Comparative Stability of Different Brands of Esomeprazole Magnesium Trihydrate of Enteric-Coated Pellets Capsules
Received: 02-Nov-2022 / Manuscript No. jpcm-22-78078 / Editor assigned: 04-Nov-2022 / PreQC No. jpcm-22-78078(PQ) / Reviewed: 18-Nov-2022 / QC No. jpcm-22-78078 / Revised: 23-Nov-2022 / Manuscript No. jpcm-22-78078(R) / Published Date: 29-Nov-2022 DOI: 10.4172/2165-7386.1000487
Abstract
The aim of study is to determine the stability of Esomeprazole magnesium trihydrate under environmental conditions and Accelerated stability chamber. The drug products of esomeprazole enteric-coated pellets are more sensitive to environmental factors like heat, humidity, and light. These factors affect the stability of the product if not stored under the necessary condition. A stability study of enteric-coated pellets of esomeprazole magnesium trihydrate was performed following the USP method. Stability testing was performed for a new pharmaceutical product. The accelerated test was performed for the evolution of the stability at climate change like 40°C±2°C and 75±5% relative humidity (RH). The newly formulated brands were kept in the accelerated chamber (40 ± 2 0C / 75 ± 5% RH) for six months. After three and six months, products were analyzed at the initial interval stage by a High- Performance liquid Chromatography with a UV detector. The other product brands at different stages were also analyzed in the mid-shelf shelf life, near to expired, and expired products by the HPLC method under environmental conditions. The chromatography method of HPLC is based on the UV detector using the mixture of mobile phase acetonitrile, Buffer, and Distilled Water with a ratio of 350 ml: 500 ml: 150 ml, respectively. The flow rate is 1ml/min and detected at 305nm. During stability studies checked the appearance, potency, and bioavailability. The newly formulated brands give results under limits and are considered stable products under accelerated conditions. But other brands at different stages give some results under the low range and out of average limit and are not stable products throughout and after the shelf life. This study helps to check the stability of the esomeprazole magnesium products throughout the shelf life under accelerated and environmental conditions.
Keywords
Esomeprazole magnesium trihydrate; High-Performance liquid Chromatography; Enteric-coated pellets; Stability; Accelerated Chamber; Environmental condition
Introduction
The open sores are present inside the lining of the human stomach and esophagus and the outer layer of the small intestine of the human body. That disease is called peptic ulcer. A physiological balance exists under normal conditions between the peptic acid secretion gastro duodenum mucosal defenses. Peptic ulcer disease occurs due to the disturbance of the balance between aggressive factors like NSAIDs, alcohol, bile salt, acid, mucus, cellular retention, and mucosal blood flow [1].
The different gastro-esophageal reflux diseases or peptic ulcers are treated using proton inhibitors. These inhibitors provide the best control for the symptomatic and recover the healing of the esophagus. Proton pump inhibitors give satisfaction for the cure of the ulcer. Esomeprazole is the S isomer of the omeprazole and developed as a single stable optical isomer. It is the best drug for the treatment of acid suppression than omeprazole [2].
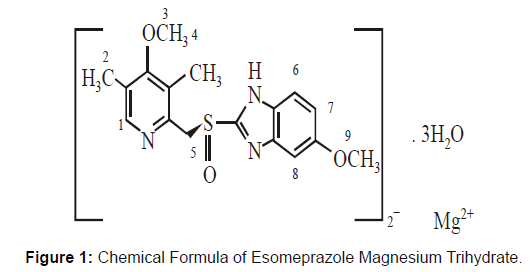
• Molecular Formula Esomeprazole molecular formula is (C17H18N3O3S) 2 Mg.3H2O.
• Chemical Formula Esomeprazole magnesium trihydrate has the chemical formula bis(5-methoxy-2-[(S)-[(4-methoxy-3,5- dimethyl-2-pyridinyl) methyl] sulfinyl]-1H-benzimidazole-1-yl) magnesium trihydrate.
Molecular Weight Esomeprazole magnesium trihydrate has a molecular weight of 767.2 and 713.1 on an anhydrous basis. Figure 1 Chemical Formula of Esomeprazole Magnesium Trihydrate The Esomeprazole magnesium stability depends upon the changes in pH. It is easily degraded and unstable under an acidic medium and has strength under an alkaline medium. The half-life of magnesium salt proton inhibitor is about "19 hours" at 25°C temperature and "8 hours" at 37°C temperature.
Esomeprazole magnesium trihydrate is used as enteric-coated pellets because it quickly degraded in the acidic condition of the stomach. The enteric coating unstable and can easily be degraded by environmental effects. Some factors, temperature, light, pH, Oxidation, and enzymatic degradation affect the stability of drugs. These factors also affect the efficiency of the product. Mainly the pH affects more on the enteric coating of the drug to prevent drug release in the stomach, which is a highly acidic medium that is useful to inhibit it from rapid degradation in the stomach.
Those factors quickly degrade and unstable the product, losing its efficiency and stability. Dosage form's chemical and physical strength is essential to ensure quality and safety. It is also beneficial for the patient. The expiry date of any product is the period during which it will stay stable if stored according to the manufacturer's instructions. As a result, an expiry date is the time limit in which the product will no longer be fit for use.
The shelf-life is the duration of time in which it will keep fit for use if stored adequately according to instructions. The shelf life is the duration on the labels of the dosage form that designates the period during which a batch of the product remains stable within the approved shelf-life specifications. Some medicines readily degraded in a short time; it depends on the formulations and use of low-cost raw material, packing materials, and due to some factors. Those medicines are not more effective for the patient and lose the standard of any pharmaceutical [3-4]
Many pharmaceutical products degraded in the storage cause the instability problem of the product become unfit for the patient use. It lacks efficiency, safety, and acceptability in a few days.
The United State Pharmacopeia and Federal Food drug administration give a well-developed method to ensure the drug stability under labeled storage conditions. The USP and FDA provide the advanced technology of the HPLC for the stability testing of the product [5]
The degradation classification of the pharmaceutical dosage form depends on the chemical, physical, and biological properties that lose their stability. Many pharmaceutical products lose their assay potency and are less than the limit of the label claim, cause to the instability of the drug [6].
Environmental factors quickly affect drugs and change their physical and chemical structure and properties. The manufacturer must ensure the product and drug quality under environmental conditions during transport, storage, and manufacture. Stability studies are very important for selecting drug packing material and storage conditions. Those studies allow avoiding the drug's physical and chemical degradation and interaction with excipients. The precautions are essential for storing the medications under severe environmental conditions.
The study's results will explore esomeprazole magnesium trihydrate's physical and chemical stability and shelf life. The study will be more beneficial for the patient and pharmaceutical industries. Deterioration of the product converts into the unstable dosage form loss the stability and activity according to the label claim. It may cause the therapy fails to result in death [7].
Stability is the time range in which a dosage form will retain all its original properties within defined parameters under specific storage circumstances and container-closure systems. The United States Pharmacopeia (USP, 2016) defines stability as the content potency of a product retains the same features and characteristics that it had at the manufacturing stage, within defined limits, and during its storage period, which is called its shelf-life [8-19].
The time in which the formulation of the product remains stable under required store conditions is called shelf life and expiration date. After the expiration rate, drugs no longer retain fitness for us. The drugs are considered to destroy more rapidly if products are not stored under critical conditions [20-22]. The storage conditions or requirements must be labeled on the product, which helps us confirm the dosage form's stability according to the expiration date. Shelf life is when we consider that drugs retain their stability until they expire. The pharmaceutical used only qualitative and semi-quantitative procedures for drug studies from about 1950. Stability testing protocols like accelerated stability, real-time stability, and validated stability claim may determine the shelf life.
It is a method by which the stability of the drug may be estimated by the storage conditions that accelerate degradation caused at high temperatures. The accelerated changes occur under stress conditions like light, temperature, moisture, pH, packing, gravity, etc. This method provides a shelf life of the product under shortened development schedule. This method allows some conditions to detect the drug's stability at a temperature from data obtained by stress testing. This statistical treatment in accelerated stability is based on the Arrhenius equation requires that four stress temperatures be used.
Most models of accelerated stability testing are based on the Arrhenius Equation [23-24].
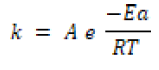
K = rate constant at temperature T.
Ea = activation energy.
R=gas constant.
This equation gives the relationship between the degradation rate and temperature storge.
This equation shows the stability of drugs from the degradation rate at the high temperature for the degradation process. USP compendia monographs specify the storage conditions retained throughout the product's expiration date, distribution, and storage. USP storage limitations fall into two major classifications, specific and nonspecific. Many monographs give particular storage conditions like "store in a cool place." The required specific storage conditions under "freezer, cold, cool room temperature, excessive heat, warm," etc.
The storage of the product under nonspecific conditions regardless of quantity where no critical store conditions are in the individual monograph, it is considered that required specific conditions include "moisture, storage at controlled room temperature and protection from the light" [25-26]. The lengthy procedure of stability studies and storage requirements should be enough to cover storage and shipment. The accelerated and long-term storage conditions and minimum period are mentioned in Table 1.
| Sr. No. | Conditions | Minimum period duration |
|---|---|---|
| 1 | Long-term testing 25 °c +/- 2 °c I 60 +/- 5% RH | 12 months |
| 2 | Accelerated testing 40 °c +/- 2 °c I 75 +/- 5%RH | 6 months |
Table 1: In This Table Mentions the Stability Testing Conditions and Periods.
Accelerated testing significant changes and testing at intermediate conditions may be used like 30 °C ±2 °C and 60 ±5% RH. The data was used to find the effect of short-term duration outside the label-specific storage condition .
The testing protocol under specific conditions is defined as testing performed every three months during the first year, every six months during the second year, and then after one year. The long-term testing should be performed after twelve months for shelf-life surety at specific test periods. The packing material used for storage condition and distribution is the same as the container used in the long-term, realtime stability evaluation .
The dissolution of a pharmaceutical product present in solid form is the process by which a solid drug is converted from its original formulation to a suitable solution under controlled and ideal conditions. In quality, control dissolution is very important and critical testing at the final manufacturing product. It is considered a standard method for checking the batch-to-batch solid oral drug delivery system like tablets and capsules. According to USP, there is seven specified dissolution testing system. Different types of dissolution systems are used in different types of dosage forms under specified conditions like medium, the volume of medium, RPM, UV wavelength detecting, etc
Aim and Objectives of the Study
The proposed method will be more reliable for the determination of the shelf life of the dosage forms. That study will help study the stability and shelf life of pharmaceuticals.
The research aims to perform a validated method for determining the stability of Esomeprazole magnesium trihydrate capsule in different batches at different stages using high-performance liquid chromatography and other equipment.
• A stability study is essential to detect whether a product is stable or unstable at the earliest stage.
• Then check the shelf life and potency of the product near expiry and expiry date.
• To perform the dissolution test of all brands at all stages.
• To check the stability of the newly formulated product under accelerated conditions.
• To perform the assay of all brands at all stages to check the potency.
• Unstable product lowering the potency of active drug in the dosage form
• Hazardous products decompose, which may lead to toxic products.
• Transportation from one place to another place changes its physical property.
Materials and Reagents
Hydrochloric acid (fuming 37%) was analytical grade purchased from Germany. Acetonotrile, Ethanol and water (all HPLC-grade) were purchased from Sigma-Aldrich. Tri-Sodium phosphate-12-hydrate, di- Sodium hydrogen phosphate-2-hydrate, Sodium phosphate monobasic (all analytical grade) were purchased from Riedel-de Haen.
Method
Instrumentation and Chromatographic Conditions The chromatographic system consisted of Hitachi multi-solvent delivery system pump, auto sampler with variable injection valve and UV– visible tunable absorbance detector. Mode of HPLC was LC and detector was UV. Separation was performed on a 4.6mm*15 cm packing L1. A mixture of analytical grade acetonitrile, buffer, and distilled water was used to make the mobile phase. The flow rate of the mobile phase through the analytical column was 1ml/min, at room temperature. The detection wavelength was set at 305 nm.
Preparation of Standard Sample Solution for Assay A solution was prepared of working standard omeprazole by using alcohol, diluent, and distilled water. This solution contains a concentration of 0.04 mg/ml of omeprazole.
The sample stock solution was prepared by taking the mixed contents of 20 capsules filled with enteric-coated Esomeprazole magnesium trihydrate. This sample stock solution was further used for prepared a concentration of 0.04 mg/ml of Esomeprazole by using alcohol, diluent, and distilled water.
Dissolution
Dissolution is an in vitro test determining the rate and time at which the dosage form dissolved into the solution. Basically in vitro test is most important for the evolution of solid oral drugs. It provides bioavailability studies. The bioavailability test was checked comparative brands of esomeprazole magnesium trihydrate through dissolution test and checked at different intervals like an initial stage, mid-stage, near expiry, and the expiry stage.
Preparation of Dissolution Medium
Preparation of 0.1 N HCl: First, carefully prepare 1N HCl solution by concentrated analytical grade HCl. Some precautions are always followed when concentrated HCl prepares 1 N HCl. 85 ml of concentrated HCl are carefully transferred into 1000ml of the cylinder and added 500 ml of distilled water and then transferred concentrated HCl; otherwise, splashes of HCl are spread in the surrounding. Carefully adjust the volume up to the mark of 1000ml of a cylinder. Then 0.1 N HCl is prepared from 1N HCl up to the volume required in dissolution.
Dissolution Test Parameters
Dissolution test was performed by using different parameters paddle type 2 with 0.1 NHCL and Phosphate Buffer pH 6.8 with 1000 ml medium. The test was performed with 100 RPM for 2 hours and 30 mints for HCL and Buffer respectively. The temperature must be 370C ± 0.50C.
Preparation of Standard Solution
An accurately weighed quantity of about 100 mg of Esomeprazole as Magnesium pellets and transferred to the 50 ml volumetric flask. Add the phosphate buffer pH 6.8 in a 50 ml volumetric flask to dilute the standard. 1ml of the standard solution was taken from the stock solution and transferred to a 100 ml flask. Then immediately added 2ml of 0.25 M NaOH to the 100 ml standard solution and adjusted the volume with phosphate buffer. The flask is covered by the cap and labeled. Protect from the light.
Preparation of Sample Solution
Some volume of the sample solution was taken from each dissolution vessel one by one. Filter these samples of dissolution of each vessel. Added the 5 ml is of the dissolution filtrate sample in the suitable glassware and added the 1 ml of 0.25 M sodium hydroxide to the filtrate solution of dissolution. Mix well and protect from sunlight and heat.
Result = ru/rs × Cs/L ×V 100
Result
Assay of different brands
Espra Capsule 40mg
The newly formulated Espra capsule 40 mg brands has checked the potency at different intervals of time under required accelerated chamber conditions by following the HPLC method.
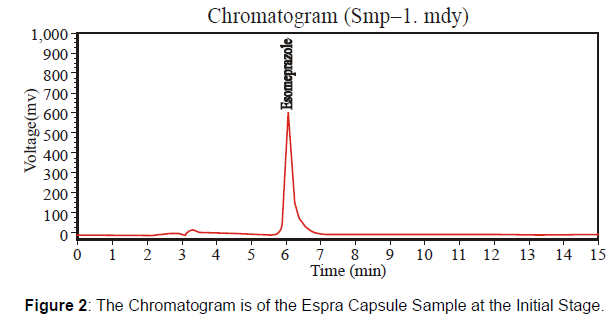
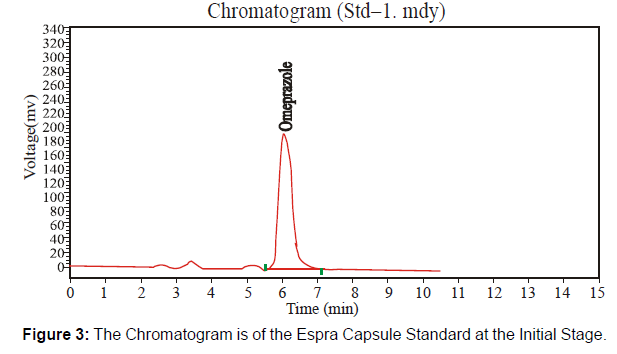
At the Initial Stage of the product: This newly formulated Espra Capsule 40 mg has the batch number "HF 413" at the initial stage. Check this batch at a different time at the initial stage, after three months, and after six months under accelerated conditions [Figure 2 and 3, Table 2]
| Solution | Retention Time | Height | Theoretical plates | Average Area |
|---|---|---|---|---|
| Sample | 6.098 | 582791938 | 3576.919 | 9690210 |
| Standard | 6.057 | 233661.406 | 2877.317 | 5322160 |
Table 2: Parameters of System Suitability.
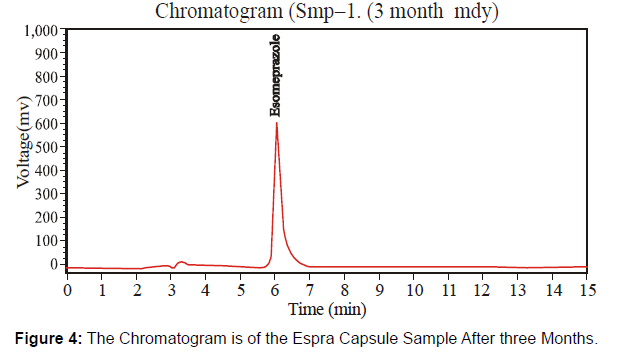
After three Months duration: This newly formulated Espra Capsule 40 mg has the batch number "HF 413" checked potency after three months under accelerated conditions by following the HPLC method. [Figure 4-5, Table 3]
Different system suitability parameter values of standard and sample chromatogram of Espra Capsule at the three months are mentioned in table 3.
| Solution | Retention Time | Height | Theoretical plates | Area |
|---|---|---|---|---|
| Sample | 6.094 | 591162.210 | 3461.812 | 9711134 |
| Standard | 6.055 | 212172.458 | 5129.778 | 5114679 |
Table 3: Parameters of System Suitability.
After Six Month Stage: This newly formulated Espra Capsule 40 mg has the batch number "HF 413" checked potency after six months under accelerated conditions by following the HPLC method. [Figure 6-7, Table 4]
| Solution | Retention Time | Height | Theoretical plates | Area |
|---|---|---|---|---|
| Sample | 6.053 | 590817 | 3913.127 | 9918157 |
| Standard | 6.012 | 211296.176 | 4319.214 | 5239617 |
Table 4: Parameters of System Suitability.
Espra Capsule 20 mg
This Espra Capsule 20 mg has the batch number "HF 373" at the mid-stage of the expired date. Following the HPLC method, I checked potency at the mid-stage of the shelf life under environmental conditions. [Figure 8-9. Table 5]
| Solution | Area | Retention Time | Height | Theoretical plates |
|---|---|---|---|---|
| Sample | 9667890 | 6.093 | 582828.125 | 3571.049 |
| Standard | 5909529 | 5.823 | 417829.531 | 4398.577 |
Table 5: Parameters of system suitability.
Espra Capsule 40mg
This Espra Capsule 40 mg has the batch number "HF 419" at the nearly expired stage. Following the HPLC method, I checked potency at the nearly expired shelf life under environmental conditions. [Figure 10-11, Table 6]
The HPLC system suitability parameter values of standard and sample chromatogram of Espra Capsule 40 mg at nearly expiring shelflife duration are mentioned in table 6.
| Solution | Area | Retention Time | Height | Theoretical plates |
|---|---|---|---|---|
| Sample | 9514709 | 6.093 | 579595.750 | 3570.852 |
| Standard | 52009305 | 6.062 | 209800.359 | 3099.268 |
Table 6: Parameters of System Suitability.
Espra Capsule 20mg
This Espra Capsule 20 mg has the batch number "HF 352" at expire stage. Following the HPLC method, I checked potency at the expired shelf life under environmental conditions. [Figure 12-13, Table 7]
The HPLC system suitability parameter values of standard and sample chromatogram of Espra Capsule 20 mg at expiring shelf-life duration are mentioned in table 7.
| Solution | Area | Retention Time | Height | Theoretical plates |
|---|---|---|---|---|
| Sample | 4713742 | 6.073 | 583034.395 | 4963.601 |
| Standard | 5787176.5 | 5.918 | 407171.40 | 5216.607 |
Table 7: Parameters of System Suitability.
Essopel Insta Capsule 40mg
The newly formulated Essopel Insta capsule 40 mg brands has checked the potency at different intervals under required accelerated chamber conditions by following the HPLC method.
At the Initial Stage of the product
This newly formulated Essopel Insta Capsule 40 mg has the batch number "111131" at the initial stage. Check this batch at a different time at the initial stage, after three months, and after six months under accelerated conditions. [Figure 14-15, Table 8]
Different system suitability parameter values of standard and sample chromatogram of Essopel Insta Capsule at the initial stage are mentioned in table 8.
| Solution | Area | Retention Time | Height | Theoretical plates |
|---|---|---|---|---|
| Sample | 9713543 | 6.098 | 582653.000 | 2084.729 |
| Standard | 5497296 | 5.857 | 264691.313 | 2452.897 |
Table 8: Parameters of System Suitability.
After three Month Stage
This newly formulated Essopel Insta Capsule 40 mg has the batch number "111131" after three months. Check this batch at a different time at the initial stage, after three months, and after six months under accelerated conditions. [Figure 16-17, Table 9]
Different system suitability parameter values of standard and sample chromatogram of Essopel Insta Capsule after the three month stage are mentioned in table 9.
| Solution | Area | Retention Time | Height | Theoretical plates |
|---|---|---|---|---|
| Sample | 10415214 | 5.512 | 485615.190 | 5721.161 |
| Standard | 5517140.5 | 5.617 | 283980.514 | 4137.095 |
Table 9: Parameters of System Suitability.
After Six Month Stage
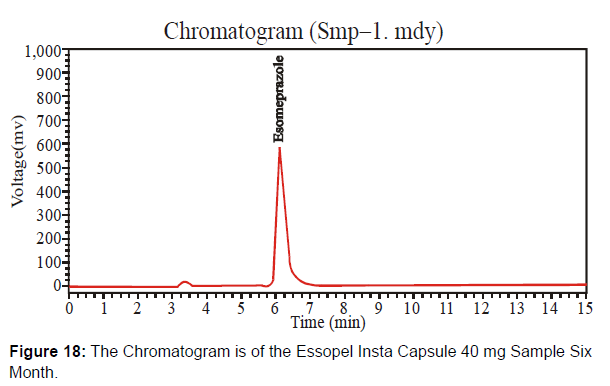
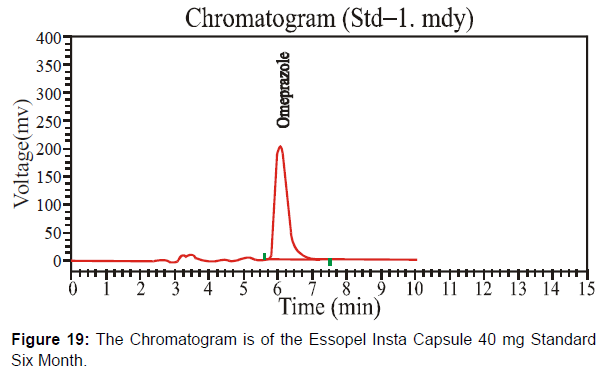
This newly formulated Essopel Insta Capsule 40 mg has the batch number "111131" after six months. Check this batch after six months under accelerated conditions. [Figure 18-19, Table 10]
Different system suitability parameter values of standard and sample chromatogram of Essopel Insta Capsule 40mg after six months are mentioned in table 10.
| Solution | Retention Time | Height | Theoretical plates | Area |
|---|---|---|---|---|
| Sample | 6.093 | 583180.063 | 3571.056 | 9749849.0 |
| Standard | 6.062 | 210286.516 | 3099.268 | 5287616.5 |
Table 10: Parameters of System Suitability.
Essopel Insta Capsule 40mg
This Essopel Insta Capsule 40 mg has the batch number "01072" at the mid-stage of the expired date. Following the HPLC method, I checked potency at the mid-stage of the shelf life under environmental conditions. [Figure 20-21, Table 11]
The HPLC system suitability parameter values of standard and sample chromatogram of Essopel Insta Capsule 40 mg at mid-stage of shelf-life duration are mentioned in table 11.
| Solution | Area | Retention Time | Height | Theoretical plates |
|---|---|---|---|---|
| Sample | 9909190 | 6.196 | 581170.619 | 4314.711 |
| Standard | 5481184 | 5.991 | 382520.181 | 3971.011 |
Table 11: Parameters of System Suitability.
Essopel Insta Capsule 40 mg
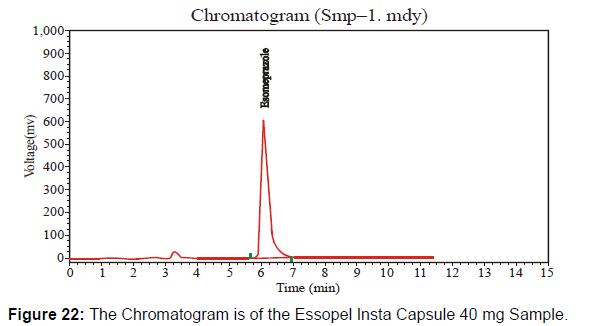
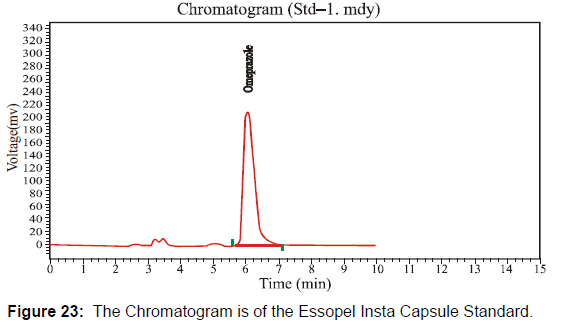
This Essopel Insta Capsule 40 mg has the batch number "00642" at the nearly expired stage. Following the HPLC method, I checked potency at the nearly expired shelf life under environmental conditions. [Figure 22-23, Table 12]
The HPLC system suitability parameter values of standard and sample chromatogram of Essopel Insta Capsule 40 mg at nearly expiring shelf-life duration are mentioned in table 12.
| Solution | Area | Retention Time | Height | Theoretical plates |
|---|---|---|---|---|
| Sample | 9514709 | 6.093 | 579595.750 | 3570.852 |
| Standard | 52009305 | 6.062 | 209800.359 | 3099.268 |
Table 12: Parameters of System Suitability.
Essopel Insta Capsule 40 mg
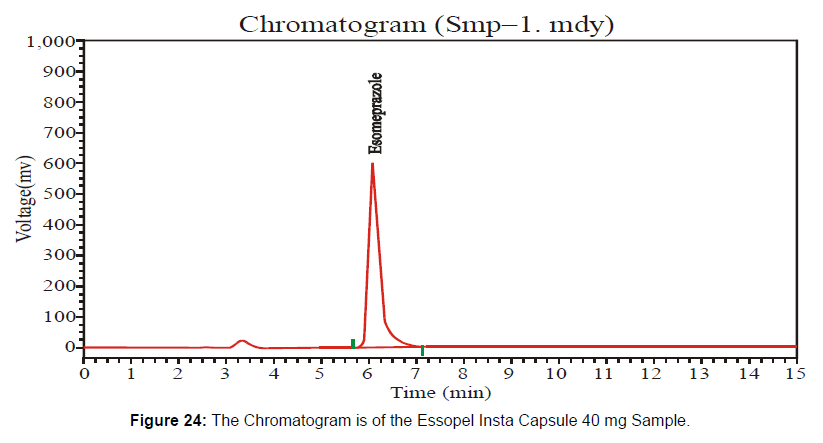
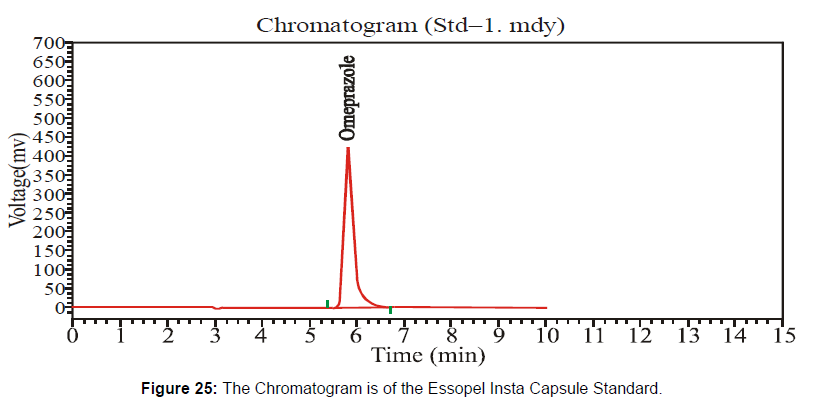
This Essopel Insta Capsule 40 mg has the batch number "911122" at the expired stage. Following the HPLC method, I checked potency at the expired shelf life under environmental conditions. [Figure 24-25, Table 13]
The HPLC system suitability parameter values of standard and sample chromatogram of Essopel Insta Capsule 40 mg at expiring shelf-life duration are mentioned in table 13.
| Solution | Area | Retention Time | Height | Theoretical Plates |
|---|---|---|---|---|
| Sample | 9621826 | 6.093 | 582477.063 | 3576.913 |
| Standard | 5798654.5 | 5.823 | 417319.875 | 4398.561 |
Table 13: Parameters of System Suitability.
The results of the newly prepared two different brands under the accelerated condition at different stages like the initial stage, after three months, and after six months.
The Espra capsule 40 mg batch number HF413 gives the same results at the initial stage, after three months, after six months 100.11%, 100.31%, 100.01%, respectively.
The Essopel Insta Capsule 40 mg batch number 111131 gives the same results at the initial stage, after three months, after six months 101.53%, 101.53%, 101.88%, respectively. It shows that they are stable under accelerated conditions. The brand's names, batch number, manufacturing date, Expire date, and assay percentages mentioned in table 14.
| Sr.No. | Brands Name | Batch No. | Mfg.Date | Exp. Date | At Initial Stage (Assay %) |
After three month (Assay %) |
After six month (Assay %) |
|---|---|---|---|---|---|---|---|
| 1 | Espra Capsule 40mg | HF413 | 11-2021 | 10-2023 | 100.11 % | 100.31 % | 100.01% |
| 2 | Essopel Insta Capsule 40mg | 111131 | 11-2021 | 11-2023 | 101.53 % | 101.32% | 101.86% |
Table 14: Results of Assay of the newly formed product.
The average standard, sample, and assay percentage results of the two different brands at different stages. The different stages of different brands at the mid of the shelf life batch ( Espra Capsule 20 mg Batch # HF373, Essopel Insta Capsule 40mg Batch #01072 ) give results 98.76%, 97.52%, respectively. Then nearly expired (Espra Capsule Batch # HG419, Essopel Insta Capsule Batch # 00642) gives results
The HPLC system suitability parameter values of standard and sample chromatogram of Essopel Insta Capsule 40 mg at nearly expiring shelf-life duration are mentioned in table 12.
| Solution | Area | Retention Time | Height | Theoretical plates |
|---|---|---|---|---|
| Sample | 9514709 | 6.093 | 579595.750 | 3570.852 |
| Standard | 52009305 | 6.062 | 209800.359 | 3099.268 |
Table 12: Parameters of System Suitability.
Essopel Insta Capsule 40 mg
This Essopel Insta Capsule 40 mg has the batch number "911122" at the expired stage. Following the HPLC method, I checked potency at the expired shelf life under environmental conditions. [Figure 24-25, Table 13]
The HPLC system suitability parameter values of standard and sample chromatogram of Essopel Insta Capsule 40 mg at expiring shelf-life duration are mentioned in table 13.
The results of the newly prepared two different brands under the accelerated condition at different stages like the initial stage, after three months, and after six months.
The Espra capsule 40 mg batch number HF413 gives the same results at the initial stage, after three months, after six months 100.11%, 100.31%, 100.01%, respectively.
The Essopel Insta Capsule 40 mg batch number 111131 gives the same results at the initial stage, after three months, after six months 101.53%, 101.53%, 101.88%, respectively. It shows that they are stable under accelerated conditions. The brand's names, batch number, manufacturing date, Expire date, and assay percentages mentioned in table 14.
The average standard, sample, and assay percentage results of the two different brands at different stages. The different stages of different brands at the mid of the shelf life batch ( Espra Capsule 20 mg Batch # HF373, Essopel Insta Capsule 40mg Batch #01072 ) give results 98.76%, 97.52%, respectively. Then nearly expired (Espra Capsule Batch # HG419, Essopel Insta Capsule Batch # 00642) gives results 95.26%, 95.26%, respectively. The expired brands batched (Espra Capsule 20 mg Batch # HF352, Essopel Insta Capsule Batch # 911122) give low results 81.45%, 85.98%, respectively, low potency than limits under the environmental conditions by the HPLC method. That result showed the instability of the product at the near and expired stages. [Table 15-18]
| Sr. No. | Brands Name | Batch No. | Stages | Mfg. Date | Exp. Date | Avg. Std Area | Avg. Smp Area | Assay % |
|---|---|---|---|---|---|---|---|---|
| Espra Capsule 20mg | HF373 | Mid | 06-20 | 05-22 | 47968041 | 9690208 | 98.76% | |
| Espra Capsule 40mg | HG419 | Near Expire | 04-20 | 03-22 | 5172201.4 | 9530137 | 95.26% | |
| Espra Capsule 20mg | HF352 | Expire | 06-19 | 05-21 | 5790251 | 9702395 | 81.45% | |
| Essopel Insta Capsule 40mg | 01072 | Mid | 10-2020 | 10-2022 | 5793691 | 9737932 | 97.52 % | |
| Essopel Capsule 40 mg | 00642 | Near Expire | 06-2020 | 06-2022 | 5172201.4 | 9530137 | 95.26% | |
| Essopel Insta Capsule 40 mg | 911122 | Expire | 11-2019 | 11-2021 | 5775386 | 9620352.5 | 85.98 % |
Table 15: Results of different brands assay at different stages.
| Sr.No. | Brands Name | Batch No. | Stages | Hard Gelatin Shell Color | Color of Pellets |
|---|---|---|---|---|---|
| 1 | Espra Capsule 40mg | HF413 | Fresh | Light Green and white | White to Off White spherical pellets |
| 2 | Espra Capsule 20mg | HF373 | Mid | Light Green and white | White to Off White spherical pellets |
| 3 | Espra Capsule 40mg | HG419 | Near Expire | Blue and white color minute change | White to Off White spherical pellets |
| 4 | Espra Capsule 20mg | HF352 | Expire | Light Green and white color properly change | White to Yellow color spherical pellets |
| 5 | Essopel Capsule 40mg | 111131 | Fresh | Amethst Blue | White to Off White spherical pellets |
| 6 | Essopel Capsule 40mg | 01072 | Mid | Amethst Blue | White to Off White spherical pellets |
| 7 | Essopel Capsule | Near Expire | Amethst Blue Color minute Change | White to Off White spherical pellets | |
| 8 | Essopel Capsule 40 mg | 911122 | Expire | Amethst Blue Color proper change |
White to Yellow color spherical pellets |
Table 16: Appearance and color of the hard gelatin shell and enteric coated –pellets.
| Sr.No | Brands Name | Batch No. | Mfg. Date | Exp. Date | At Initial Stage (Avg. Dissolution Assay %) |
After three month (Avg. Dissolution Assay %) |
After six month (Avg. Dissolution Assay %) |
|---|---|---|---|---|---|---|---|
| 1 | Espra Capsule 40mg | HF413 | 11-2021 | 10-2023 | 93.98 % | 93.52 % | 91.73% |
| 2 | Essopel Insta Capsule 40mg | 111131 | 11-2021 | 11-2023 | 91.04 % | 90.67 % | 90.11% |
Table 17: Results of the dissolution of the newly formulated product at different stages.
| Sr. No. |
Brands Name | Batch No. | Stages | Assay% (Cap-1) |
Assay% (Cap-2) |
Assay% (Cap-3) |
Assay% (Cap-4) |
Assay% (Cap-5) |
Assay% (Cap-6) |
Average Assay % |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Espra Capsule 20mg | HF373 | Mid | 85.26% | 85.82 % | 88.52 % | 86.47% | 86.01% | 85.99 % | 86.35 % |
| 2 | Espra Capsule 40mg | HG419 | Near Expire | 81.03% | 85.48 % | 80.99 % | 96.50 % | 71.99% | 79.11 % | 82.52 % |
| 3 | Espra Capsule 20mg | HF352 | Expire | 60.08% | 45.38 % | 63.15 % | 52.11 % | 49.73% | 54.15 % | 54.10 % |
| 4 | Essopel Insta Capsule 40mg | 01072 | Mid | 94.08% | 80.67 % | 103.26% | 76.62 % | 80.92% | 76.43% | 85.34 % |
| 5 | Essopel Insta Capsule 40 mg | 00642 | Near Expire | 88.29% | 69.53 % | 102.81% | 49.88 % | 54.27% | 98.71% | 77.26 % |
| 6 | Essopel Insta Capsule 40 mg | 911122 | Expire | 50.86% | 73.39 % | 57.80 % | 45.43 % | 58.73% | 45.23 % | 55.41 % |
Table 18: Results of the dissolution of different products at different stages.
Discussion
Stability Study
The different batch of capsules dosage form was stored in different required conditions. Newly formulated and stored under accelerated conditions (40°C and 75% temperature and humidity) for six months. The other brands store under environmental conditions. Then observed were the different brands' physical appearance, shape, and colors at different stages. The appearance of the newly formed two brands of dosage form Espra Capsule 40 mg (Batch No. HF 413) and Essopel Insta Capsule 40 mg (Batch No.111131) have the same physical appearance at the initial stage, after three months and after six months even under the accelerated conditions. They showed the stability of the product under required conditions. The dosage forms are in the mid of the shelf life duration Espra Capsule 20 mg (Batch No. HF 373) and Essopel Insta Capsule 40 mg (Batch No. 01072) do not change their physical appearance under environmental conditions. The dosage forms are at the near-to-expire brands Espra Capsule 40 mg (Batch No. HG 419) and Essopel Insta Capsule 40 mg (Batch No.00642) had a minute change in the shell color. The expired brands Espra capsule 20 mg (Batch No. HF 352), and Essopel Insta Capsule 40 mg (Batch No.911122) changed their Shell color and enteric-coated pellets color under environmental conditions. It shows that they were not stable after shelf-life duration.
The different product brands were subjected to stability studies under accelerated and environmental conditions. The entire test was performed according to the USP method to check the product's stability at three and six-month intervals. The results were checked and compared with the initial results to evaluate the stability parameters of the dosage form. All results of Espra Capsule 40 mg (Batch No. HF 413) and Essopel Insta Capsule 40 mg (Batch No.111131) showed no significant difference at the interval of time at the initial, after three months, and six months. Hence they proved that the formulation of the product was well stable under accelerated conditions (40°C and 75% temperature and humidity).
The other product at the mid of the shelf life, near expiration, and expired checked the assay potency under the environmental conditions. The results were of the mid-stage of the shelf life, and near expired and expired product assay potency was not the same as at the initial stage. The product results at the mid of the shelf life duration and near expired were within limits but the low potency as compared to the initial stage. The expired brands failed because they had very low assay potency. That product was not stable and unfit for the patients. The dissolution test is a bioavailability test. The dissolution was performed according to the USP method and parameters by using the different product brands at different stages. The newly formulated two brands of Espra capsule 40 mg (Batch No. HF 413) and Essopel Insta capsule 40 mg (Batch No.111131) were put under accelerated conditions. They checked the bioavailability after a while initial, after three months, and after six months. The results of the bioavailability test showed the stability of the product.
The other product was in the mid of the shelf life, near to expiration, and expired checked the dissolution test under the environmental conditions. The mid-stage of the shelf life and near expired and expired product dissolution test was not the same as at the initial stage. The dissolution test results at the mid of the shelf life duration and near expired were within limits but had low bioavailability compared to the initial stage. The expired brands that failed in the bioavailability test showed the unstable behavior of the product and were not suitable for the patients.
Conclusion
This method helps check the stability and the shelf life of the product. It is more beneficial for the pharmaceutical as well as the patient. The stability studies of pharmaceutical products is very important in the development program for new drugs as well as new formulations and these tests has become easy to predict the shelf life under accelerated conditions. Any deviation from the established stability profile could affect its quality, safety and efficacy. The stability shows the quality standard of any product. If product is not stored according to the manufacture instruction, then it easily degraded before the shelf life under environmental conditions. Esomeprazole never stores above the 3 0C otherwise unstable before expired stage.
Acknowledgement
I pay my sincere and humble gratitude to my Research Supervisor, Prof. Dr. Syeda Rubina Gilani, and Zubia Gulzar for her keen interest, guidance, encouragement, and especially her keen behavior.
References
- Ramakrishnan K Salinas R C (2007) Peptic ulcer disease. Am Fam Physician 76: 1005-1012.
- Lind T, Rydberg L, Kylebäck A, Jonsson A, Andersson T, et al. (2000) Esomeprazole provides improved acid control vs. omeprazole In patients with symptoms of gastro-oesophageal reflux disease. Aliment Pharmacol Ther 14: 861-867.
- BronsteinA C, Spyker DA, Cantilena JrL R, Rumack BH, DartR C (2012) 2011 annual report of the American Association of Poison Control Centers’ National Poison data system (NPDS): 29th annual report. Clin Toxicol 50: 911-1164.
- Deshpande AA, RhodesCT, Shah NH Malick AW (1996) Controlled-release drug delivery systems for prolonged gastric residence: an overview. Drug Dev. Ind. Pharm. 22: 531-539.
- Mounica P, Pavani S Mounica RaniP (2018) A review on recent advances in enteric coating and enteric polymers. World J Pharm Res7: 475-495.
- Bishara RH (2006) Cold chain management–an essential component of the global pharmaceutical supply chain. American Pharmaceutical Review 9: 105-109.
- AhmedI, DayP (1987) Stability of cefazolin sodium in various artificial tear solutions and aqueous vehicles. Am J Hosp Pharm 44: 2287-2290.
- Achanta AS, Adusumilli PS, James KW, Rhodes CT (1997) Development of hot melt coating methods. Drug development and industrial pharmacy 23: 441-449.
- Mandil H, Sakur AA, Allabban AA (2013) New sensitive spectrophotometric methods for determination of esomeprazole magnesium trihydrate in dosage forms. Int j pharm 5.
- Bajaj S, Singla D, Sakhuja N (2012) Stability testing of pharmaceutical products. J Appl Pharm Sci 2: 129-138.
- Pawar MPD (2016). Doctoral dissertation, Bharati Vidyapeeth Development ofsome chromatographic methods and their validation for simultaneous estimation of some drugs in bulk and formulations.
- Planchart A, Mattingly C J, AllenD, Ceger P, Casey, et al. (2016) Advancing toxicology research using in vivo high throughput toxicology with small fish models. Altex 33: 435.
- KuriharaT, Min JZ, Toyo'okaT, Fukushima T, Inagaki S (2007) Determination of fluorescence-labeled asparaginyl-oligosaccharide in glycoprotein by reversed-phase ultraperformance liquid chromatography with electrospray ionization time-of-flight mass spectrometry. Analytical chemistry79: 8694-8698.
- Dong MW (2006) Regulatory aspects of HPLC analysis: HPLC system and method validation. Modern HPLC for practicing scientists. John Wiley & Sons 230.
- Ackermann BL, Berna MJ (2007) Coupling immune affinity techniques with MS for quantitative analysis of low-abundance protein biomarkers. Expert review of proteomics 4: 175-186.
- Suksiriworapong J, Rungvimolsin T, Junyaprasert VB, Chantasart D (2014) Development and characterization of lyophilized diazepam-loaded polymeric micelles. Aaps PharmScitech 15: 52-64.
- Saccone CD, Tessore J, Olivera SA, Meneces NS (2004) Statistical Properties of the Dissolution Test of the USP. Dissolution Technologies 11: 25-29.
- Wiklund A KE, Broman BSD (2005) Toxicity evaluation by using intact sediments and sediment extracts. Marine pollution bulletin 50: 660-667.
- KwokY C, Hsieh DPH, Wong PK (2005) Toxicity identification evaluation (TIE) of pore water of contaminated marine sediments collected from Hong Kong waters. Marine pollution bulletin 51: 1085-1091.
- Ain QU, Farooq M A, Caliskan B, Ahsan A, Aquib M, et al.(2020) Stability Studies of Parenteral Products. In Drug Stability and Chemical Kinetics 247-263.
- Hongxia Y, Jing C, Yuxia C, Huihua S, Zhonghai D& Hongjun J (2004) Application of toxicity identification evaluation procedures on wastewaters and sludge from a municipal sewage treatment works with industrial inputs. Ecotoxicol Environ Saf 57: 426-430.
- AyrtonJ (1981) Assay of ceftazidime in biological fluids using high-pressure liquid chromatography. J Antimicrob Chemother 8: 227-231.
- Carstensen JT, Rhodes CT (1986) cyclic testing in stability programs. Drug Development and Industrial Pharmacy 12: 1219-1225.
- Huynh-Ba K, Zahn M (2009) Understanding ICH guidelines applicable to stability testing. In Handbook of stability testing in pharmaceutical development.
- Black JC, Layloff T (1996) Summer of 1995-Mailbox Temperature Excursions in St. Louis. In Pharmacopeial Forum 22: 3305-3305.
- Önal, A., Kepekçi, E. Ş., & Öztunç, A. (2005). Spectrophotometric methods for the determination of the antidepressant drug paroxetine hydrochloride in tablets. Journal of AOAC International 88: 490-495.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Fozia, Gilani R, Zubia, Gul N (2022) Comparative Stability of Different Brands of Esomeprazole Magnesium Trihydrate of Enteric-Coated Pellets Capsules. J Palliat Care Med 12: 487. DOI: 10.4172/2165-7386.1000487
Copyright: © 2022 Fozia, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Conferences
42nd Global Conference on Nursing Care & Patient Safety
Toronto, CanadaRecommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 3438
- [From(publication date): 0-2022 - Apr 02, 2025]
- Breakdown by view type
- HTML page views: 3069
- PDF downloads: 369