Research Article Open Access
Comparative Analysis of Nasal Therapy with Soluble and Liposomal Forms of Curcumin on Rats with Alzheimer’s Disease Model
Sokolik VV1, Berchenko OG1 and Shulga SM2*1Laboratory of Neurophysiology, Immunology and Biochemistry SE, Institute of Neurology, Psychiatry and Narcology of the National Academy of Medical Science of Ukraine, Kharkiv, Ukraine
2Department of Industrial and Food Biotechnology SE, Institute for Food Biotechnology and Genomics of the National Academy of Sciences of Ukraine, Kyiv, Ukraine
- Corresponding Author:
- Shulga SM
Department of Industrial and Food Biotechnology SE
Institute for Food Biotechnology and Genomics of the National Academy of Sciences of Ukraine, Kyiv, Ukraine
Tel: +38067 4400531
E-mail: shulga5@i.ua
Received date: May 04, 2017; Accepted date: July 25, 2017; Published date: August 01, 2017
Citation: Sokolik VV, Berchenko OG, Shulga SM (2017) Comparative Analysis of Nasal Therapy with Soluble and Liposomal Forms of Curcumin on Rats with Alzheimer’s Disease Model. J Alzheimers Dis Parkinsonism 7:357. doi:10.4172/2161-0460.1000357
Copyright: © 2017 Sokolik VV, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Alzheimers Disease & Parkinsonism
Abstract
The aim of this study was a comparative analysis of the efficacy of nasal therapy of curcumin soluble and liposomal forms of animals with a model of Alzheimer’s disease. Cognitive tests and immunity-enzyme analysis of cytokines were completed. On experimental model of Alzheimer’s disease in rats (intrahippocampus administration of 15 nÐ�? Ðβ42_Human) installed more efficient nasal therapy (1 month treatment) of curcumin liposome form (3.5 μg/animal daily) has turned out more efficient compared to its aqueous solution, both in terms of cognitive tests (the portion of the positive responses and latent period of the conditioned reflex reaction elimination) and in neuroinflammation (cytokines content (interleukin-1β, interleukin-6, interleukin-10, tumor necrosis factor α) in the brain sections: cerebral cortex and hippocampus of the rats with Alzheimer’s disease model). The data indicate a high anticytokine potential specifically of the liposomal form of curcumin.
Keywords
Curcumin; Liposome; Alzheimer’s disease; Memory; Cytokines; Brain
Introduction
Alzheimer’s disease (AD) is a neurodegenerative disease, which is characterised clinically by the progressive loss of short-term memory and cognitive functioning. Major pathological features of an AD brain include the accumulation of extracellular plaques and fibrils with Aβ peptides, intracellular neurofibrillary tangles (NFT), as well as chronic inflammation and widespread synaptic and neuronal loss, leading to brain atrophy and dysfunction [1-3]. With the ageing of many populations worldwide, it is predicted that over the next few decades there will be a marked increase in the number of people with dementia. According to the World Health Organization (WHO), 5% of men and 6% of woman above the age of 60 years are affected with Alzheimer's type dementia worldwide. Current estimations show that 36 million people worldwide have dementia, which is predicted to more than triple to 115 million by 2050 (Figure 1) [4].
The “amyloid cascade hypothesis,” in which mutations in amyloid precursor protein, β-secretase (BACE-1), apolipoprotein E, presenilin-1 or presenilin-2 genes lead to increased production of β-amyloid, is now widely considered to contribute to the neurodegeneration seen in AD [5]. Mutations in these genes are linked to some forms of AD [6-8] and although generally responsible for early-onset disease, they have also been reported in some patients with late-onset disease [9,10]. Nevertheless, only about 5% of AD cases are caused by these mutations, and so it seems that there must be other factors that lead to an overproduction and deposition of β-amyloid. The remaining 95% of cases of this neurodegenerative pathology are caused by agerelated metabolic disorders: chronic neuroinflammation, oxidative stress, epigenetic alterations and other. Therefore, the main focus of the prevention and treatment of AD may be a correction of nonspecific age-related disorders caused by broad spectrum agents [11,12].
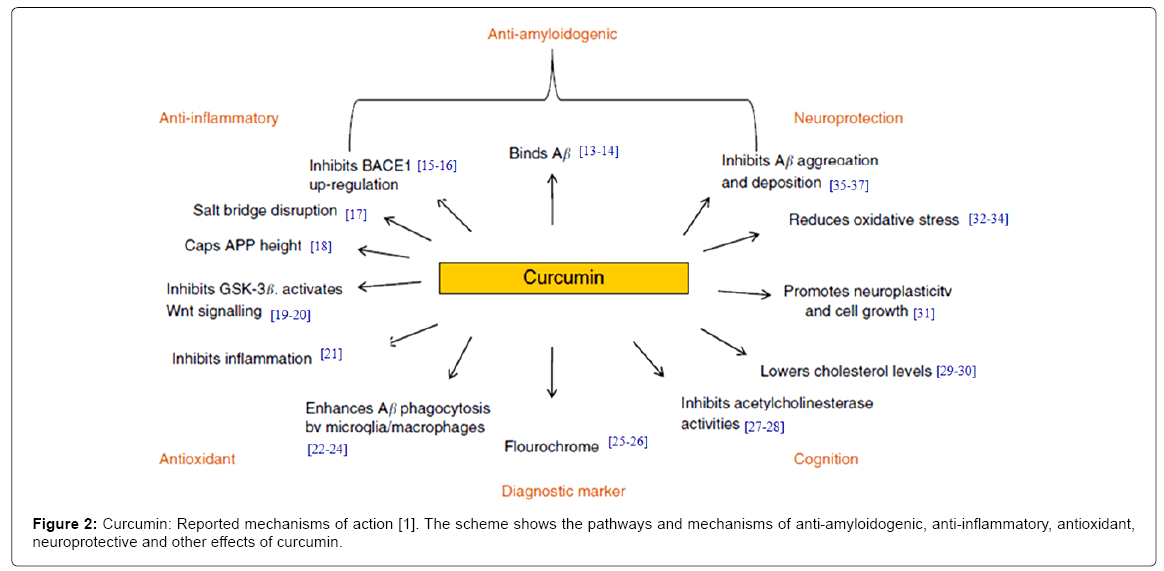
With no current effective disease-modifying treatments available, finding pharmacological/non-pharmacological strategies to halt or slow disease progression is of significant importance. The continuing lack of effective pharmaceutical drugs has also prompted the evaluation of alternative therapeutics, such as nutraceuticals. Extensive studies in the last two decade suggested that curcumin possesses antiinflammatory, antioxidant, Aβ-lowering agent and Aβ aggregation inhibitor properties; it shows potential as a therapeutic for AD [13-37]. The mechanism for these effects involves modulation of several signaling transduction pathways (Figure 2).
However, curcumin’s clinical application is severely limited because of its poor stability under physiological conditions that limits its systemic bioavailability [38,39]. Major reasons contributing to the low plasma and tissue levels of curcumin appear to be due to poor absorption, rapid metabolism, and rapid systemic elimination. Therefore, many technologies have been developed and applied to overcome this limitation and development of nano-sized delivery systems for curcumin, including liposomes, polymeric nanoparticles and micelles, conjugates, peptide carriers, cyclodextrins, solid dispersions, lipid nanoparticles and emulsions [40-46].
Still one problem is physiological barriers (including a blood brain barrier) limiting curcumin absorption after oral or intravenous administrations [47]. This natural polyphenol was more permeable under acidic conditions, but the permeability was substantially below the permeability of highly permeable standards by its non-specific binding. This can be explained by greater stability of keto form of curcumin, which becomes dominant in a tautomeric mixture of keto and enol forms in curcumin’s solution. Therefore, the aim of this study was a comparative analysis of the efficacy of nasal therapy of curcumin soluble and liposomal forms of animals with a model of Alzheimer’s disease.
Materials and Methods
Study design
The effect of nasal therapy with curcumin in two forms was studied, namely its aqueous solution and its liposomal form (composed of phospholipid/cholesterol liposomes). Experimental protocols were complied with the rules of the European Convention for Protection of Vertebrate Animals used in experiments and for other scientific purposes. The rats (n=60) were randomly distributed into 7 groups. The reference group included the intact animals (n=12); Group 1 included rats 1 month after intrahippocampal injection of Aβ42_Human (Human Amyloid β Protein Fragment 1-42, Sigma-Aldrich, USA) – experimental model of AD (n=12); Group 2 included sham-operated animals (n=12); Group 3 included rats with experimental model of AD, who received curcumin aqueous solution of (Sigma-Aldrich, USA) delivered by intranasal administration daily for 1 month (n=6); Group 4 included the animals with experimental model of AD, who received bidistilled water by intranasal administration daily for 1 month (n=6); Group 5 included rats with experimental model of AD, who received liposomal curcumin delivered by intranasal administration daily for 1 month (n=6) and Group 6 included animals with experimental model of AD, who received intranasal administration of empty liposomes daily for 1 month, as well (n=6).
Cognitive tests
Preliminarily, 20 days before a conditioned reflex reaction was formed in all the rats on the basis of non-conditioned reflex elimination [48]. Infallible conditioned reflex responses to metronome sound were considered to be positive results. Next to the positive response portion (number) (in percent, %), the duration of latent period of conditioned reflex reaction elimination was registered in the study (in seconds, s). The animals of all the groups were tested for these values of conditioned reflex reaction elimination after the AD experimental model was formed in them and after intranasal administration of curcumin, respectively.
Experimental model of Alzheimer’s disease
Aβ42_Human solved in bidistilled water was aggregated for 24 h at 37°C. Large rough conglomerates of Aβ42_Human were dispersed, using the ultrasonic homogenizer (Musson-1, Russia) for 5 min and sterilized immediately before injection. The effect of β-amyloid peptide 42_Human in homoaggregate form was studied one month after its single injection in the dosage of 15 nM Aβ42_Human (65 μg) to the brain hippocampus of the rats. The volume of the solution: 10 μl per animal. Aβ42_Human solution volume was 10 μl, the rate of introduction of a needle syringe chromatographic – 0.03 μl/s and duration of administration – 5 min. The stereotaxic coordinates of the left hippocampus were determined by the map of the rat brain [49], which corresponds to the distance from the point of intersection of the sagittal seam with bregma (zero point): distally – 2 mm, laterally – 2 mm and in depth – 3.5 mm. Stereotaxic operations in the investigated animals ran under general narcosis using intraperitoneal injections of thiopental, 50 mg/kg of body mass.
Nasal therapy with curcumin
Since curcumin has low solubility in water, its concentrated solution in 96% ethanol was first prepared. Curcumin remained stable in ethanol at the room temperature for three weeks but degraded fast in water at neutral or weak basic pH [50]. Therefore the outgoing curcumin solution was dissolved in the bidistilled water to 0.7 g/l immediately before the nasal administration into the rats in the dosage of 3.5 μg/animal.
To prepare liposomes with curcumin, lecithin/cholesterol was dissolved in the round-bottom flask at ratio 18:1 in 50% ethanol. After the lipid film was formed as a result of the solvent evaporation, 28.85 mM CUA in 5 ml of PBS buffer (10 mM Na2HPO4, 1.76 mM KH2PO4, 137 mM NaCl, 2.7 mM KCl, pH 7.4) was added and thoroughly mixed for suspension of liposomes with curcumin formation [51]. The suspension of empty liposomes was prepared using the similar protocol but at the final stage PBS buffer without CUA was added. Liposome suspension with CUA was dissolved with PBS buffer to 0.7 g/l CUA immediately before nasal administration to the rats in the dose of 3.5 μg/animal. Suspension of empty liposomes was diluted similarly. Daily nasal therapy of the rats with AD experimental model lasted for 1 month. Administration of liposome form curcumin by nasal method is determined by the fact that, unlike peripheral blood circulation, this is the shortest way to the target regions of the rat neocortex. It is known that after entering the body the dissolved curcumin is nearly unable to overcome the hematoencephalic barrier whereas its liposome form is actively and non-specifically entrapped by the formed elements of blood, which requires big doses of the preparation.
After the processing was finished the animals were decapitated. The samples of the cerebral cortex and hippocampus were frozen and stored for further measurement. The tissues of the brain sections were homogenized in Tris buffer (50 mM tris-HCl, 150 mM NaCl, pH 7.5), centrifuged at 14,000 g (RS-6, RF) for 5 min and then the supernatant was collected.
Immunity-enzyme analysis of cytokines
The samples of hippocampus supernatant and cerebral cortex were used to determine cytokines by the ELISA method in accordance with the protocols (Rat ELISA Kits Invitrogen BCM DIAGNOSTICS, USA) for IL-1β, IL-6, IL-10 and TNFα. Assay absorption was read out by GBG Stat FAX 2100 (USA) microplate analyzer at 450 nm with wavelength correction at 630 nm. The ELISA data (μg/l cytokins) were recalculated to the general protein. Concentration of general protein was quantitated by Lowry method [52].
Statistical processing of the study results
The obtained results were statistically processed, the average values and standard deviations being calculated. The statistical analysis of differences was calculated using Student t-test. Values at p<0.05 were considered significant.
Results and Discussion
The rats with Alzheimer’s disease model (Group 1) showed memory impairment in cognitive tests by such indicators as the portion of the positive responses and latent period of the conditioned reflex reaction elimination (Table 1). In particular, the percentage of positive responses decreased and the length of the latent period increased as compared to the intact animals (Reference group). However, the first indicator also decreased in sham-operated rats (Group 2), calling into question its specificity. In addition, the latter did not show any credible changes in the latent period. Therefore, this particular indicator of animal cognitive tests is a sensitive marker of memory impairment at early stages of dementia. The first clinic symptoms of dement changes manifest themselves primarily in the extension of recollection intervals, and later in the impossibility to recall anything at all.
| Group | Portion of positive responses (%) | Latent periods (seconds, s) |
|---|---|---|
| Reference | 88.9 ± 1.2 | 5.8 ± 0.4 |
| Group 1 | 76.4 ± 3.3 1) | 7.8 ± 0.1 1) |
| Group 2 | 71.0 ± 1.2 1) | 7.1 ± 0.3 |
| Group 3 | 87.2 ± 2.0 2) 3) | 6.7 ± 0.2 |
| Group 4 | 78.4 ± 3.1 1) | 7.0 ± 0.2 1) |
| Group 5 | 91.1 ± 3.7 2) 3) | 6.1 ± 0.3 3) 4) |
| Group 6 | 83.4 ± 3.4 | 6.4 ± 0.4 |
Remark: 1)�? ≤ 0.05 compared to the Reference (intact animals, n=12); 2)�? ≤ 0.05 at comparison of the Group 1 (Alzheimer’s disease model, n=12) and Group 2 (shamoperated animals, n=12), Group 3 (nasal therapy of the AD model rats with soluble CUA, n=6) and Group 4 (nasal therapy of the AD model rats with H2O, n=6) and Group 5 (nasal therapy of the AD model rats with liposomal CUA, n=6) and Group 6 (nasal therapy of the AD model rats with empty liposomes, n=6), respectively; 3) �? ≤ 0.05 compared to the Group 1 (Alzheimer’s disease model, n=12); 4)�? ≤ 0.05 at comparison of the Group 3 (nasal therapy of the AD model rats with soluble CUA, n=6) and Group 5 (nasal therapy of the AD model rats with liposomal CUA, n=6)
Table 1: Effect of curcumin soluble and liposomal forms on memory parameters in the rats with Alzheimer’s disease model.
Daily nasal therapy with aqueous solution of curcumin for 1 month in AD model rats (Group 3) recovered only the portion of the positive responses, whereas latent period remained somewhat extended (Table 1). In the reference group, which received only the solvent, (Group 4) the cognitive indicators under study did not improve. Nasal therapy with liposomal form of curcumin (Table 1) within the same period of time led to a specific recovery of both indicators of the conditioned reflex reaction elimination in animals of Group 5, as compared to Group 1 (AD model animals) and Group 6 (nasal therapy of the AD model rats with empty liposomes). In particular: the portion of the positive responses increased by 19% and 9%, but latent period decreased by 28% and 5%, respectively.
Comparison of the effects of nasal therapy with CUA soluble and liposomal forms on the memory of animals with a model of Alzheimer’s disease, Table 1 shows that the indicators of cognitive tests in Group 5 (nasal therapy of the AD model rats with liposomal CUA) and Group 3 (nasal therapy of the AD model rats with soluble CUA) differ only by 5% and 10% for the portion of the positive responses and latent period, respectively. However, the difference was in favor of liposomal curcumin. Thus, greater efficacy of nasal therapy with liposomal curcumin was established based on the results of cognitive tests.
To determine the leading mechanism of curcumin action at the molecular level, we investigated its impact on the cytokine link of inflammation in targeted parts of the brain of rats (hippocampus and cerebral cortex), which are responsible for the memory. Since brain neurons form a single neuronal network with projections of the neocortex axons in the zones of the hippocampus, the spread of the inflammatory process is expected when the hippocampus is damaged in the cerebral cortex.
Homoaggregates Aβ42_Human in the hippocampus of rats with Alzheimer’s disease model caused chronic neuroinflammation specifically and predominantly at the injection site (Table 2). In particular: hippocampal levels IL-1β and IL-10 in rats with Alzheimer’s disease model were increased compared with the control group (by 221% and 111%, respectively) and group 2 (110% and 78%, respectively). In this part of the brain, concentration of IL-6 in rats of group 1 did not differ from the values of intact animals (control group), but was reduced by 44% compared to the concentration of IL-6 in the shamoperated rats. However, the activation of the inflammatory process was also found in the cerebral cortex of the rats, although to a lesser extent: increased levels of IL-1β (by 109%) and IL-6 (by 54%) compared with those of intact animals (control), and increased concentrations of IL-6 (by 29%) and reduced IL-10 (by 31%) compared to sham-operated animals. The content of TNFα in the cerebral cortex and hippocampus of rats of groups 1 and 2 did not differ from the benchmark and among themselves (Table 2). This result confirms the conclusion of previous studies [53], in which it was shown that homoaggregates Aβ40_Human, injected into the cerebral cortex of rats, cause greater cytokine response specifically in the area of administration.
| Group | IL-1β (ng/g protein) | TNFα (ng/g protein) | IL-6 (ng/g protein) | IL-10 (ng/g protein) |
|---|---|---|---|---|
| Cerebral cortex | ||||
| Reference | 166.3 ± 18.5 | 50.8 ± 2.5 | 52.5 ± 4.2 | 179.5 ± 13.0 |
| Group 1 | 347.5 ± 11.8 1) | 46.2 ± 2.4 2) | 80.8 ± 7.4 1) | 150.8 ± 10.6 2) |
| Group 2 | 340.8 ± 13.3 1) | 40.6 ± 2.9 1) 3) | 68.5 ± 5.8 | 206.4 ± 24.2 3) |
| Group 3 | 222.0 ± 16.1 1) 2) 3) | 26.1 ± 3.7 1) 2) 3) | 45.2 ± 5.7 2) 3) | 133.6 ± 10.2 1) 2) |
| Group 4 | 430.5 ± 20.6 1) 3) | 58.6 ± 2.5 3) | 79.1 ± 5.6 1) | 281.3 ± 8.3 1) 3) |
| Group 5 | - | 13.2± 0.9 1) 2) 3) 4) | 26.8 ± 2.0 1) 2) 3) 4) | 89.4 ± 7.6 1) 2) 3) 4) |
| Group 6 | - | 47.1 ± 2.8 | 49.3 ± 3.9 3) | 177.0 ± 12.8 3) |
| Hippocampus | ||||
| Reference | 174.0 ± 18.8 | 50.7 ± 2.1 | 57.3 ± 8.3 | 130.4 ± 11.0 |
| Group 1 | 558.0 ± 18.8 1) 2) | 63.8 ± 3.5 1) 2) | 72.8 ± 6.8 1) 2) | 254.3 ± 16.7 1) 2) |
| Group 2 | 365.4 ± 19.1 1) 3) | 46.8 ± 1.9 3) | 98.3 ± 6.8 1) 3) | 152.8 ± 12.9 3) |
| Group 3 | 523.6 ± 14.8 1) 2) | 68.0 ± 4.3 1) 2) | 101.1 ± 7.1 1) 2) 3) | 362.0 ± 21.1 1) 2) 3) |
| Group 4 | 581.3 ± 16.9 1) | 80.0 ± 7.5 1) 3) | 120.6 ± 11.8 1) 3) | 505.3 ± 20.8 1) 3) |
| Group 5 | - | 28.3 ± 1.7 1) 2) 3) 4) | 44.7 ± 5.9 3) 4) | 122.4 ± 13.4 2) 3) 4) |
| Group 6 | - | 46.3 ± 2.3 3) | 58.5 ± 8.2 3) | 151.0 ± 12.4 3) |
Remark: 1)�? ≤ 0.05 compared to the Reference (intact animals, n=12); 2)�? ≤ 0.05 at comparison of the Group 1 (Alzheimer’s disease model, n=12) and Group 2 (shamoperated animals, n=12), Group 3 (nasal therapy of the AD model rats with soluble CUA, n=6) and Group 4 (nasal therapy of the AD model rats with H2O, n=6) and Group 5 (nasal therapy of the AD model rats with liposomal CUA, n=6) and Group 6 (nasal therapy of the AD model rats with empty liposomes, n=6), respectively; 3)�? ≤ 0.05 compared to the Group 1 (Alzheimer’s disease model, n=12); 4)�? ≤ 0.05 at comparison of the Group 3 (nasal therapy of the AD model rats with soluble CUA, n=6) and Group 5 (nasal therapy of the AD model rats with liposomal CUA, n=6)
Table 2: Effect of curcumin soluble and liposomal forms on cytokines content in the brain sections of the rats with Alzheimer’s disease model.
Thus, the predictor and catalyst of AD is the chronication of the nonspecific neuroinflammatory process, which provokes the toxicity of Aβ40/42 aggregates. One of the mechanisms of the pathogenesis of amyloidosis is activation of the cytokine response to a local excess of β-amyloid peptides. On the other hand, an excess of proinflammatory cytokines (IL-1β, TNFα, IL-6) against a background of lack of antiinflammatory interleukins (IL-10) results in an amyloidogenic processing scenario for amyloid precursor protein and new portions of secreted Aβ as a signal peptide of the inflammatory response.
The results obtained with regard to the cytokine system activation in the brain of rats with Alzheimer’s disease model are consistent with other studies on the activation of neuroinflammation with Aβ aggregates [54-56]. Aβ deposits are responsible for the activation of microglia [57]. Aβ enhances the inflammatory response to NFκB stimulation, which is involved in the regulation of extracellular signal-regulated kinase (ERK) and mitogen-activated protein kinase (MAPK), leading to the production of cytokines and chemokines [58]. Toll-like receptors (TLR), along with inflammatory cytokine receptors, are important for the regulation of microglial response to Aβ. Modification of microglia inflammatory state plays a leading role in the course of amyloidosis [59]. Generally, neuroinflammation is implicated in the pathogenesis of many neurodegenerative disorders. In AD, several mediators in the inflammation cascade contribute both to neurodegeneration and to the production and accumulation of the β-amyloid peptide, including proinflammation cytokines, phospho-c-Jun NH2-terminal kinase (pJNK), Wnt signalling, reactive oxygen species, inducible nitricoxide synthase-mediated production of reactive NO species, and lipid peroxidation products [60].
The effect of an aqueous solution of CUA in cerebral cortex of rats showed specific inhibition of inflammatory cytokine activation (Table 2): normalized levels of IL-1β and IL-6; TNFα levels decreased by 49% compared to control; concentration of IL-10 did not change. In group 4 (nasal administration of solvent - H2O) we observed further aggravation of the neuroinflammatory process induced by the intrahippocampal administration Aβ42_Human. Concentrations of IL-1β and IL-10 in this part of the brain increased by 50% and 73%, respectively, for one month of bidistillate nasal therapy. In the hippocampus of animals, the impact of curcumin on cytokine indicators had a similar orientation (Table 2). However, the concentration of any of the cytokines did not normalize, while the levels of IL-6 (49%) and IL-10 (83%) increased compared with the figures earlier this month. But a comparison of cytokines in hippocampus of rats in groups 3 and 4 clearly indicates a specific reduction in levels of IL-1β (by 33%), TNFα (by 24%), IL-6 (34%) and IL-10 (by 99%) due to a depressing effect of curcumin. The anticytokine effect of CUA can be explained by its ability to inhibit the activation of the inflammatory transcription factor NFκB, inhibiting phosphorylation and degradation of IκBα (NFκB inhibitor) [61,62]. Recently discovered a network of routes anti-inflammatory impact of curcumin in conditions of neurotoxicity units of β-amyloid peptide: inhibit cyclooxygenase (COX-2), phospholipases, pJNK, transcription factor AP-1 and NF-κB [63-65], which makes its properties as a potent inhibitor of pro-inflammatory cytokine production [66].
The effect of liposomal curcumin on the cytokines performance in the hippocampus of animals after the intrahippocampal administration of Aβ42_Human was marked with a significant inhibition of inflammation (Table 2): TNFα levels decreased by 56%, IL-6 – by 39% and IL-10 – by 52%, respectively. However, the concentration of cytokines did not normalize. The effect of CUA in the composition of liposomes in cerebral cortex of rats with Alzheimer’s disease model showed similar inhibition of cytokine reactions: TNFα levels decreased by 71%, IL-6 – by 67% and IL-10 – by 41%, respectively.
Conclusion
The results obtained coincide with the data on the aqueous solution of curcumin only for cerebral cortex, because the liposomal form of CUA in the hippocampus of rats with Alzheimer’s disease model showed a more intense suppression of cytokine level of neuroinflammation compared to its aqueous solution. The above data indicate a high anticytokine potential specifically of the liposomal form of curcumin.
References
- Goozee KG, Shah TM, Sohrabi HR, Rainey-Smith SR, Brown B, et al. (2016) Examining the potential clinical value of curcumin in the prevention and diagnosis of Alzheimer’s disease. Br J Nutr 115: 449-465.
- Prince M, Jackson J (2009) Alzheimer’s Disease International: World Alzheimer Report, Executive Summary. London: Alzheimer’s Disease International.
- Lee VM, Goedert M, Trojanowski JQ (2001) Neurodegenerative tauopathies. Annu Rev Neurosci 24: 1121-1159.
- Ferri CP, Prince M, Brayne C (2005) Global prevalence ofdementia: A Delphi consensus study. Lancet 366: 2112-2117.
- Hardy J, Selkoe DJ (2002) The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 297: 353-356.
- Goate A, Hardy J (2012) Twenty years of Alzheimer’s disease-causing mutations. J Neurochem 120: 3-8.
- Borchelt DR, Thinakaran G, Eckman CB, Lee MK, Davenport F, et al. (1996) Familial Alzheimer’s disease-linked presenilin 1 variants elevate Aβ1–42/1–40 ratio in vitro and in vivo. Neuron 17: 1005-1013.
- Hunt CE, Turner AJ (2009) Cell biology, regulation and inhibition of β-secretase (BACE-1). FEBS J 276: 1845-1859.
- Cruchaga C, Haller G, Chakraverty S (2012) Rare variants in APP, PSEN1 and PSEN2 increase risk for AD in late-onset Alzheimer’s disease families PloS ONE 7: e31039.
- Crean WS, Mercaldi CJ, Collins JM, Boyd D, Cook MN, et al. (2012) Prevalence of apolipoprotein E4 genotype and homozygotes (APOE e4/4) among patients diagnosed with Alzheimer’s disease: A systematic review and meta-analysis. Neuroepidemiology 38: 1-17.
- Potter PE (2013) Curcumin: A natural substance with potential efficacy in Alzheimer’s disease. J Exp Pharmacol 5: 23-31.
- Aggarwal BB, Sung B (2009) Pharmacological basis for the role of curcumin in chronic diseases: An age-old spice with modern targets. Trends Pharmacol Sci 30: 85-94.
- Garcia-Alloza M, Borrelli LA, Rozkalne A, Hyman BT, Bacskai BJ (2007) Curcumin labels amyloid pathology in vivo, disrupts existing plaques and partially restores distorted neurites in an Alzheimer mouse model. J Neurochem 102: 1095-1104.
- Mutsuga M, Chambers JK, Uchida K, Tei M, Makibuchi T, et al. (2012) Binding of curcumin to senile plaques and cerebral amyloid angiopathyin the aged brain of various animals and to neurofibrillary tangles in Alzheimer’s brain. J Vet Med Sci 74: 51-57.
- Liu H, Li Z, Qiu D (2010) The inhibitory effects of different curcuminoids on β-amyloid protein, β-amyloid precursor protein and β-site amyloid precursor protein cleaving enzyme 1 in swAPP HEK293 cells. Neurosci Lett 485: 83-88.
- Wang X, Kim JR, Lee SB, Kim YJ, Jung MY, et al. (2014) Effects of curcuminoids identified in rhizomes of Curcuma longa on BACE-1inhibitory and behavioral activity and lifespan of Alzheimer’s disease Drosophila models. BMC Complement Altern Med 14.
- Mithu VS, Sarkar B, Bhowmik D, Das AK, Chandrakesan M, et al. (2014) Curcumin alters the salt bridge-containing turn region in amyloid β(1-42) aggregates. J Biol Chem 289: 11122-11131.
- Fu Z, Aucoin D, Ahmed M, Ziliox M, Van Nostrand WE, et al. (2014) Capping of aβ42 oligomers by small molecule inhibitors. Biochemistry 53: 7893-7903.
- Olivia CA, Vargas JY, Inestrosa NC (2013) Wnt signaling: Role in LTP, neural networks and memory. Ageing Res Rev 12: 786-800.
- Parr C, Mirzaei N, Christian M, Sastre M (2015) Activation of the Wnt/beta-catenin pathway represses the transcription of the beta-amyloid precursor protein cleaving enzyme (BACE-1) via binding of T-cell factor-4 to BACE1 promoter. FASEB J 29: 623-635.
- Shi X, Zheng Z, Li J, Xiao Z, Qi W, et al. (2015) Curcumin inhibitsAbeta-induced microglial inflammatory responses in vitro: Involvement of ERK1/2 and p38 signaling pathways. Neurosci Lett 594: 105-110.
- Zhang L, Fiala M, Cashman J, Sayre J, Espinosa A, et al. (2006) Curcuminoids enhance amyloid-beta uptake by macrophages of Alzheimer’s disease patients. J Alzheimers Dis 10: 1-7.
- Fiala M, Liu PT, Espinosa-Jeffrey A, Rosenthal MJ, Bernard G, et al. (2007) Innate immunityand transcription of MGAT-II and toll-like receptors inAlzheimer’s disease patients are improved by bisdemethoxycurcumin. Proc Natl Acad Sci USA 104: 12849-12854.
- Masoumi, Goldenson B, Ghirmai S, Avagyan H, Zaghi J, et al. (2009) 1 alpha, 25-Dihydroxy vitamin D3 interacts with curcuminoids to stimulate amyloid-beta clearance by macrophages of Alzheimer’s disease patients. J Alzheimers Dis 17: 703-717
- KK Cheng, PS Chan, S Fan, SM Kwan, KL Yeung, et al. (2015) Curcumin-conjugated magnetic nanoparticles for detecting amyloid plaques in Alzheimer’s disease mice using magnetic resonance imaging (MRI). Biomaterials 44: 155-172.
- Patil R, GangalumPR, Wagner S, Portilla-Arias J, Ding H, et al. (2015) Curcumin targeted, polymalic acid-based MRI contrast agent for the detection of abeta plaques in Alzheimer’s disease. Macromol Biosci 15: 1212-1217
- Ahmed T, Gilani AH (2009) Inhibitory effect of curcuminoids on acetylcholinesterase activity and attenuation of scopolamine-induced amnesia may explain medicinal use of turmeric in Alzheimer’s disease. Pharmacol Biochem Behav 91: 554-559.
- Peeyush KT, Antony S, Sonan S, Kuruvilla KP, George N, et al. (2011) Role of curcuminin the prevention of cholinergic mediated cortical dysfunctions in streptozotocin-induced diabetic rats. Mol Cell Endocrinol 331: 1-10.
- Kim M, Kim Y (2010) Hypocholesterolemic effects of curcumin via up-regulation of cholesterol 7α-hydroxylase inrats fed a high fat diet. Nutr Res Pract 4: 191-195.
- Tu Y, Sun D, Zeng X, Yao N, Huang X, et al. (2014) Piperine potentiates the hypocholesterolemic effect of curcumin in rats fed on a highfat diet. Exp Ther Med 8: 260-266.
- Kim SJ, Son TG, Park HR, Park M, Kim MS (2008) Curcumin stimulates proliferation of embryonic neural progenitor cells and neurogenesis in the adult hippocampus. J Biol Chem 283: 14497-14505.
- Huang HC, Chang P, Dai XL, Jiang ZF (2012) Protective effects of curcumin on amyloid-beta-induced neuronal oxidative damage. Neurochemical Research 37: 1584-1597.
- Xiao Z, Zhang A, Lin J, Zheng Z, Shi X, et al. (2014) Telomerase: A target for therapeutic effects of curcumin and a curcumin derivative in Abeta insult in vitro. PLoS ONE 9: e101251.
- Belviranli M, Okudan N, Atalik KEN, Öz M (2013) Curcuminim proves spatial memeory and decreases oxidative damagein aged female rats. Biogerontology 14: 187-196.
- Zhang C, Browne A, Child D, Tanzi RE (2010) Curcumin decreases amyloid-beta peptide levels by attenuating the maturation of amyloid-beta precursor protein. J Biol Chem 285: 28472-28480.
- Yang F, Lim GP, Begum AN, Ubeda OJ, Simmons MR, et al. (2005) Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques and reduces amyloid in vivo. J Biol Chem 280: 5892-5901.
- Wang YJ, Thomas P, Zhong JH, Bi FF, Kosaraju SH, et al. (2009) Consumption of grape seed extract prevents amyloid-β deposition and attenuates inflammation in brain of an Alzheimer’s disease mouse. Neurotox Res 15: 3-14.
- Chakraborti S, Dhar G, Dwivedi V, Das A, Poddar A, et al. (2013) Stable and potent analogues derived from the modification of the dicarbonyl moiety of curcumin. Biochemistry 52: 7449-7460.
- Lee WH, Loo CY, Bebawy M, Luk F, Mason RS, et al. (2013) Curcumin and its derivatives: Their application in neuropharmacology and neuroscience in the 21st century. Curr Neuropharmacol 11: 338-378.
- Naksuriya O, Okonogi S, Schiffelers RM, Hennink WE, (2014) Curcumin nanoformulations: A review of pharmaceutical properties and preclinical studies and clinical data related to cancer treatment. Biomaterials 35: 3365-3383.
- Ranjan P, Mukerjee A, Helson L, Gupta R, Vishwanatha JK (2013) Efficacy of liposomal curcumin in a human pancreatic tumor xenograft model: Inhibition of tumor growth and angiogenesis. Anticancer Res 33: 3603-3609.
- Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB, (2007) Bioavailability of curcumin: Problems and promises. Mol Pharm 4: 807-818.
- Mourtas S, Lazar AN, Markoutsa E, Duyckaerts C, Antimisiaris SG, (2014) Multifunctional nanoliposomes with curcumin-lipid derivative and brain targeting functionality with potential applications for Alzheimer disease. Eur J Med Chem 80: 175-183.
- Mohanty C, Sahoo SK (2010) The in vitro stability and in vivo pharmacokinetics of curcumin prepared as an aqueous nanoparticulate formulation. Biomaterials 31: 6597-6611.
- Ghosh M, Singh AT, Xu W, Sulchek T, Gordon LI, et al. (2011) Curcumin nanodisks: Formulation and characterization. Nanomedicine 7: 162-167.
- NK Gupta, VK Dixit (2011) Bioavailability enhancement of curcumin by complexation with phosphatidyl choline. J Pharm Sci 100: 1987-1995.
- Berginc K, Trontelj J, Basnet NS, Kristl A (2012) Physiological barriers to the oral delivery of curcumin. Pharmazie 67: 518-524.
- Vorobjova TM (1969) Role of limbic and reticular systems in self stimulation. Neurosci Behav Physiol 70: 95-101.
- Bures J, Petran M, Zachar J (1960) Electrophysiological methods in biological research. Ed.2 Publishing House.
- Wang YJ, Pan MH, Cheng AL, Lin LI, Ho YS, et al. (1997) Chengetal stability of curcumin in buffer solutions and characterization of its degradation products. J Pharm Biomed Anal15: 1867-1876.
- Sokolik VV, Shulga SM (2015) Effect of curcumin liposomal form on angiotensin converting activity, cytokines and cognitive characteristics of the rats with Alzheimer’s disease model. Biotechnologia Acta 8: 48-55.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with Folin phenol reagent. J Biol Chem 193: 265-275.
- Sokolik VV, Maltsev AV (2015) Cytokines neuroinflammatory reaction to the action of β-amyloid 1-40 administered to rats in homoaggregated and liposomal forms. Biochemistry, (Moscow) Supplement Series B: Biomedical Chemistry 9: 355-361.
- Smith JA, Das A, Ray SK, Banik NL (2012) Role of pro-inflammatory cytokines released from microglia in neurodegenerative diseases. Brain Res Bull 87: 10-20.
- Salminen, Ojala J, Kauppinen A, Kaarniranta K, Suuronen T (2009) Inflammation in Alzheimer’s disease: amyloid-β oligomers trigger innate immunity defence via pattern recognition receptors. Prog Neurobiol 87: 181-194.
- Combs K, Karlo JC, Kao SC, Landreth GE (2001) β-Amyloid stimulation of microglia and monocytes results in TNF-dependent expression of inducible nitric oxide synthase and neuronal apoptosis. J Neurosci 21: 1179-1188.
- Sadigh-Eteghad S, Sabermarouf B, Majdi A, Talebi M, Farhoudi M, et al. (2015) Amyloid-beta: A crucial factor in Alzheimer’s disease. Med Princ Pract 24: 1-10.
- Ridolfi E, Barone C, Scarpini E, Galimberti D, (2013) The role of the innate immune system in Alzheimer’s disease and frontotemporal lobar degeneration: An eye on microglia Clin Dev Immunol 2013: 939786.
- Boutajangout A, Wisniewski T (2013) The innate immune system in Alzheimer’s disease. Int J Cell Biol 2013: 576383.
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, et al. (2000) Inflammation and Alzheimer’s disease. Neurobiol Aging 21: 383-421.
- Aggarwal BB, Gupta SC, Sung B (2013) Curcumin: An orally bioavailable blocker of TNF and other pro-inflammatory biomarkers. Br J Pharmacol 169: 1672-1692.
- Pan MH, Lin-Shiau SY, Lin JK (2000) Comparative studies on the suppression of nitric oxide synthase by curcumin and its hydrogenated metabolites through downregulation of IB kinase and NF-κB activation in macrophages. Biochem Pharmacol 60:1665-1676.
- Park SY, Kim DS (2002) Discovery of natural products from Curcuma longa that protect cells from beta-amyloid insult: A drug discovery effort against Alzheimers disease. J Nat Prod 65: 1227-1231.
- Kim GY, Kim KH, Lee SH, Yoon MS, Lee HJ, et al. (2005) Curcumin inhibits immunostimulatory function of dendritic cells: MAPKs and translocation of NF-B as potential targets. J Immunol 174: 8116-8124.
- Weber WM, Hunsaker LA, Gonzales AM, Heynekamp JJ, Orlando RA, et al. (2006) TPA-induced up-regulation of activator protein-1 can be inhibited or enhanced by analogs of the natural product curcumin. Biochem Pharmacol 72: 928-940.
- Begum N, Jones MR, Lim GP, Morihara T, Kim P, et al. (2008) Curcumin structure-function, bioavailability and efficacy in models of neuroinflammation and Alzheimer’s disease. J Pharmacol Exp Ther 326:196-208.
Relevant Topics
- Advanced Parkinson Treatment
- Advances in Alzheimers Therapy
- Alzheimers Medicine
- Alzheimers Products & Market Analysis
- Alzheimers Symptoms
- Degenerative Disorders
- Diagnostic Alzheimer
- Parkinson
- Parkinsonism Diagnosis
- Parkinsonism Gene Therapy
- Parkinsonism Stages and Treatment
- Stem cell Treatment Parkinson
Recommended Journals
Article Tools
Article Usage
- Total views: 3741
- [From(publication date):
August-2017 - Jul 06, 2025] - Breakdown by view type
- HTML page views : 2876
- PDF downloads : 865