Research Article Open Access
Comparative Efficacy and Safety of Linezolid and Quinupristin- Dalfopristin in the Treatment of Vancomycin-Resistant Enterococcus Infections: A Meta-Analysis
Tomoki Watanabe1,2*, Rika Uchida1, Satoko Handa2, Manabu Shindo1, Ichiro Kato1 and Yasuhisa Kato21Department of Pharmacy, National Hospital Organization Chiba Medical Center, Chiba, Japan
2Department of Drug Information, Division of Drug Information Analytics, School of Pharmacy, Showa University, Tokyo, Japan
- *Corresponding Author:
- Tomoki Watanabe
Department of Pharmacy, National Hospital Organization Chiba Medical Center
4-1-2 Tsubakimori, Chuo-ku, Chiba-Shi, Chiba 260-0042, Japan
Tel: +81-043-251-5311
Fax: +81-043-251-8922
E-mail: www.tomoki@gmail.com
Received date: April 10, 2017; Accepted date: April 26, 2017; Published date: April 27, 2017
Citation: Watanabe T, Uchida R, Handa S, Shindo M, Kato I, et al. (2017) Comparative Efficacy and Safety of Linezolid and Quinupristin-Dalfopristin in the Treatment of Vancomycin-Resistant Enterococcus Infections: A Meta-Analysis. J Infect Dis Ther 5:318. doi: 10.4172/2332-0877.1000318
Copyright: © 2017 Watanabe T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Infectious Diseases & Therapy
Abstract
Introduction: Vancomycin-resistant Enterococcus (VRE) is one of the most important causative organisms of nosocomial infections. Once VRE outbreaks occur in hospitals, enormous efforts must be made to control them, especially in wards housing neutropenic or transplant patients. The purpose of this meta-analysis was to investigate the efficacy and adverse event profile of linezolid versus that of Quinupristin-Dalfopristin for the treatment of VRE infections.
Methodology: Literature searches of PubMed, MEDLINE, and EMBASE databases were performed on April 5, 2017 using combined text words with the following MeSH/EMTREE terms: “linezolid” and “Quinupristin-Dalfopristin” and “Enterococcus” and “human.” The odds ratios (ORs) with 95% confidence intervals (CIs) for individual studies were calculated and pooled separately. The pooled estimates were combined using the inverse variance weighting scheme and random effect method.
Results: A systematic search identified 674 articles, and five involving 333 patients were included in the final analysis. One study was a prospective randomized controlled trial, and four were retrospective studies. The mortality rate in the groups of patients treated with linezolid was significantly lower than that in patients treated with Quinupristin-Dalfopristin (OR: 0.47; 95% CI: 0.23 to 0.97; heterogeneity P=0.13, Z=2.05, P=0.04; I2=44%; Begg’s test: P=0.33; Egger’s test: P=0.78). The clinical and microbiological responses indicated no significant differences between the linezolid and Quinupristin- Dalfopristin groups (58% and 43%, respectively, P=0.6; OR: 1.51; 95% CI: 0.75 to 3.04; heterogeneity P=0.32; Z=1.15, P=0.25; I2=0%). The adverse event proiles differed between the Linezolid and quinupristin-dalfopristin groups.
Conclusion: Our results suggest a significantly lower mortality rate in patients treated with linezolid than in those treated with Quinupristin-Dalfopristin for VRE infections; however, this was limited by a variety of factors (mostly retrospective).
Keywords
Vancomycin-resistant Enterococcus; Linezolid; Quinupristin-Dalfopristin; Meta-analysis
Abbreviations
VRE: Vancomycin-Resistant Enterococcus
Introduction
Vancomycin-resistant Enterococcus (VRE) is one of the most important causative organisms of nosocomial infections. VRE was first reported in the UK and France in 1988 [1,2]. Since then, VRE has spread in medical settings worldwide including the US and European countries [3-5]. Linezolid, Quinupristin-Dalfopristin, and daptomycin are anti-infective agents used to treat vancomycin-resistant Enterococcus faecium (VREF) infections. Linezolid was the first oxazolidinone anti-infective agent approved in the US in 2000. It can be administered either intravenously or orally; however, its use poses the potential risk of bone marrow toxicity and neuropathy. Serotonergic drug interactions are another drawback of linezolid therapy. Birmingham et al. [6] showed that the evaluable clinical and microbiological response rates of linezolid in the treatment of VRE infections were 73% and 84%, respectively.
Quinupristin-Dalfopristin is a streptogramin antibiotic used for the treatment of VREF that was approved in the US and the UK in 1999. It should be used with caution owing to the risk of side effects (arthralgias, myalgias, and infusion-related side effects), potential drug-drug interactions, and low efficacy against E. faecalis [7]. Linden et al. [8] showed that the clinical response rate of Quinupristin-Dalfopristin in the treatment of VREF infections was 68.8% in an evaluable subset. Both linezolid and Quinupristin-Dalfopristin are bacteriostatic against VRE [9]. There are currently few studies comparing the outcomes of patients treated with linezolid versus those of Quinupristin-Dalfopristin for VRE infections. Thus, more studies are needed to evaluate the efficacy outcomes of linezolid and Quinupristin-Dalfopristin in the treatment of VRE infections.
Daptomycin is a cyclic lipopeptide antibiotic with activity against VRE [9]. Two recent meta-analyses showed lower mortality for VRE bacteremia treatment with linezolid than with daptomycin, but these studies were limited by a variety of factors [10-12].
The purpose of this meta-analysis was to compare the efficacy and adverse event profiles of linezolid versus those of Quinupristin-Dalfopristin in the treatment of VRE infections.
Materials and Methods
Literature search
This meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [13]. Literature searches of the PubMed MEDLINE database (from January 1, 1966) and EMBASE (from January 1, 1974) were performed on April 5, 2017, using combined text words with the following MeSH/EMTREE terms: “linezolid” and “Quinupristin-Dalfopristin” and “Enterococcus” and “human.” The references of the identified studies and reviews were also scanned to identify additional relevant studies, and no language restriction was imposed.
Study selection and data extraction
Study selection and data extraction were conducted independently by two investigators (TW and RU), and disagreements or uncertainties were resolved by discussion among all the authors to reach a consensus. Studies were included in this meta-analysis if they were published clinical trials or observational studies that simultaneously reported the outcome (mortality) of the treatment of VRE infections with linezolid and Quinupristin-Dalfopristin. Articles were excluded from the analysis if they were reviews or letters, and all prospective and retrospective studies were included. Only the most recent study was included when duplicated studies on the same population were identified. The following information was extracted from each study: author, publication year, country, study duration, study design, study size, mean age, type of infection, acute physiology and chronic health evaluation (APACHE) II score, duration of treatment, mortality, clinical response, and microbiological response. The primary outcome assessed in this meta-analysis was overall mortality. The clinical and microbiological responses were also evaluated. Attempts were made to contact the corresponding authors of studies with insufficient data when necessary.
Statistical analysis
The odds ratios (ORs) with 95% confidence intervals (CIs) were calculated and pooled separately for individual studies. The pooled estimates were combined using the inverse variance weighting scheme and random effect method [14]. The heterogeneity among the studies was estimated using the I2 and Cochran’s Q statistics [14]. The publication bias was investigated using funnel plots and further assessed using Begg’s rank correlation and Egger’s linear regression tests [15,16]. All statistical analyses were performed using the Review Manager Version 5.3 (Revman, The Cochrane Collaboration, Oxford, UK).
Results
Search results
The systematic search identified 674 articles, which included 20 duplicates. After title and abstract screening, 175 full-text articles were reviewed, and 170 were subsequently excluded. During the process of abstracting data from the identified articles and reviews, no additional references were identified, and five articles were subsequently included in the final analysis (Figure 1).
Study characteristics
The characteristics of the eligible studies are shown in Table 1. One study was a prospective randomized controlled trial, and four were retrospective studies [17-21]. All the studies were single-center experiences published between 2004 and 2010 in the US and Korea, and 333 patients were identified. Sample sizes of 208 and 125 patients were selected for the linezolid and Quinupristin-Dalfopristin groups, respectively. The attempts to contact the corresponding authors of the four studies with insufficient data yielded no additional data [18-21].
| Author and year | Country | Study duration | Study design | Study size (no. of patients) | Mean age (SD) | Type of infection (no. of patients) | APACHE II, mean (SD) | Duration of treatment, mean days (SD) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LZD | Q-D | LZD | Q-D | LZD | Q-D | LZD | Q-D | ||||||||
| Raad et al., 2004 [17] | US | 1998–2001 | Prospective, randomized study | 19 | 21 | 54.2 (16.0) | 53.4 (14.4) | Bacteremia (37), surgical wound infection (2), and upper urinary tract infection (1) | 13.8 (4.1) | 14.3 (3.8) | 14.7 (9.6) | 10.9 (4.6) | |||
| Gearhart M et al., 2005 [18] | US | 1995–2002 | Retrospective study | 6 | 8 | NA | NA | Sites of VRE cultures in the infected patients were blood, peritoneal fluid, bile, urine culture, feces, others (detailed data was not shown). |
NA | NA | NA | NA | |||
| Erlandson et al., 2008 [19] | US | 1993–2005 | Retrospective study | 71 | 20 | 34.0 (29.0) | 44.7 (19.2) | Bacteremia (91) | 17.0 (6.6) | 21 (6.9) | 14 (range, 3–70) | 12 (range, 2–42) | |||
| Han et al., 2009 [20] | Korea | 1998–2007 | Retrospective study | 51 | 24 | 58 (44–66) a | Bacteremia (75) | 14 (12–20) a | 14 (7–20) a | ||||||
| Chong et al., 2010 [21] | Korea | 2003–2007 | Retrospective study | 61 | 52 | 50 (17) | 51 (16) | Bacteremia (113) | 18 (7) | 16 (6) | 16 (9) | 12 (8) | |||
| APACHE, Acute physiology and chronic health evaluation; LZD, linezolid; NA, not applicable; Q-D, quinupristin-dalfopristin; SD, standard deviation; VRE, vancomycin-resistant Enterococcus | |||||||||||||||
| a interquartile range. | |||||||||||||||
Table 1: Characteristics of eligible studies.
Meta-analyses
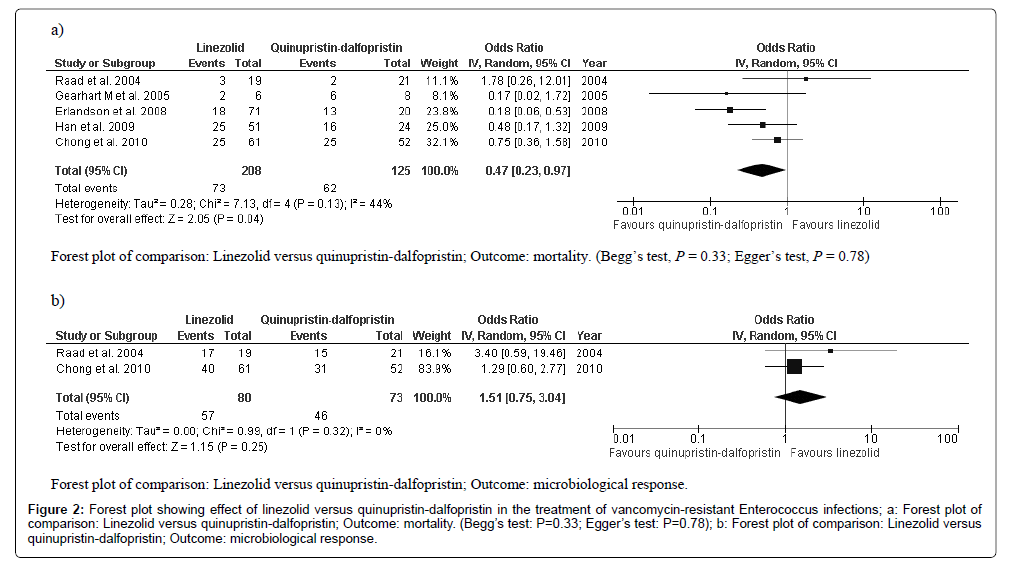
The five included studies reported the mortality of 333 patients treated with linezolid (n=208) and Quinupristin-Dalfopristin (n=125) [17-21]. The mortality rate was significantly lower in patients treated with linezolid than it was in those treated with Quinupristin-Dalfopristin (OR: 0.47; 95% CI, 0.23 to 0.97; heterogeneity P=0.13; Z=2.05, P=0.04; I2=44%; Begg’s test: P=0.33; Egger’s test: P=0.78 (Figure 2a). Clinical responses were reported in only one study and, therefore, we could not perform a meta-analysis. The clinical response was comparable between the linezolid and Quinupristin-Dalfopristin groups (58% and 43%, respectively, P=0.6) [17]. Two studies reported microbiological responses, and the results indicated no significant difference between the linezolid and Quinupristin-Dalfopristin groups (OR: 1.51; 95% CI, 0.75 to 3.04; heterogeneity P=0.32; Z=1.15, P=0.25; I2=0%, (Figure 2b) [17-21]. Begg’s and Egger’s tests were not carried out for microbiological response because only two studies were included.
Figure 2: Forest plot showing effect of linezolid versus quinupristin-dalfopristin in the treatment of vancomycin-resistant Enterococcus infections; a: Forest plot of comparison: Linezolid versus quinupristin-dalfopristin; Outcome: mortality. (Begg’s test: P=0.33; Egger’s test: P=0.78); b: Forest plot of comparison: Linezolid versus quinupristin-dalfopristin; Outcome: microbiological response.
Discussion
The results of this meta-analysis suggest that the mortality rate of the groups of patients treated with linezolid was significantly lower than that of the Quinupristin-Dalfopristin-treated groups. The Quinupristin-Dalfopristin group tended to show a lower rate of clinical and microbiological responses than the linezolid group did, but the differences were not significant. More studies would be needed to make reliable quantitative statements about the differences in the clinical and microbiological responses between the linezolid and Quinupristin-Dalfopristin groups.
Linezolid treatment has been associated with reversible myelosuppression as well as thrombocytopenia and a slight increase in the risk of developing anemia [22]. Conversely, the most common systemic adverse events related to treatment with Quinupristin-Dalfopristin are arthralgias and myalgias [23]. The adverse event profiles for the eligible studies in our report are shown in Table 2. Raad et al. [17] showed that the rate of myalgias/arthralgias was 33% (7/21) in the Quinupristin-Dalfopristin study group (P<0.01); however, in all seven cases, myalgias/arthralgias resolved when the drug was discontinued. Chong et al. [21] reported that antibiotic-induced thrombocytopenia was observed only in three patients in the linezolid group (5%), but their platelet counts recovered after linezolid therapy was discontinued. These findings suggest differences in adverse event profiles of linezolid and Quinupristin-Dalfopristin groups.
| Author and year | Adverse event | Linezolid, n (%) | Quinupristin-dalfopristin, n (%) | P-value a |
|---|---|---|---|---|
| Raad et al., 2004 [17] | Increased bilirubin | 7/19 (37) | 4/21 (19) | 0.293 |
| Myalgias/arthralgias | 0/19 (0) | 7/21 (33) | 0.009 | |
| Nausea/vomiting, diarrhea | 5/19 (26) | 3/21 (14) | 0.442 | |
| Thrombocytopenia/leukopenia | 2/19 (11) | 0/21 (0) | 0.219 | |
| Erlandson et al., 2008 [19] | Clostridium difficile colitis | 1/71 (1) | NA | |
| Diarrhea (non–Clostridium difficile) | 1/71 (1) | NA | ||
| Lactic acidosis | 1/71 (1) | 1/20 (5) | 0.393 | |
| Leukocytosis | NA | 2/20 (10) | ||
| Myalgias | NA | 4/20 (20) | ||
| Neuropathy | 1/71 (1) | NA | ||
| Thrombocytopenia | 1/71 (1) | NA | ||
| Chong et al., 2010 [21] | Thrombocytopenia | 3/61 (5) | 0/52 (0) | 0.248 |
| NA, not applicable; | ||||
| a Fisher's exact test two-sided P-value | ||||
Table 2: Adverse event profile for eligible studies.
The increasing incidence of antibiotic-resistant nosocomial pathogens including linezolid- and Quinupristin-Dalfopristin-resistant Enterococcus is a global problem [7,24,25]. Univariable analysis of a retrospective study revealed the risk factors for reduced linezolid susceptibility. These factors included allogeneic hematopoietic stem cell or solid organ transplants or both, as well as the administration of immunosuppressive medications including corticosteroids, non-corticosteroids, and linezolid within 1 year prior to the infection [26]. The intensive use of linezolid was subsequently associated with the development of decreased VREF susceptibility to this antibiotic [27]. Therefore, caution should be exercised in empiric therapy or therapy in patients with reduced susceptibility. Two recent meta-analyses showed that linezolid treatment of VRE bacteremia was associated with a lower mortality than daptomycin treatment was [10,11]. However, Anastasiou et al. [28] reported that resistance to linezolid and Quinupristin-Dalfopristin appeared to be independent of daptomycin susceptibility, and no significant cross-resistance was noted. Therefore, daptomycin has the potential to be a useful therapeutic agent for treating VRE infections.
Our study had several limitations that are worth mentioning. The majority of the included studies had some methodological limitations (mostly retrospective), and many lacked detailed case information. Furthermore, the attempts to contact the corresponding authors of studies with insufficient data were unproductive, as no additional data were obtained. There was also evidence of heterogeneity and publication bias. The differences in baseline risks between the groups in the included studies likely played a role in the bias since the linezolid-treated groups appeared to include fewer transplant recipients, APACHE II scores, and renal dysfunction than the Quinupristin-Dalfopristin-treated groups did. However, each of these studies targeted linezolid versus Quinupristin-dalfopristin in the treatment of VRE bacteremia and, therefore, we believe that our study contributes valuable information to the current efforts and strategies to control VRE infections.
Conclusion
Enterococcus is one of the most important causative organisms of nosocomial infection, and VRE outbreaks in hospitals need to be controlled aggressively and promptly to prevent the associated mortalities. Our results suggest that the mortality rate was significantly lower in the groups of patients treated with linezolid than in those treated with Quinupristin-Dalfopristin; however, these findings are limited by a variety of factors. Additional larger, randomized, controlled trials are needed to evaluate the efficacy and safety of linezolid and Quinupristin- Dalfopristin in the treatment of VRE infections. In addition, the antibiotic choice in the treatment of VRE for individual patients should be considered based on local availability, antibiotic resistance patterns, the risk of adverse events, and cost, and our findings would be useful in making informed decisions.
Acknowledgments
We thank all the authors of the included studies.
Funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical Statement
Not required.
Authors Contributions
TW and RU performed the literature search, as well as the data collection, analysis, and interpretation. All authors conceived and designed the study, and read and approved the final manuscript.
Competing Interests
The authors declare that they have no competing interests.
References
- Uttley AH, Collins CH, Naidoo J, George RC (1988) Vancomycin-resistant Enterococci. Lancet 1: 57–58.
- Leclercq R, Derlot E, Duval J, Courvalin P (1988) Plasmid-mediated resistance to vancomycin and teicoplanin in Enterococcus faecium. N Engl J Med 319: 157–161.
- Ziakas PD, Thapa R, Rice LB, Mylonakis E (2013) Trends and significance of VRE colonization in the ICU: a meta-analysis of published studies. PLoS One 8: e75658.
- Ramsey AM, Zilberberg MD (2009) Secular trends of hospitalization with vancomycin-resistant Enterococcus infection in the United States, 2000-2006. Infect Control Hosp Epidemiol 30: 184–186.
- Werner G, Coque TM, Hammerum AM, Hope R, Hryniewicz W, et al. (2008) Emergence and spread of vancomycin resistance among Enterococci in Europe. Euro Surveill 13: 1–11.
- Birmingham MC, Rayner CR, Meagher AK, Flavin SM, Batts DH, et al. (2003) Linezolid for the treatment of multidrug-resistant, gram-positive infections: experience from a compassionate-use program. Clin Infect Dis 36: 159–168.
- Luh KT, Hsueh PR, Teng LJ, Pan HJ, Chen YC, et al. (2000) Quinupristin-Dalfopristin resistance among gram-positive bacteria in Taiwan. Antimicrob Agents Chemother 44: 3374–3380.
- Linden PK, Moellering RC Jr, Wood CA, Rehm SJ, Flaherty J, et al. (2001) Treatment of vancomycin-resistant Enterococcus faecium infections with quinupristin/dalfopristin. Clin Infect Dis 33: 1816–1823.
- Rybak MJ, Hershberger E, Moldovan T, Grucz RG (2000) In vitro activities of daptomycin, vancomycin, linezolid, and quinupristin-dalfopristin against Staphylococci and Enterococci, includingvancomycin- intermediate and -resistant strains. Antimicrob Agents Chemother 44: 1062–1066.
- Chuang YC, Wang JT, Lin HY, Chang SC (2014) Daptomycin versus linezolid for treatment of vancomycin-resistant enterococcal bacteremia: systematic review and meta-analysis. BMC Infect Dis 14: 687.
- Balli EP, Venetis CA, Miyakis S (2014) Systematic review and meta-analysis of linezolid versus daptomycin for treatment of vancomycin-resistant enterococcal bacteremia. Antimicrob Agents Chemother 58: 734–739.
- McKinnell JA, Arias CA (2015) Editorial Commentary: Linezolid vsDaptomycin for vancomycin-resistant Enterococci : The evidence gap between trials and clinical experience. Clin Infect Dis 61: 879-882.
- Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339: b2535.
- Woodward M (2013) Epidemiology: Study Design and Data Analysis, 3rd Ed. Chapman & Hall/CRC, Boca Raton, FL.
- Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50: 1088–1101.
- Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315: 629–634.
- Raad I, Hachem R, Hanna H, Afif C, Escalante C, et al. (2004) Prospective, randomized study comparing quinupristin-dalfopristin with linezolid in the treatment of vancomycin-resistant Enterococcus faecium infections. J AntimicrobChemother 53: 646–649.
- Gearhart M, Martin J, Rudich S, Thomas M, Wetzel D, et al. (2005) Consequences of vancomycin-resistant Enterococcus in liver transplant recipients: a matched control study. Clin Transplant 19: 711–716.
- Erlandson KM, Sun J, Iwen PC, Rupp ME (2008) Impact of the more-potent antibiotics quinupristin-dalfopristin and linezolid on outcome measure of patients with vancomycin-resistant Enterococcus bacteremia. Clin Infect Dis 46: 30–36.
- Han SH, Chin BS, Lee HS, Jeong SJ, Choi HK, et al. (2009) Vancomycin-resistant enterococci bacteremia: risk factors for mortality and influence of antimicrobial therapy on clinical outcome. J Infect 58: 182–190.
- Chong YP, Lee SO, Song EH, Lee EJ, Jang EY, et al. (2010) Quinupristin-dalfopristin versus linezolid for the treatment of vancomycin-resistant Enterococcus faecium bacteraemia: efficacy and development of resistance. Scand J Infect Dis 42: 491–499.
- Gerson SL, Kaplan SL, Bruss JB, Le V, Arellano FM, et al. (2002) Hematologic effects of linezolid: summary of clinical experience. Antimicrob Agents Chemother 46: 2723–2726.
- Moellering RC, Linden PK, Reinhardt J, Blumberg EA, Bompart F, et al. (1999) The efficacy and safety of quinupristin/dalfopristin for the treatment of infections caused by vancomycin-resistant Enterococcus faecium. Synercid Emergency-Use Study Group. J Antimicrob Chemother 44: 251–261.
- Jevitt LA, Smith AJ, Williams PP, Raney PM, McGowan JE Jr, et al. (2003) In vitro activities of Daptomycin, Linezolid, and Quinupristin-Dalfopristin against a challenge panel of Staphylococci and Enterococci, including vancomycin-intermediate Staphylococcus aureus and vancomycin-resistant Enterococcus faecium. Microb Drug Resist 9: 389–393.
- Patel SN, Memari N, Shahinas D, Toye B, Jamieson FB, et al. (2013) Linezolid resistance in Enterococcus faecium isolated in Ontario, Canada. DiagnMicrobiol Infect Dis 77: 350–353.
- Santayana EM, Grim SA, Janda WM, Layden JE, Lee TA, et al. (2012) Risk factors and outcomes associated with vancomycin-resistant Enterococcus infections with reduced susceptibilities to linezolid. DiagnMicrobiol Infect Dis 74: 39–42.
- Raad II, Hanna HA, Hachem RY, Dvorak T, Arbuckle RB, et al. (2004) Clinical-use-associated decrease in susceptibility of vancomycin-resistant Enterococcus faecium to linezolid: a comparison with quinupristin-dalfopristin. Antimicrob Agents Chemother 48: 3583–3585.
- Anastasiou DM, Thorne GM, Luperchio SA, Alder JD (2006) In vitro activity of daptomycin against clinical isolates with reduced susceptibilities to linezolid and quinupristin/dalfopristin. Int J Antimicrob Agents 28: 385–388.
Relevant Topics
- Advanced Therapies
- Chicken Pox
- Ciprofloxacin
- Colon Infection
- Conjunctivitis
- Herpes Virus
- HIV and AIDS Research
- Human Papilloma Virus
- Infection
- Infection in Blood
- Infections Prevention
- Infectious Diseases in Children
- Influenza
- Liver Diseases
- Respiratory Tract Infections
- T Cell Lymphomatic Virus
- Treatment for Infectious Diseases
- Viral Encephalitis
- Yeast Infection
Recommended Journals
Article Tools
Article Usage
- Total views: 4484
- [From(publication date):
April-2017 - Jul 15, 2025] - Breakdown by view type
- HTML page views : 3604
- PDF downloads : 880