Research Article Open Access
Comparative Analysis of Intrahippocampal Amyloid Beta (1-42) and Intracerbroventricular Streptozotocin Models of Alzheimer's Disease: Possible Behavioral, Biochemical, Mitochondrial, Cellu lar and Histopathological Evidences
Arti Singh and Anil Kumar*
Pharmacology Division, University Institute of Pharmaceutical Sciences, UGC Centre of Advanced Study, Panjab University, Chandigarh-160014, India
- Corresponding Author:
- Anil Kumar
Pharmacology Division
University Institute of Pharmaceutical Sciences
UGC Centre of Advanced Study, Panjab University
Chandigarh-160014, India
Tel: 919478692293
E-mail: kumaruips@yahoo.com
Received date: December 16, 2015; Accepted date: January 23, 2016; Published date: January 30, 2016
Citation:Singh A, Kumar A (2016) Comparative Analysis of Intrahippocampal Amyloid Beta (1-42) and Intracerbroventricular Streptozotocin Models of Alzheimer’s Disease: Possible Behavioral, Biochemical, Mitochondrial, Cellular and Histopathological Evidences. J Alzheimers Dis Parkinsonism 6:208. doi:10.4172/2161-0460.1000208
Copyright: © 2016 Singh A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Alzheimers Disease & Parkinsonism
Abstract
Background: Alzheimer’s disease (AD), one of the most common progressive neurodegenerative disorders that leads to dementia in aged humans. Amyloid beta [Aβ (1-42)] model has an ability to mimic many pathological aspects of human AD. Intracerebroventricular streptozotocin (icv-STZ) is an another relevant model for sporadic dementia of Alzheimer’s type. However, both the models are frequently used to AD in experimental animals.
Objective: Aim of the present study was to compare these two animal models of AD in order to comment on the best and reliable model of AD as well as screening of the neuroprotectants.
Materials and Methods: Animals received a single bilateral intrahippocampal (ih) injection of Aβ (1-42) (1 μg/μl; 4 μl/site) and single bilateral icv injection of STZ (3 mg/kg; 4 μl/site). Galantamine (2 mg/kg) was used as a standard drug and administered for a period of 21 days. Various neurobehavioral parameters were evaluated, followed by biochemical (oxidative stress parameters), molecular (TNF-α level), mitochondrial respiratory enzyme complexes (Complex I, II, III, IV) and histopathological (H&E staining) parameters.
Results: In the present study, ih-Aβ (1-42) administration significantly impaired cognitive performance on MWM test, increased oxidative stress markers (raised lipid peroxidation, nitrite concentration, reduced glutathione, catalase activity), increased AChE level and neuroinflammation (increased TNF-α levels), reduced mitochondrial respiratory enzyme complexes (complex I,II,III,IV) and histopathological alterations as compared to single bilateral icv-STZ administration. The effect of galantamine (2 mg/kg) was significantly produced its protective effect in reversing these neurobehavioral, biochemical, cellular and histopathological parameters as compared to their respective controls [ih-Aβ (1-42) and icv-STZ]
Conclusions: Result of the present study suggests that the single bilateral ih-Aβ (1-42) model is the most effective and reliable model of AD as compared to single bilateral icv-STZ.
Keywords
Alzheimer’s disease, galantamine, oxidative stress, mitochondrial dysfunction, Aβ (1-42), STZ
Introduction
Alzheimer’s disease (AD), a common progressive and incapacitating neurodegenerative disorders [1]. The disease is characterized by progressive loss of neurons in the parts of brain involved in cognition [2]. It accounts for more than 60-70% cases of dementia and 35 million people worldwide. The major neuropathological hallmarks of AD include progressive accumulation of the amyloid beta (A) plaques outside the neurons in the brain and neurofibrillary tangles (twisted strands of the tau protein) inside the neurons, which eventually accompanied by the damage and death of neurons [3-5]. AD is also symptomatically characterized by memory loss and disoriented behavior in language, comprehension, and spatial skills etc.
According to the “amyloid cascade hypothesis” accumulation of Aβ (1-42) leads to the aggregation of a monomeric form of Aβ (1-42) and converting it to Aβ (1-42) oligomers. Aβ (1-42) oligomers further leads to hyperphosphorylation of tau. Aggregation of Aβ (1-42) oligomers increases reactive oxygen species (ROS) and also causes cholinergic dysfunction, mitochondrial dysfunction, synaptic dysfunction, oxidative stress and neuroinflammation [6]. So, Aβ (1-42) oligomers plays central role in the pathogenesis and progression of AD. And Aβ (1-42) model is considered to be a relevant model of AD as it develops symptoms that are pathologically similar to human AD.
Studies have shown that intracerebroventricular (icv) administration of streptozotocin (STZ) at sub-diabetogenic dose causes desensitization of insulin receptors [7]. It further causes prolonged impairment of brain energy metabolism and leads to cognitive dysfunction by inhibiting the synthesis of adenosine triphosphate (ATP) and acetyl-CoA. Also, this impaired energy metabolism may result in the production of increased amounts of free radicals that cause oxidative damage [7]. This cholinergic deficiency, followed by reducing choline acetyltransferase (ChAT) activity in the hippocampus, thus provides a model for senile dementia of Alzheimer’s type (SDAT) [7,8].
Galantamine (or galanthamine) is an alkaloid found in the bulbs of snowdrops and several Amaryllidaceae plants. Based on the ability of acetylcholinesterase inhibition [3], galantamine has been approved by U.S. FDA for the treatment of AD [4].
In order to develop any rodent model for AD disease, it is mandatory that it must mimic the disease pathology, can reproduce the complexity of human behavior in rodents as well in order to develop an effective therapy. In AD, varieties of pathophysiological events are involved so, the animal model that explains these cognitive, biochemical, behavioral and histopathological features could be treated as best suited for the screening of newer drugs. In the present study, the authors have tried to compare and comments on two different and frequently used experimental models of AD. Therefore, the present study has been designed to perform the comparative analysis of two different animal models of AD induced by single bilateral ih-Aβ (1-42) and icv-STZ administration by using standard drug galantamine.
Materials and Methods
Animals
Adult male Sprague–Dawley rats (180–200 g) from Central Animal House of the Panjab University, Chandigarh, were used. Animals were acclimatized to laboratory conditions prior to experimentation at room temperature. The animals were kept under standard conditions of 12 h light and dark cycle with food and water ad libitum. All the experiments were carried out between 09:00 and 15:00 h. The protocol was approved by the Institutional Animal Ethics Committee (IAEC) and carried out in accordance with the guidelines of Committee for Control and Supervision of Experimentation on Animals (CPCSEA), Government of India on animal experimentation.
Surgery
Animals were anesthetized indivudally with thiopental sodium (45 mg/kg, i.p.) and head was positioned in a stereotaxic apparatus and the skull was exposed. A midline sagittal incision was made in the scalp and two holes were drilled in the skull. The stereotaxic coordinates for ih region were 2.00 mm posterior to bregma, 1.5 mm lateral to the sagittal suture and 1.0 mm beneath the cortical surface. Similarly icv of STZ administration was as per the following coordinates 0.8 mm posterior to bregma, 1.8 mm lateral to the sagittal suture and 3.6 mm beneath the cortical surface set according to rat brain atlas [9]. Through a skull hole, a 28-gauge Hamilton micro syringe of 10 μl units and piston of the syringe was lowered with the help of sterotaxic apparatus into each hippocampal region. Rats were infused with Aβ (1-42) (1 μg/μl) and STZ (3 mg/kg) dissolved artificial cerebrospinal fluid (ACSF; in mmol/L: 147 NaCl, 2.9 KCl, 1.6 MgCl2, 1.7 CaCl2 and 2.2 dextrose) [4,7]. The scalp was then closed with a suture. After surgery, all animals received gentamicin (5 mg/kg, i.p.) to prevent sepsis. In sham group, surgery was identical in both the models and they receive the same volume of ACSF instead of Aβ (1-42) or STZ. To promote the diffusion from the micro syringe, it was left in place for a period of 2 min following ih-Aβ (1- 42) and icv-STZ administration. Special care of the animals was taken during the post-operative period.
Drug treatment
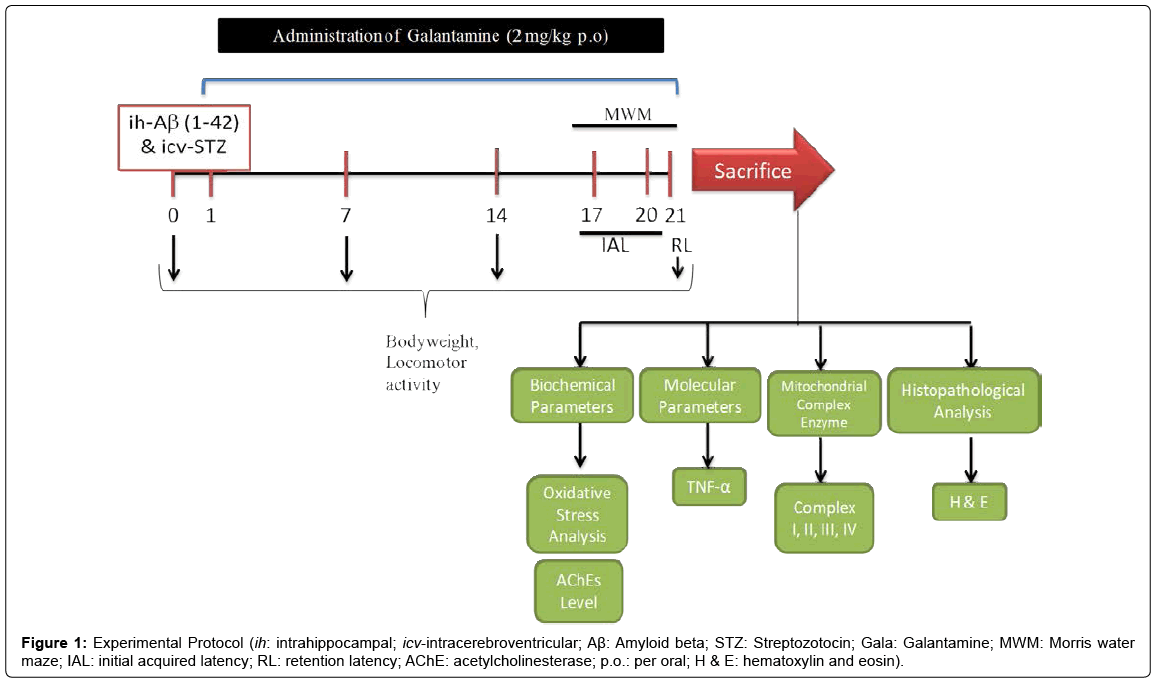
Aβ (1-42), STZ and galantamine (Gala) were purchased from Sigma Chemicals, Pvt. Ltd., India. Aβ (1-42) and STZ was prepared in ACSF and administered ih and icv at a dose of 1 μg/μL and 3 mg/kg using Hamilton syringe. For drug administration Gala (per oral; p.o.) were suspended in 0.25% sodium-carboxy-methyl-cellulose (Na-CMC) and administered at the dose of 2.0 ml/kg body weight for 21 days. Animals were selected randomly and divided on the basis of their body weight into several groups (12 animals per group; n=12). Study was performed as shown in experimental protocol (Figure 1). The doses of Aβ (1-42), STZ and Gala were selected on the basis of previous literature [3,4,7,8]. Study protocol was lasted for 21 days (3 weeks) (Table 1).
| Group | Group Name | Treatment |
|---|---|---|
| 1. | Naïve | Healthy animals |
| 2. | Sham | Surgery Performed, ACSF administered(4µl/site) |
| 3. | Aβ (1-42) | Single Bilateral ih-Aβ (1-42) (1µg/µl; 4µl/site) administration |
| 4. | STZ | Single Bilateral icv-STZ (3 mg/kg; 4µl/site) administration |
| 5. | Aβ (1-42) + Gala | Galantamine (2 mg/kg, p.o) administered inih-Ab (1-42) treated animals |
| 6. | STZ + Gala | Galantamine (2 mg/kg, p.o) administered in icv-STZ treated animals |
Table 1: Treatment groups.
Body weight
The body weights of animals were recorded 0, 7, 14 and day 21.
Behavioral parameters
Measurement of locomotor activity (ambulation) by actophotometer: The locomotor activity (ambulatory activity) was recorded by using actophotometer (IMCORP, India). Before locomotor task, animal was placed individually in the actophotometer chamber for 3 min for habituation. Thereafter, locomotor activity was recorded using actophotometer for a period of 5 min. Ambulatory activity was recorded and values expressed in terms of counts per 5 min [10].
Morris Water Maze: Animal were than examined for acquisition and retention of a spatial navigation task using Morris water maze (MWM) [10,11]. Animals were trained to swim in a circular water tank (150 cm diameter, 40 cm high, filled to a depth of 30 cm with water at 28 ± 1°C). A platform (4.5 cm diameter) was placed in any one quadrant of the pool 1 cm above the water level for acquisition test and same platform was placed 1 cm below the water level for retention phase. Animals received training during four day of the acquisition phase with the help of several brightly colored cues that were visible from the pool. During each trial animal was subjected to four consecutive trials with gap of 5 min. The animal was gently placed between any one of the quadrant, facing the wall of pool with drop location, change for each trial, and allowed 120 s to locate the platform. Then, animals were guided to remain on the platform for 20 s. If animal failed to reach the platform within 120 s, same was guided to reach the platform and remained there for next 20 s.
During acquisition Phase (or training phase) animals received four consecutive daily training sessions from day 17 to 20. Each rat was put into the water in any one of four starting positions, the sequence of which was selected randomly. The time latency to reach the visual platform for escaping from water (IAL; initial acquisition latency) also known as escape latency time (ELT) was measured.
During retention Phase after 24 hour i.e., on day 21, after IAL, animal was released randomly at one of the edges facing the wall of the pool to assess for memory retention. Time taken by animal to find the hidden platform on day 21 known as time spent in the target quadrant (TSTQ) were recorded.
Biochemical analysis
Tissue preparation: Biochemical tests were carried out 24 h after the last behavioral test on day 21. After 3 weeks of post treatment, animals (n=5) were sacrificed and their brains were taken out quickly to dissect hippocampus and cerebral cortex by using rat brain atlas [4]. The dissected brain parts were harvested and rinsed with ice-cold isotonic saline. Brains were homogenized in ice cold 10 mM phosphate buffer (pH 7.4). The homogenate (10% w/v) was then centrifuged at 10,000 g for 15 min and the supernatant so formed was used for the biochemical estimations.
Measurement of lipid peroxidation: The extent of lipid peroxidation (LPO) was determined by method as described by Wills, 1966 [12]. The amount of malondialdehyde (MDA) was measured by reaction with thiobarbituric acid at 532 nm using Perkin Elmer Lambda 20 spectrophotometer. The values were calculated using the molar extinction co-efficient of chromophore (1.56 X 105 (mol/l)-1 cm-1).
Reduced glutathione (GSH) content estimation: GSH was estimated according to the method as described by Ellman, 1959 [13]. The change in absorbance was measured at 412 nm using Perkin Elmer Lambda 20 spectrophotometer. Results were calculated using molar extinction co-efficient of the chromophore (1.36 X 104 (mol/l)-1 cm-1).
Estimation of nitrite: For the estimation of nitrite, an indicator of the production of nitric oxide (NO), colorimetric assay was performed with Greiss reagent (0.1% N-(1- napththyl) ethylene diamine dihydrochloride, 1% sulphanilamide and 5% phosphoric acid) [14]. The change in absorbance was measured at 540 nm using Perkin Elmer Lambda 20 spectrophotometer. The concentration of nitrite in the supernatant was determined from the sodium nitrite standard curve.
Superoxide dismutase activity: Superoxide dismutase (SOD) activity was assayed by the method of Kono, 1978 [15]. The change in absorbance was measured for 2 min at 30 s intervals by measuring absorbance at 560 nm using Perkin Elmer Lambda 20 spectrophotometer.
Catalase activity: Catalase activity was assessed by the method of Luck, 1978 [16], wherein the breakdown of H2O2 is measured. The change in absorbance was recorded for 2 min at 30 sec interval at 240 nm using Perkin Elmer Lambda 20 spectrophotometer. The results were expressed as micromoles of H2O2 decomposed/min/mg of protein.
Acetylcholinesterase (AChE) activity: The AChE activity was assessed by Ellman method [17]. The change in absorbance was measured for 2 min at 30 s interval at 412 nm using Perkin Elmer Lambda 20 spectrophotometer. Results were expressed as micromoles of acetylthiocholine iodide hydrolyzed/min/mg of protein.
Protein content estimation: The protein content was estimated by Biuret method [18] using bovine serum albumin as a standard.
Mitochondrial enzyme complex estimation
Isolation of rat brain (cortex and hippocampus) mitochondria for enzyme complex assay
The cortex and hippocampus was homogenized in an isolation buffer with ethylene glycol tetra acetic acid (EGTA; 215 mM Mannitol, 75 mM sucrose, 0.1% BSA, 20 mM HEPES, 1 mM EGTA, pH-7.2) (n=5). Homogenates were centrifuged at 13,000 g for 5 min at 4°C. Pellet was resuspended in isolation buffer with EGTA and spun again at 13,000 g for 5 min. The resulting supernatants were transferred to new tubes and topped off with isolation buffer with EGTA and again spun at 13,000 g for 10 min. Pellets containing pure mitochondria were resuspended in isolation buffer without EGTA [19].
NADH dehydrogenase (Complex I) activity
Complex I activity was measured spectro photometrically by the method of King and Howard (1967) [20]. The method involves catalytic oxidation of NADH to NAD+ with subsequent reduction in cytochrome C. The change in absorbance was measured at 550 nm for 2 min.
Succinate dehydrogenase (SDH) (Complex II) activity: Complex II was measured spectrophotometrically according to King (1967) [21]. The method involves oxidation of succinate by an artificial electron acceptor, potassium ferricyanide. The change in absorbance was measured at 420 nm for 2 min.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT) or Mitochondrial redox (Complex III) assay: Complex III assay employed in the present study is based on the in vitro studies to evaluate mitochondrial redox activity through the conversion of MTT tetrazolium salt to formazan crystals by mitochondrial respiratory chain reactions in isolated mitochondria by the method of Liu et al. (1997) [22]. The absorbance of the resulting medium was measured by an ELISA reader at 580 nm wavelength.
Cytochrome oxidase (Complex IV) activity: Complex IV activity was assayed in brain mitochondria according to the method of Sottocasa et al. (1967) [23]. The change in absorbance was recorded at 550 nm for 2 min.
Estimation of TNF-α activity
The molecular analysis was done to assess the levels of cytokine TNF-α by the help and instructions provided by R&D Systems Quantikine rat TNF-α immunoassay kit [4].
Histopathological analysis by Hematoxylin and Eosin Staining (H&E staining)
For histopathological analysis rats were sacrificed by decapitation immediately after the last behavioral test. The brains were removed and transferred to formalin (10%, v/v). The brain tissues were embedded in paraffin blocks and sectioned into 3 mm thickness with the help of a microtome. The brain sections (5–10 μm) thick were de-waxed and stained with hematoxylin and eosin. The stained sections were viewed under a binocular microscope and photographed [24].
Statistical analysis
Graph Pad Prism (Graph Pad Software, San Diego, CA) was used for all statistical analysis. Results are expressed as mean ± SEM. The behavioral assessment data were analyzed by a repeated measures two-way analysis of variance (ANOVA). The biochemical estimations were analyzed by one-way ANOVA followed by Tukey’s test. Post hoc comparisons between groups were made using Tukey’s test. The p-value <0.05 was considered significant.
Results
Effect of ih-Aβ (1-42) and icv-STZ on memory performance on MWM task in rats
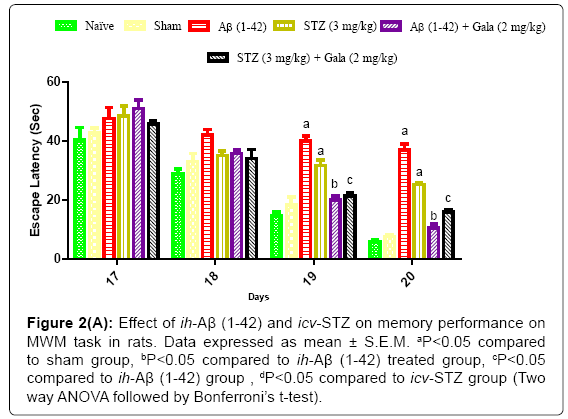
Sham treated animals quickly learned to swim directly to the platform in the MWM during the acquisition phase as compared to naïve. However, a significant difference was observed in the mean IAL of ih-Aβ (1-42) and icv-STZ treated groups as compared to sham group during acquisition phase indicating that ih-Aβ (1-42) and icv-STZ induced impairment in spatial memory and learning (P<0.05). Further, chronic treatment of galantamine (2 mg/kg) significantly decreased IAL to reach the platform during acquisition phase as compared to their respective control groups [ih-Aβ (1-42) and icv-STZ treated groups] (P<0.05) (Figure 2).
Figure 2a: Effect of ih-Aβ (1-42) and icv-STZ on memory performance on MWM task in rats. Data expressed as mean ± S.E.M. aP<0.05 compared to sham group, bP<0.05 compared to ih-Aβ (1-42) treated group, cP<0.05 compared to ih-Aβ (1-42) group , dP<0.05 compared to icv-STZ group (Two way ANOVA followed by Bonferroni’s t-test).
Figure 2b: Effect of ih-Aβ (1-42) and icv-STZ on TSTQ in the MWM retention phase (21th day) in Aβ (1-42) treated rats. Data expressed as mean ± S.E.M. aP<0.05 compared to sham group, bP<0.05 compared to ih-Aβ (1-42) treated group, cP<0.05 compared to ih-Aβ (1-42) group, dP<0.05 compared to icv-STZ group (One way ANOVA followed by Tukey’s test).
In a sham treated group, animals did not show any significant effect on TSTQ as compared to naïve. On the contrary, ih-Aβ (1-42) and icv- STZ treated groups showed significant (P<0.05) decrease in TSTQ as compared to sham treated animals suggesting poor memory to relocate the submerged platform as compared to sham treatment. Further, administration of galantamine (2 mg/kg) significantly improved TSTQ in both ih-Aβ (1-42) and icv-STZ treated groups and improved retention performance of the spatial navigation task as compared to their respective control group (P<0.05) (Figure 2).
Effect of ih-Aβ (1-42) and icv-STZ on locomotor activity in rats
In this study, mean values of locomotor activity of each group of animal were invariable before Aβ (1-42) and icv-STZ administration. The mean values in naïve (vehicle), sham-operated and ih-Aβ (1-42) and icv-STZ treated rats remains unchanged during the experiment. Chronic administration of galantamine(2 mg/kg) did not produce any significant effect on locomotor activity as compared to sham operated rats on day 7, 14 and 21 (Data not shown).
Effect of ih-Aβ (1-42) and icv-STZ on oxidative stress parameters in rats
Sham treated animals did not show any significant effect on oxidative stress parameters among themselves. However, ih-Aβ (1- 42) and icv-STZ administration caused a significant rise in LPO and NO activities, and reduced GSH, SOD and catalase activities in hippocampus and cortex as compared to sham groups (P<0.05). Further, chronic administration of galantamine (2 mg/kg) significantly attenuated oxidative stress markers (attenuated elevated LPO, NO activities, restored SOD, catalase and reduced GSH) as compared to control groups (P<0.05) (Table 2).
| Treatment group (mg/kg) | Brain region | LPO (nmol MDA/mg protein) (% of naïve) | Nitrite (µmol/mg protein)(% of naïve) | GSH (nmol/mg protein) (% of naïve) | Catalase (µmol of H2O2 decomposed /min/mg protein) (% of naïve) | SOD (units/ mg protein) (% of naïve) |
|---|---|---|---|---|---|---|
| Naïve | Cortex | 0.10 ± 0.6 (100) | 189 ± 2.9 (100) | 0.88 ± 0.2 (100) | 8.12 ± 0.2 (100) | 0.68 ±0.1 (100) |
| Hippocampus | 0.15 ± 0.2 (100) | 143 ± 2.1 (100) | 0.70 ± 0.6 (100) | 8.15 ± 0.3 (100) | 0.61 ±0.3 (100) | |
| Sham (ACSF; 4µl/site) | Cortex | 0.18 ± 0.1 (180) | 201 ± 1.6 (106) | 0.67 ± 0.3(76) | 7.5 ± 0.1 (92.3) | 0.55± 0.2(80.8) |
| Hippocampus | 0.21 ± 0.4 (140) | 166 ± 1.5 (116) | 0.61 ± 0.4(87.1) | 7.1 ± 0.2 (87.1) | 0.59 ± 0.1(96.7) | |
| Aβ (1-42) (1µg/µl) | Cortex | 0.59±0.4a(393.3) | 625 ± 6.1a(330) | 0.11 ±0.2a(12.5) | 1.5 ± 0.1a(18.4) | 0.02±0.7a(2.94) |
| Hippocampus | 0.54 ± 0.3a(540) | 698±5.1a(488.1) | 0.09±0.1a(12.8) | 1.1 ± 0.8a(13.4) | 0.05±0.6a(8.19) | |
| STZ (3 mg/kg) | Cortex | 0.42 ± 0.2 a(280) | 488±8.3 a(258.2) | 0.15±0.2a17.04) | 2.9 ± 0.6a(35.7) | 0.18±0.1a(26.4) |
| Hippocampus | 0.37 ± 0.8a(370) | 501 ± 3.2 a(350) | 0.20 ±0.2a(28.5) | 2.3 ± 0.1 a(28.2) | 0.11±0.1a(18) | |
| Aβ (1-42) + Gala | Cortex | 0.25 ± 0.1b(250) | 245±9.1b(129.6) | 0.61 ±0.3b(69.3) | 7.1 ± 0.2 b(87.4) | 0.47±0.8 b(69.1) |
| Hippocampus | 0.22 ±0.4 b(146.6) | 233 ± 2.1 b(163) | 0.59 ±0.2b(84.2) | 6.7 ± 0.6 b(82.2) | 0.53±0.2 b(86.8) | |
| STZ+ Gala | Cortex | 0.27 ± 0.3c(270) | 299±9.4c(158.2) | 0.45 ± 0.4 c(51.1) | 5.1 ± 0.4 c(62.8) | 0.31±0.2c(45.5) |
| Hippocampus | 0.21 ± 0.5 c(140) | 315 ± 2.5 c(220) | 0.51 ±0.2 c(72.8) | 4.7±0.42c(57.6) | 0.39±0.2c(63.9) |
Data expressed as mean ± S.E.M.
aP<0.05 compared to sham group, bP<0.05 compared to ih-Aβ (1-42) treated group,cP<0.05 compared to icv-STZ treated group (One way ANOVA followed by Tukey’s test).
Table 2: Effect of ih-Aβ (1-42) and icv-STZ on oxidative stress parameters in rats.
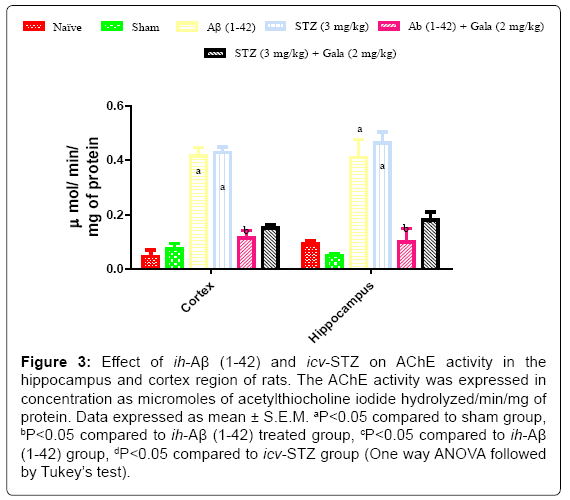
Effect of ih-Aβ (1-42) and icv-STZ on AChEs activity in rats
Sham treated animals did not produce any significant changes in AChEs activity as compared to naïve treatment. Administration of ih-Aβ (1-42) and icv-STZ significantly increased AChEs activity in hippocampus and cortex as compared to sham treated animals (P<0.05). Chronic galantamine (2 mg/kg) treatment significantly attenuated AChEs activity as compared to their respective control groups (P<0.05) (Figure 3).
Figure 3: Effect of ih-Aβ (1-42) and icv-STZ on AChE activity in the hippocampus and cortex region of rats. The AChE activity was expressed in concentration as micromoles of acetylthiocholine iodide hydrolyzed/min/mg of protein. Data expressed as mean ± S.E.M. aP<0.05 compared to sham group, bP<0.05 compared to ih-Aβ (1-42) treated group, cP<0.05 compared to ih-Aβ (1-42) group, dP<0.05 compared to icv-STZ group (One way ANOVA followed by Tukey’s test).
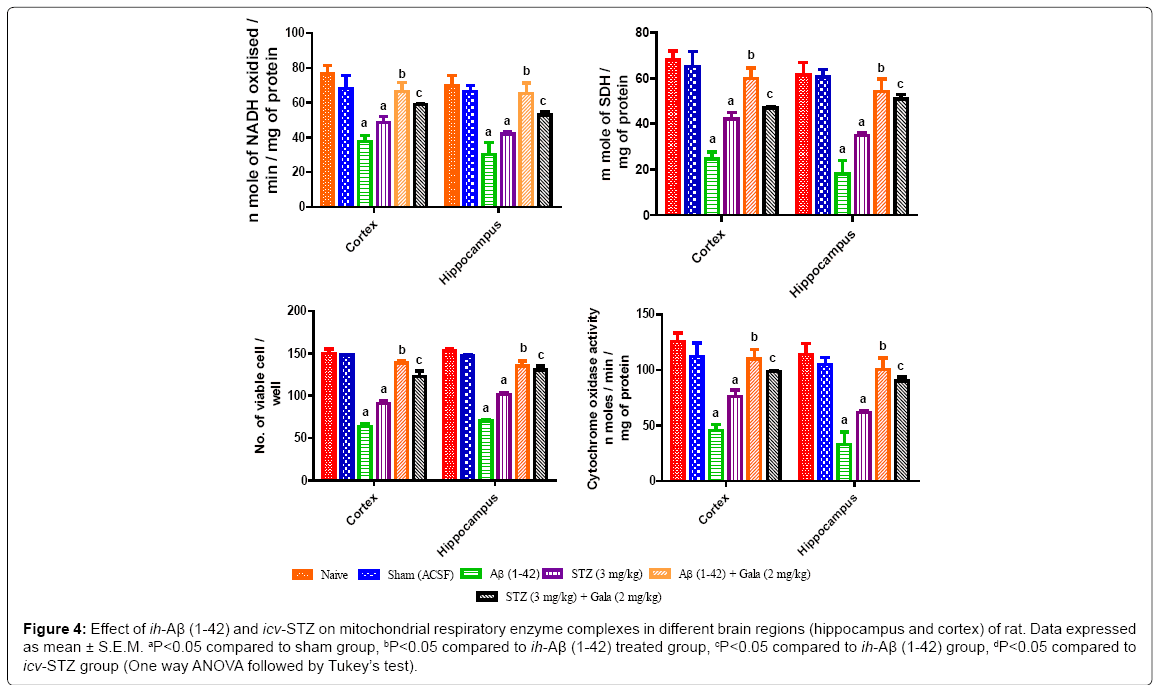
Effect of ih-Aβ (1-42) and icv-STZ on mitochondrial enzyme complexes in rats
Sham treated animals did not show any significant alteration on mitochondrial respiratory enzymes complex (I to IV). Administration of ih-Aβ (1-42) and icv-STZ significantly impaired mitochondrial enzyme complexes (NADH dehydrogenase, succinate dehydrogenase, cytochrome oxidase and cell viabilities activities) in both hippocampus and cortex as compared to sham operated rats. Further, chronic treatment of galantamine (2 mg/kg) significantly restored mitochondrial enzymes complex activities and increased cell viability as compared to their respective control groups (P<0.05) (Figure 4).
Figure 4: Effect of ih-Aβ (1-42) and icv-STZ on mitochondrial respiratory enzyme complexes in different brain regions (hippocampus and cortex) of rat. Data expressed as mean ± S.E.M. aP<0.05 compared to sham group, bP<0.05 compared to ih-Aβ (1-42) treated group, cP<0.05 compared to ih-Aβ (1-42) group, dP<0.05 compared to icv-STZ group (One way ANOVA followed by Tukey’s test).
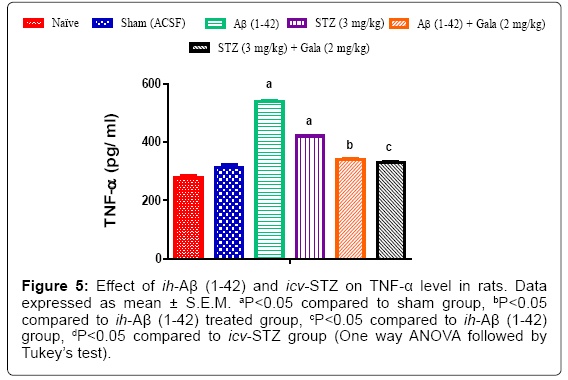
Effect of ih-Aβ (1-42) and icv-STZ on inflammatory marker (TNF-α) in rats
Sham treated animals had not shown any significant alteration in inflammatory markers as compared to naïve group. However, administration of ih-Aβ (1-42) and icv-STZ significantly increased TNF-α levels as compared to sham operated rats. Further, chronic treatment with galantamine (2 mg/kg) significantly attenuated increased TNF- α levels as compared to control group (P<0.05) (Figure 5).
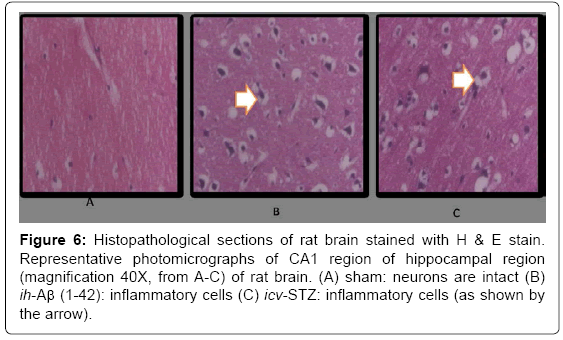
Histopathological examination
In the histopathological study, sham-treatment had not shown any damaged neuronal cells as compared to naïve group. Histopathological alterations were seen in hippocampal regions in ih-Aβ (1-42) and icv- STZ treated animals which was significant as compared to sham group. Further, the chronic galantamine (2 mg/kg) administration significantly protected the brain cells from the neuronal damage as compared to their respective control groups (P<0.05) (Figure 6).
Figure 6: Histopathological sections of rat brain stained with H & E stain. Representative photomicrographs of CA1 region of hippocampal region (magnification 40X, from A-C) of rat brain. (A) sham: neurons are intact (B) ih-Aβ (1-42): inflammatory cells (C) icv-STZ: inflammatory cells (as shown by the arrow).
Discussion
As we know that AD is a neurological disorder that involves progressive deterioration of learning and memory [25]. It has been well characterized by two major hallmarks intracellular neurofibrillary tangles (NFTs) and extracellular β-amyloid plaques [26]. Evidences have shown that Aβ peptides represents a crucial role in the pathogenesis of AD [4,25]. In AD, there is an imbalance between the production and clearance of Aβ peptides that results into its aggregation and finally form Aβ (1-42) oligomers. Aβ plaque is the major pathological hallmark in AD and direct injection or continuous infusion of Aβ into the brain causes brain dysfunction, neurodegeneration and learning and memory impairment which produces very much similar characteristic of AD pathogenesis. It is considered as a good reliable model of AD as it mimics the various pathophysiological aspects of human AD [27]. It is evidenced that these toxic Aβ (1-42) oligomers results in oxidative stress, promote tau hyperphosphorylation, mitochondrial dysfunction, results in toxic effects on synapses and microglial activation which further results in the activation of inflammatory cascade (release of proinflammatory cytokines, including IL-1β, TNF-α, and IFN-γ) [25, 28]. On the other hand, icv-STZ animal model has been considered as an appropriate model for sporadic dementia of Alzheimer’s type. The model is characterized by progressive deterioration of memory, cerebral energy metabolism and oxidative stress [1,2,26]. This impaired energy system and reduced acetyl CoA synthesis leads to the defects in cholinergic transmission.
The purpose of the present study was to compare the extent of neurodegeneration occurring in two different, well practiced, animal models of AD with the help of various behavioral, biochemical, cellular, inflammatory and histopathological parameters.
In the present study, sham treated animals quickly learned to swim in the MWM test during the acquisition phase. However, ih-Aβ (1-42) and icv-STZ administration significantly impaired cognitive performance (as evidenced by prolonged ELT and reduced TSTQ) as compared to sham treatment. However, extent of memory impairment was significantly higher as compared to icv-STZ groups, suggesting that its reliability in spatial memory assessment.
The present study showed that both ih-Aβ (1-42) and icv-STZ treatment raised AChEs activity further supporting an impairment of cholinergic system in AD. This further indicates that both models comparably raised AChEs activity as compared to sham treatment. Further, both icv STZ and ih-Aβ (1-42) administration causes significant increase in lipid peroxidation, nitrite concentration, depleted reduced glutathione, super oxide dismutase and catalase activities suggesting the occurrence of oxidative stress as compared to sham group. However, ih- Aβ (1-42) treated groups produced more oxidative damage as compared to icv-STZ.
Mitochondrial dysfunction has been well documented in the pathophysiology of AD [29,30]. Similarly, Previous reports have shown a strong link between inflammation mediated by proinflammatory cytokines and oxidative stress in AD [4,25,31]. Oxidative stress is supposed to be the major factor that shifts the cell signaling pathway towards neuroinflammation. [25,31]. Recent studies provided evidence that in–vitro activation of microglia from Alzheimer’s patient’s results in release of proinflammatory cytokines, including IL-1β, IL-6 and TNF-α [4,25,31,32]. Further, in the present study, both ih-Aβ (1-42) and icv-STZ administration significantly altered mitochondrial respiratory enzyme complex activity, neuroinflammation and histopathological alteration as compared to sham group. However, ih-Aβ (1-42) treatment caused more alteration of mitochondrial enzyme complex (I to IV) activity, neuroinflammation (TNF-α) and neuronal damage in histopathological examination as compared to icv-STZ treatment suggesting its reliability in AD studies. Further, icv-STZ treatment also produced these changes which are non-selective in nature. Hence, cannot rule out the involvement of other cellular and pathological mechanisms in causing cognitive impairment.
In a nutshell, the present study highlighted the importance of both ih-Aβ (1-42) as well as icv-STZ model of AD. The present study suggests that both models represent several histopathological features of AD. However, ih-Aβ (1-42) model is more reliable and a specific model for AD in terms of cognitive dysfunction, oxidative damage, neuroinflammation, mitochondrial dysfunction and histopathological alteration as compared to icv-STZ model of AD.
Acknowledgements
Authors are thankful to the financial support of UGC funded Rajiv Gandhi National Fellowship (RGNF) with grant no. F1-17.1/2012-13/RGNF-2012-13- SC-HAR-19724, New Delhi and University Institute of Pharmaceutical Sciences, Panjab University, Chandigarh for providing infrastructure facilities for carrying out this work.
References
- Alzheimer's Association (2014) 2014 Alzheimer's disease facts and figures. Alzheimers Dement 10: e47-92.
- Braak H, Del Tredici K (2012) Alzheimer's disease: pathogenesis and prevention. Alzheimers Dement 8: 227-233.
- Zhong SZ, Ge QH, Li Q, Qu R, Ma SP (2009) PeoniflorinattentuatesAbeta((1-42))-mediated neurotoxicity by regulating calcium homeostasis and ameliorating oxidative stress in hippocampus of rats. J NeurolSci 280: 71-78.
- Prakash A, Kumar A (2014) Implicating the role of lycopene in restoration of mitochondrial enzymes and BDNF levels in β-amyloid induced Alzheimer׳s disease. Eur J Pharmacol 741: 104-111.
- Christensen R, Marcussen AB, Wörtwein G, Knudsen GM, Aznar S (2008) Abeta(1-42) injection causes memory impairment, lowered cortical and serum BDNF levels, and decreased hippocampal 5-HT(2A) levels. ExpNeurol 210: 164-171.
- Prince M, Albanese E, Guerchet M (2014) World Alzheimer Report 2014
- Ishrat T, Khan MB, Hoda MN, Yousuf S, Ahmad M, et al. (2006) Coenzyme Q10 modulates cognitive impairment against intracerebroventricular injection of streptozotocin in rats. Behav Brain Res 171: 9-16.
- Kumar A, Prakash A, Pahwa D (2011) Galantamine potentiates the protective effect of rofecoxib and caffeic acid against intrahippocampalKainic acid-induced cognitive dysfunction in rat. Brain Res Bull 85: 158-168.
- Paxinos G, Watson C (2006) the rat brain in stereotaxic coordinates 2nd (Edn). Academic Press, New York.
- Kumar P, Padi SS, Naidu PS, Kumar A (2007) Possibleneuroprotective mechanisms of curcumin in attenuating 3-nitropropionic acid-induced neurotoxicity. Methods Find ExpClinPharmacol 29: 19-25.
- Mueller SC, Temple V, Oh E, VanRyzin C, Williams A, et al. (2008) Early androgen exposure modulates spatial cognition in congenital adrenal hyperplasia (CAH). Psychoneuroendocrinology 33: 973-980.
- Wills ED (1966) Mechanisms of lipid peroxide formation in animal tissues. Biochem J 99: 667-676.
- ELLMAN GL (1959) Tissue sulfhydryl groups. Arch BiochemBiophys 82: 70-77.
- Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, et al. (1982) Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem 126: 131-138.
- Kono Y (1978) Generation of superoxide radical during autoxidation of hydroxylamine and an assay for superoxide dismutase. Arch BiochemBiophys 186: 189-195.
- Luck H (1971) Catalase in methods of enzymatic analysis. (Bergmeyer HU Edn) Academic Press, New York. 885-893.
- ELLMAN GL, COURTNEY KD, ANDRES V Jr, FEATHER-STONE RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. BiochemPharmacol 7: 88-95.
- GORNALL AG, BARDAWILL CJ, DAVID MM (1949) Determination of serum proteins by means of the biuret reaction. J BiolChem 177: 751-766.
- Berman SB, Hastings TG (1999) Dopamine oxidation alters mitochondrial respiration and induces permeability transition in brain mitochondria. J Neurochem 73:1127-1137.
- King TE, Howard RL (1967) [52] Preparations and properties of soluble NADH dehydrogenases from cardiac muscle. Methods Enzymol 10: 275-294.
- King TE (1967) [58] Preparation of succinate dehydrogenase and reconstitution of succinate oxidase. Methods Enzymol 10: 322-331.
- Liu Y, Peterson DA, Kimura H, Schubert D (1997) Mechanism of cellular 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction. J Neurochem 69: 581-593.
- Sottocasa GL, Kuylenstierna B, Ernster L, Bergstrand A (1967) An electron-transport system associated with the outer membrane of liver mitochondria. A biochemical and morphological study. J Cell Biol 32: 415-438.
- Galpern WR, Cudkowicz ME (2007) Coenzyme Q treatment of neurodegenerative diseases of aging. Mitochondrion 7 Suppl: S146-153.
- Kumar A, Singh A, Ekavali (2015) A review on Alzheimer's disease pathophysiology and its management: an update. Pharmacol Rep 67: 195-203.
- Anand R, Gill KD, Mahdi AA (2014) Therapeutics of Alzheimer's disease: Past, present and future. Neuropharmacology 76 Pt A: 27-50.
- Hampel H, Prvulovic D, Teipel S, Jessen F, Luckhaus C, et al. (2011) The future of Alzheimer's disease: the next 10 years. ProgNeurobiol 95: 718-728.
- Parihar MS, Hemnani T (2004) Alzheimer's disease pathogenesis and therapeutic interventions. J ClinNeurosci 11: 456-467.
- Mehta A, Prabhakar M, Kumar P, Deshmukh R, Sharma PL (2013) Excitotoxicity: bridge to various triggers in neurodegenerative disorders. Eur J Pharmacol 698: 6-18.
- Burchell VS, Gandhi S, Deas E, Wood NW, Abramov AY, et al. (2010) Targeting mitochondrial dysfunction in neurodegenerative disease: Part I. Expert OpinTher Targets 14:369-385.
- Smith JA, Das A, Ray SK, Banik NL (2012) Role of pro-inflammatory cytokines released from microglia in neurodegenerative diseases. Brain Res Bull 87: 10-20.
- Burchell VS, Gandhi S, Deas E, Wood NW, Abramov AY, et al. (2010) Targeting mitochondrial dysfunction in neurodegenerative disease: Part II. Expert OpinTher Targets 14: 497-511.
Relevant Topics
- Advanced Parkinson Treatment
- Advances in Alzheimers Therapy
- Alzheimers Medicine
- Alzheimers Products & Market Analysis
- Alzheimers Symptoms
- Degenerative Disorders
- Diagnostic Alzheimer
- Parkinson
- Parkinsonism Diagnosis
- Parkinsonism Gene Therapy
- Parkinsonism Stages and Treatment
- Stem cell Treatment Parkinson
Recommended Journals
Article Tools
Article Usage
- Total views: 13835
- [From(publication date):
March-2016 - Jul 05, 2025] - Breakdown by view type
- HTML page views : 12596
- PDF downloads : 1239