et al. (2012) Weight loss and rapid cognitive decline in community-dwelling patients with Alzheimer's disease. J Alzheimers Dis 28: 647-654.
- Clark TA, Lee HP, Rolston RK, Zhu X, Marlatt MW, et al. (2010) Oxidative Stress and its Implications for Future Treatments and Management of Alzheimer Disease. Int J Biomed Sci 6: 225-227.
- Ono K, Yamada M (2012) Vitamin A and Alzheimer's disease. Geriatr Gerontol Int 12: 180-188.
- Farina M, Lara FS, Brandão R, Jacques R, Rocha JB (2002) Effects of aluminum sulfate on erythropoiesis in rats. Toxicol Lett 132: 131-139.
- Kim MS, Clesceri LS (2001) Aluminum exposure: a study of an effect on cellular growth rate. Sci Total Environ 278: 127-135.
- Nayak P (2002) Aluminum: impacts and disease. Environ Res 89: 101-115.
- Mattson MP (2004) Pathways towards and away from Alzheimer's disease. Nature 430: 631-639.
- Exley C (2005) The aluminium-amyloid cascade hypothesis and Alzheimer's disease. Subcell Biochem 38: 225-234.
- Bus AI, Huang X, Fairlie DP (1999) The possible origin of free radicals from amyloid beta peptides in Alzheimer's disease. Neurobiol Aging 20: 335-337.
- Holmquist L, Stuchbury G, Berbaum K, Muscat S, Young S, et al. (2007) Lipoic acid as a novel treatment for Alzheimer's disease and related dementias. Pharmacol Ther 113: 154-164.
- Holscher C, Gengler S, Gault VA, Harriott P, Mallot HA (2007) Soluble beta-amyloid[25-35] reversibly impairs hippocampal synaptic plasticity and spatial learning. Eur J Pharmacol 561: 85-90.
- Aksenov MY, Aksenova MV, Butterfield DA, Geddes JW, Markesbery WR (2001) Protein oxidation in the brain in Alzheimer's disease. Neuroscience 103: 373-383.
- Ishrat T, Khan MB, Hoda MN, Yousuf S, Ahmad M, et al. (2006) Coenzyme Q10 modulates cognitive impairment against intracerebroventricular injection of streptozotocin in rats. Behav Brain Res 171: 9-16.
- Ishrat T, Parveen K, Khan MM, Khuwaja G, Khan MB, et al. (2009) Selenium prevents cognitive decline and oxidative damage in rat model of streptozotocin-induced experimental dementia of Alzheimer's type. Brain Res 1281: 117-127.
- Ernster L, Dallner G (1995) Biochemical, physiological and medical aspects of ubiquinone function. Biochim Biophys Acta 1271: 195-204.
- Tsuneki H, Sekizaki N, Suzuki T, Kobayashi S, Wada T, et al. (2007) Coenzyme Q10 prevents high glucose-induced oxidative stress in human umbilical vein endothelial cells. Eur J Pharmacol 566: 1-10.
- McDonald SR, Sohal RS, Forster MJ (2005) Concurrent administration of coenzyme Q10 and alpha-tocopherol improves learning in aged mice. Free Radic Biol Med 38: 729-736.
- Dumont M, Kipiani K, Yu F, Wille E, Katz M, et al. (2011) Coenzyme Q10 decreases amyloid pathology and improves behavior in a transgenic mouse model of Alzheimer's disease. J Alzheimers Dis 27: 211-223.
- Vranesić-Bender D (2010) The role of nutraceuticals in anti-aging medicine. Acta Clin Croat 49: 537-544.
- Shinde S, Patil N, TendolkarA (2005) Coenzyme Q10: A review of essential functions. Internet J Nutr Wellness.
- Tran MT, Mitchell TM, Kennedy DT, Giles JT (2001) Role of coenzyme Q10 in chronic heart failure, angina, and hypertension. Pharmacotherapy 21: 797-806.
- Clarke R, Smith AD, Jobst KA, Refsum H, Sutton L, et al. (1998) Folate, vitamin B12, and serum total homocysteine levels in confirmed Alzheimer disease. Arch Neurol 55: 1449-1455.
- Isobe C, Abe T, Terayama Y (2009) Increase in the oxidized/total coenzyme Q-10 ratio in the cerebrospinal fluid of Alzheimer's disease patients. Dement Geriatr Cogn Disord 28: 449-454.
- Shixian Q, VanCrey B, Shi J, Kakuda Y, Jiang Y (2006) Green tea extract thermogenesis-induced weight loss by epigallocatechin gallate inhibition of catechol-O-methyltransferase. J Med Food 9: 451-458.
- Naidu PS, Singh A, Kulkarni SK (2002) Carvedilol attenuates neuroleptic-induced orofacial dyskinesia: possible anti-oxidant mechanisms. Br J Pharmacol 136: 193-200.
- Ahmed S, Rahman A, Hasnain A, Lalonde M, Goldberg VM, et al. (2002) Green tea polyphenol epigallocatechin-3-gallate inhibits the IL-1 beta-induced activity and expression of cyclooxygenase-2 and nitric oxide synthase-2 in human chondrocytes. Free Radic Biol Med 33: 1097-1105.
- Ali AA, Ezzeldin E (2004) Evaluation of the Effect of Caffeine and Nicotine on Postnatal Neuorobehavioral and Functional Development in Normally-fed and Protein Malnourished Rats. J. Egypt. Soc. Toxicol 31: 77-89.
- Ali AA, Ahmed HI, Abu-Elfotuh K (2015) Modeling stages of Alzheimer's disease induced by different doses of aluminum in rats: focus on progression of the disease in response to time. J Alzheimers Dis Parkinsonism 5: 94.
- Rasoolijazi H, Joghataie MT, Roghani M, Nobakht M (2007) The beneficial effect of (-)-epigallocatechin-3-gallate in an experimental model of Alzheimer's disease in rat: a behavioral analysis. Iran Biomed J 11: 237-243.
- Andreassen OA, Weber C, Jørgensen HA (1999) Coenzyme Q10 does not prevent oral dyskinesias induced by long-term haloperidol treatment of rats. Pharmacol Biochem Behav 64: 637-642.
- Bradford MM (1976) Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248-254.
- Mihara M, Uchiyama M (1978) Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem 86: 271-278.
- Nishikimi M, Rao NA, Yagi K (1972) The occurrence of superoxide anion in the reaction of reduced phenazine methosulphate and molecular oxygen. Biochem Biophys Res Comm 46: 849-864.
- Koracevic D, Koracevic G, Djordjevic V, Andrejevic S, Cosic V (2001) Method for the measurement of antioxidant activity in human fluids. J Clin Pathol 54: 356-361.
- Bancroft JD, Gamble M (2008) Theory and Practice of Histological Techniques. Churchill Livingstone, Elsevier, China.
- Rondeau V, Jacqmin-Gadda H, Commenges D, Helmer C, Dartigues JF (2009) Aluminum and silica in drinking water and the risk of Alzheimer's disease or cognitive decline: findings from 15-year follow-up of the PAQUID cohort. Am J Epidemiol 169: 489-496.
- Miu AC, Benga O (2006) Aluminum and Alzheimer's disease: a new look. J Alzheimers Dis 10: 179-201.
- Platt B, Fiddler G, Riedel G, Henderson Z (2001) Aluminium toxicity in the rat brain: histochemical and immunocytochemical evidence. Brain Res Bull 55: 257-267.
- Yokel RA (2000) The toxicology of aluminum in the brain: a review. Neurotoxicology 21: 813-828.
- Gulya K1, Rakonczay Z, Kása P (1990) Cholinotoxic effects of aluminum in rat brain. J Neurochem 54: 1020-1026.
- Kumar S (1998) Biphasic effect of aluminium on cholinergic enzyme of rat brain. Neurosci Lett 248: 121-123.
- Bihaqi SW, Sharma M, Singh AP, Tiwari M (2009) Neuroprotective role of Convolvulus pluricaulis on aluminium induced neurotoxicity in rat brain. J Ethnopharmacol 124: 409-415.
- Kaizer RR, Corrêa MC, Spanevello RM, Morsch VM, Mazzanti CM, et al. (2005) Acetylcholinesterase activation and enhanced lipid peroxidation after long-term exposure to low levels of aluminum on different mouse brain regions. J Inorg Biochem 99: 1865-1870.
- Kumar A, Dogra S, Prakash A (2009) Effect of carvedilol on behavioral, mitochondrial dysfunction, and oxidative damage against D- galactose induced senescence in mice. Naunyn Schmiedebergs Arch Pharmacol 380: 431-441.
- Prakash NT, Rao KS (1995) Modulations in antioxidant enzymes in different tissues of marine bivalve Pernaviridis during heavy metal exposure. Mol Cell Biochem 146: 107-113.
- Mahdy K, Shaker O, Wafay H, Nassar Y, Hassan H, et al. (2012) Effect of some medicinal plant extracts on the oxidative stress status in Alzheimer's disease induced in rats. Eur Rev Med Pharmacol Sci 16 Suppl 3: 31-42.
- Anane R, CreppyE (2001) Lipid peroxidation as pathway of aluminum cytotoxicity in human skin fibroblast cultures: prevention by superoxide dismutase + catalase and vitamins E and C. Hum Exp Toxicol 20: 477-481.
- Nedzvetsky VS, Tuzcu M, Yasar A, Tikhomirov AA, Baydas G (2006) Effects of vitamin E against aluminum neurotoxicity in rats. Biochemistry (Mosc) 71: 239-244.
- Jyoti A, Sethi P, Sharma D (2007) Bacopa monniera prevents from aluminium neurotoxicity in the cerebral cortex of rat brain. J Ethnopharmacol 111: 56-62.
- Yang YX, Niu Q, Niu PY, Lou J (2006) [Effects of aluminum on lipid peroxidation in rat's brain and its sex - related difference]. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi 24: 281-283.
- Strong MJ, Garruto RM, Joshi JG, Mundy WR, Shafer TJ (1996) Can the mechanisms of aluminum neurotoxicity be integrated into a unified scheme? J Toxicol Environ Health 48: 599-613.
- Padurariu M, Ciobica A, Hritcu L, Stoica B, Bild W, et al. (2010) Changes of some oxidative stress markers in the serum of patients with mild cognitive impairment and Alzheimer's disease. Neurosci Lett 469: 6-10.
- El-Demerdash FM (2004) Antioxidant effect of vitamin E and selenium on lipid peroxidation, enzyme activities and biochemical parameters in rats exposed to aluminium. J Trace Elem Med Biol 18: 113-121.
- Newairy AS, Salama AF, Hussien HM, Yousef MI (2009) Propolis alleviates aluminium-induced lipid peroxidation and biochemical parameters in male rats. Food Chem Toxicol 47: 1093-1098.
- Manal S, Azza H, Eman T (2010) The Protective Effect of Vitamin E against the neurotoxic Effect of Aluminum Cholorid in Male Albino Rat. J Am Science 6: 978-991.
- Stevanovic ID, Jovanovic MD, Jelenkovic A, Colic M, Stojanovic I, et al. (2009) Effects of L-NAME, a non-specific nitric oxide synthase inhibitor, on AlCl3- induced toxicity in the rat forebrain cortex. J Vet Sci 10: 15-22.
- Ahmed HH, Shousha WG, Hussein RM, Farrag AH (2011) Potential role of some nutraceuticals in the regression of Alzheimer’s disease in an experimental animal model. Turk J Med Sci 41: 455-466.
- Geremia E, Baratha D, Zafarana S, Giordano R, Pinizzotto MR, et al. (1990) Antioxidant enzymatic systems in neuronal and glial cell enriched fractions of rat brain. Neurochem Res 15: 719-723.
- Vyas SB, Duffy LK (1995) Stabilization of secondary structure of Alzheimer beta-protein by aluminum(III) ions and D-Asp substitutions. Biochem Biophys Res Commun 206: 718-723.
- Lynch T, Cherny RA, Bush AI (2000) Oxidative processes in Alzheimer's disease: the role of abeta-metal interactions. Exp Gerontol 35: 445-451.
- Feoli AM, Siqueira IR, Almeida L, Tramontina AC, Vanzella C, et al. (2006) Effects of protein malnutrition on oxidative status in rat brain. Nutrition 22: 160-165.
- Worobey J, Tepper BJ, and Kanarek R (2005) Nutrition and behavior. Cambridge MA: CABI Publishing.
- Morgane PJ, Galler JR, Mokler DJ (2005) A review of systems and networks of the limbic forebrain/limbic midbrain. Prog Neurobiol 75: 143-160.
- Lister JP, Tonkiss J, Blatt GJ, Kemper TL, DeBassio WA, et al. (2006) Asymmetry of neuron numbers in the hippocampal formation of prenatally malnourished and normally nourished rats: a stereological investigation. Hippocampus 16: 946-958.
- Lee SJ, Lee KW (2007) Protective effect of (-)-epigallocatechin gallate against advanced glycation endproducts-induced injury in neuronal cells. Biol Pharm Bull 30: 1369-1373.
- He M, Zhao L, Wei MJ, Yao WF, Zhao HS, et al. (2009) Neuroprotective effects of (-)-epigallocatechin-3-gallate on aging mice induced by D-galactose. Biol Pharm Bull 32: 55-60.
- Ehrnhoefer DE, Bieschke J, Boeddrich A, Herbst M, Masino L, et al. (2008) EGCG redirects amyloidogenic polypeptides into unstructured, off-pathway oligomers. Nat Struct Mol Biol 15: 558-566.
- Levites Y, Amit T, Mandel S, Youdim MBH (2003) Neuroprotection and neurorescue against Aß toxicity and PKCdependent release of non-amyloidogenic soluble precursor protein by green tea polyphenol (-) - epigallocatechin-3-gallate. FASEB J 17: 952-954.
- Ostrowski RP (1999) Effect of coenzyme Q10 (CoQ10) on superoxide dismutase activity in ET-1 and ET-3 experimental models of cerebral ischemia in the rat. Folia Neuropathol 37: 247-251.
- Ebadi M, Govitrapong P, Sharma S, Muralikrishnan D, Shavali S, et al. (2001) Ubiquinone (coenzyme q10) and mitochondria in oxidative stress of parkinson's disease. Biol Signals Recept 10: 224-253.
- Kwong LK, Kamzalov S, Rebrin I, Bayne ACV, Jana CK, et al. (2002) Effects of coenzyme Q (10) administration on its tissue concentrations, mitochondrial oxidant generation, and oxidative stress in the rat. Free Radical Biol Med 33: 627- 638.
- Gómez-Díaz C, Burón MI, Alcaín FJ, González-Ojeda R, González-Reyes JA, et al. (2003) Effect of dietary coenzyme Q and fatty acids on the antioxidant status of rat tissues. Protoplasma 221: 11-17.
- Ostrowski RP (2000) Effect of coenzyme Q(10) on biochemical and morphological changes in experimental ischemia in the rat brain. Brain Res Bull 53: 399-407.
- Hoyer S, Lannert H (1999) Inhibition of the neuronal insulin receptor causes Alzheimer-like disturbances in oxidative/energy brain metabolism and in behavior in adult rats. Ann N Y Acad Sci 893: 301-303.
- Markesbery WR (1997) Oxidative stress hypothesis in Alzheimer's disease. Free Radic Biol Med 23: 134-147.
- Liu R, Liu IY, Bi X, Thompson RF, Doctrow SR, et al. (2003) Reversal of age-related learning deficits and brain oxidative stress in mice with superoxide dismutase/catalase mimetics. Proc Natl Acad Sci U S A 100: 8526-8531.
- Morrison JH, Hof PR (1997) Life and death of neurons in the aging brain. Science 278: 412-419.
- Beal MF (2004) Therapeutic effects of coenzyme Q10 in neurodegenerative diseases. Methods Enzymol 382: 473-487.
- Mancuso M, Coppede F, Migliore L, Siciliano G, Murri L (2006) Mitochondrial dysfunction, oxidative stress and neurodegeneration. J Alzheimers Dis 10: 59-73.
- Durán-Prado M, Frontiñán J, Santiago-Mora R, Peinado JR, Parrado-Fernández C, et al.(2104) Coenzyme Q10 Protects Human Endothelial Cells from ß-Amyloid Uptake and Oxidative Stress-Induced Injury. PLOS One 9: e109223.
- Dumont M, Kipiani K, Yu F, Wille E, Katz M, et al. (2011) Coenzyme Q10 decreases amyloid pathology and improves behavior in a transgenic mouse model of Alzheimer's disease. J Alzheimers Dis 27: 211-223.
Relevant Topics
- Advanced Parkinson Treatment
- Advances in Alzheimers Therapy
- Alzheimers Medicine
- Alzheimers Products & Market Analysis
- Alzheimers Symptoms
- Degenerative Disorders
- Diagnostic Alzheimer
- Parkinson
- Parkinsonism Diagnosis
- Parkinsonism Gene Therapy
- Parkinsonism Stages and Treatment
- Stem cell Treatment Parkinson
Recommended Journals
Article Tools
Article Usage
- Total views: 13862
- [From(publication date):
June-2016 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 12751
- PDF downloads : 1111