Research Article Open Access
Compaction Characteristics of B.C Soil through Pore Fluids
Md Khaja Moniuddin1* and Manjularani P21Assistant Professor, Department of Civil Engineering, B.K.I.T, Bhalki, Bidar, Karnataka, India
2Assistant Professor, Don Bosco Institute of Technology, Bangalore, Karnataka, India
- *Corresponding Asuthor:
- Md Khaja Moniuddin
Assistant Professor, Department of Civil Engineering
B.K.I.T, Bhalki, Bidar
Karnataka, India
Tel: 084842-62288
E-mail:mdkhajamoniuddin@gmail.com
Received date: October 02, 2015; Accepted date: December 29, 2015; Published date: January 01, 2016
Citation: Moniuddin K, Manjularani P (2015) Compaction Characteristics of B.C Soil through Pore Fluids. J Archit Eng Tech 5:156. doi:10.4172/2168-9717.1000156
Copyright: © 2015 Moniuddin K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Architectural Engineering Technology
Abstract
The industrial structure and their foundations are exposed to hazardous environments and hence behavior should be given a due consideration in the design and execution. Similarly are employed in design of the landfills as impermeable membranes due to their low permeability. Clay liners eliminate or limit the movement of leachate from the landfills. The landfills liners are exposed to various chemical, biological and physical events, due to movement of leachate through them. The effects of pollutants on clays are complex due primarily to exchange or nature of pore fluid. There are number of mechanisms through which individual contaminants affect the engineering properties. Including chemical reactions such as dissolution or precipitation and physic-chemical phenomena, affecting intermolecular forces of water solutions. The compressive effective stress in liners, when applied during permeation may play a key role in controlling the chemically induced changes in hydraulic conductivity and compressibility.
Keywords
Black cotton soils; Compaction; Montmorillonite; Maximum dry density; Optimum moisture content
Introduction
Contaminant migration from landfills or contaminated sites depends on different site specific conditions like-geology and hydrology of the site, climate, type of waste material, type of contamination and type of liner system if any. There are several of mechanisms through which individual contaminants affect the engineering properties, including chemical reaction such as dissolutions or precipitation and physico-chemical phenomena affecting intermolecular forces in water solutions. The compressive effective stress in liners, when applied during permeation may play a key role in controlling the chemically induced changes in hydraulic conductivity and compressibility. The role of mechanical and chemical effects in controlling the engineering properties of clay is of great importance. Expansive soils are commonly found in arid and semi aired regions. In India, about 20% of the soil cover is comprises of expansive soils also commonly known as black cotton soil. Principally such soils contain montmorillonite as main clay mineral and they exhibit high swelling and shrinkage with the seasonal moisture fluctuations. The B.C soils are also varying in their clay content and activity from region to region.
Currently the influence of organic and inorganic contaminates on the properties of black cotton soils is under focus. In this paper the behavior of black cotton soils, essentially containing montmorillonite as principal clay mineral and having clay fraction in different proportions is investigated, with specific reference to its compaction in the presence of inorganic chemical fluids as pore fluids. The effects of various chemicals on the Index and compressibility characteristics are reported in the literature review. The object of proposed investigation is to conduct a systematic investigation on the compaction characteristics of black cotton soils with the pore solutions having chloride anions.
Literature Review
Literature is reviewed with reference to the effect of pore fluids on the geotechnical properties of the soils. The published research on the effect of contaminants on the shear strength followed by the work on compression characteristics is presented.
Physico-chemical interaction between clays and contaminants depends upon Structure of clay minerals.
1. Clay and pore fluid interaction.
2. Surface reaction and surface charge
3. Diffuse ion layer.
4. Ion exchange.
5. Interaction between clay particles.
The physic-chemical interaction taking place at Microscopic level is exhibited macroscopically and can be quantified by simple liquid limit test. The variation in the Liquid limit and plastic limit of black cotton soils with the chemical solutions having chloride, carbonate and sulphate and hydroxide has been investigated and reported by Mutalikdesai [1]. According to him the liquid limit and plastic limit values reduce in the presence of pore fluids having chlorides as anion, irrespective of cation present in the solution. Compression index varies linearly with the liquid limit for all of the pore fluids and soil under consideration. Scope of this work is in understanding the macroscopic behavior of clays in the presence of electrolyte solutions.
Sridharan and Venkatappa Rao [2] have studied the effect of pore fluid on the liquid limit of clays. Using a cone penetrometer liquid limit was measured for kaolinite and montmorillonite is various organic solvents. Since the unit weights of the fluids used differ from one another the liquid limits were calculated on volume basis to facilitate comparison. The results were plotted against dielectric constants of the pore fluids show that the Kaolinite and montmorillonite behaved in a strikingly opposite manner with respect to the change in the pore fluid. Whereas a decrease in the liquid limit was observed for kaolinite with increase in dielectric constant of pore fluids, and increase in liquid limit was recorded for montmorillonite. In montmorillonite the liquid limit decreased from 866% for water (dielectric constant = 80.4) to 149.2% for hexane (dielectric constant =1.89). But for kailonite the liquid limit decreased from 230% for hexane to 127% for water. Based on these observations the mechanisms controlling the liquid limit behavior of clays are explained.
It was observed that through the liquid limit is a measure of shearing resistance, with the use of organic pore fluids and water of different dielectric constants, it was shown that the liquid limit of the clay was primarily controlled by the shearing resistance at the particulate level and the thickness of the diffuse double layer.
An increase in dielectric constant decrease antiparticle shearing resistance and increase the double layer thickness. A decrease in the shearing resistance results in lower liquid limit where as an increase in the double layer thickness shows higher liquid limit. These effects obviously oppose each other and the liquid limit of the particular clay will depend on which of the two predominates. For kaolinite, a non – expanding lattice type of clay, the contribution due to diffuse double layer is significant and the liquid limit is primarily governed by shearing resistance at particulate level. Hence an increase in dielectric constant results in lower liquid limits.
According to Nelson and Miller [3] the macro scale soil properties reflect the micro scale nature of the soil and they are more conveniently measured in engineering works than micro scale factors, macro scale factors are primary indicators of soil behavior. Commonly determined property such as plasticity can provide a great deal of insight in to the soil behavior. Soil consistency as quantified by Atterberg limits is the most widely used indicator. Most expansive soils can exist in plastic condition over a wide range of moisture contents. This behavior results from the capacity of clay minerals to contain large amount of water between particles and yet retain coherent structure through the inter particle electrical forces. The soil plasticity is influenced by the same micro scale factors that control swell potential and provides a useful indicator of swell potential.
Although for montmorillonite the liquid limit should be governed by shearing resistance, because it is expanding lattice type of clay, the contribution of diffuse double layer overrides and governs the liquid limit. Hence, an increase in the dielectric constant results in higher liquid limit. The effect of increasing salt concentration (inorganic solutions) on the liquid limit of clays is reported by Schmitz and Passen [4]. Depending on their mineralogy, clays show considerable change in their properties when they are exposed to salt solutions. Four different clays were exposed to various concentrations of three salt solutions. The salts used were NaCl, KC1 and CaCl2. It was observed that there was a significant decrease in liquid limit up to the concentration of 0.1M (molarities) of salt. Further increase in the concentration does not cause significant changes in liquid limits. The decrease followed the second order exponential decay function. An empirical formulation describing the decay in the value of the liquid limit, as a function of clay fraction was proposed. Effect of inorganic salt solutions on the consistency limits of two clays is reported by Arasan and Yetimoglu [5]. The effect of four salt solutions (i.e. ammonium chloride (NH4Cl), Potassiums chloride (KCl), Copper sulpate (CuSO4) and Iron sulphate (FeSO4) as leachate compounds on the consistency limits of two commercial clays : one having low plasticity (CL) and another having high plasticity (CH) is reported. Tests were performed using both distilled water and salt solutions at eight different concentrations varying between 0.0001 and 1M. It was observed that for CL clay, the liquid limit and plastic limit increased with increase in the salt concentration up to 0.2 M beyond which the clays behaved as non-plastic soil. All the salt solutions with concentration up to 0.2 M significantly reduced the liquid limit of CH clays. For NH4Cl and KCI the liquid limit of CH clay remained constant beyond 0.2 M, while for FeSO4 and CuSO4 the liquid limit decreased at low concentrations but increased with increase in concentration between 0.001 and 0.2 M. Beyond 0.2 M the plastic limit values approached those of the raw clay sample. For the conditions investigated. CL and CH Class clays were transformed into ML and MH class soils respectively according to the Unified Soil Classification System (USCS).
The effect of different commonly available electrolytes consisting of various cations and anions on index properties of two naturally occurring soils containing kaolinite or montmorillonite as principal clay mineral was reposted by Sivapullaiah [6]. It is reported that the type of anion on electrolyte plays of more dominant role than that of cation. At the same electrolyte concentrations hydroxides give higher liquid limit than chlorides. Hydroxides increase shrinkage limit of soils. Good relationship exists between liquid limit and free swell index of soils.
The effect of illite – bentonite mixture on their liquid limit with water also in electrolyte solutions is reported by Sivapullaih and Savitha [7]. It is observed that the liquid limit of the mixture increases with percent bentonite but is generally lower than that calculated from noninteracting situation. With 0.5N Nacl and KCl solutions the liquid limit of the mixtures decreases with increase in percent bentonite. At high concentrations the liquid limit decreases with increase in bentonite content. The plastic limit illite-bentonite mixtures continuously decrease with increase in bentonite content. The variation of plasticity index of illite bentonite mixtures are similar to those observed for liquid limit. Shrinkage limit of illite bentonite mixtures decreases continuously with increase in percent bentonite. It was concluded that the effects of electrolytes on index properties are much more on bentonite and on mixtures containing higher amount of bentonite than on illite and on mixtures containing higher amounts of illite.
These studies were useful to understand some aspects of expansive behavior However; they do not provide a conceptual frame work to interpret engineering behavior. This is because different soils having the same Atterberg limits can exhibit different volume change behavior and shear strength characteristics as many parameters such as soil structure, chemical composition and the minerals present in soil can influence their behavior.
Effect of Pollutants on Geotechnical Properties
The effect of isolated pollutants on index properties, volume change behavior, shear strength properties and permeability of different types of clayey soils with different groups of pollutants are critically reviewed by Sivapullaiah [7]. The Factors that influence the behavior of polluted soils are type and amount of clay present in soils and the type of pollutant. In clays and other fine soils, the presence of surface forces and molecular force must be accounted for as they govern clay behavior. The physical properties of the soil are not constant but may change as a result of external and internal factors. The macro behavior of the clays is mainly governed by forces acting between the clay particles in addition to those caused by external loads. This depends on the mineralogical properties of the clay and physicochemical interactions of the clay-electrolyte system. Different clay minerals exhibit wide range of properties. In any particular soil the different constituents of the clay may not influence properties in direct or even predictable proportions to the quantity present. This is because of structural difference of clay mineral.
Thus the pollution effects on geotechnical properties depend on type and amount of chemical present in them. The effects are transmitted through changes in ion distribution near clay surface and subsequent changes in soil structure. Diffuse double layer theory can be used to explain changes in soil properties in different environments.
In the literature limited numbers of studies are reported on the effect of solutions comprising the compounds of chloride on the compaction. Most of the research is focused on the investigation on Index properties and consistency limits of higher activity clays.
Methodology
Selection of soil
Expansive soils are commonly found in many arid and semi-arid regions in the world such as Australia, Canada, China, India, Israel, South Africa and the United states. These soils typically exhibit moderate to high plasticity, low to moderate strength and high swell and shrink characteristics (Holtz, and Gibbs, 1956). In India, such soils cover about one fifth (20%) of the area and are generally known as 'Black cotton soils'. They exhibit high swelling and shrinkage characteristics due to seasonal moisture fluctuations.
They contain montmorillonite as the principal clay mineral. The black cotton soils found in different regions show a wide range in their clay contents and hence the activity. Their liquid limit is found to vary from 50% to 120% in different regions of Deccan plateau. Their behavior, especially in the presence of different contaminant is not thoroughly investigated. Therefore the present investigation focuses on the behaviors of black cotton soils when they come in contact with different contaminants. Black cotton soil are collected from different regions having liquid limits of 60%. The geotechnical properties of these soils are presented in Table 1.
Selection of contaminant
One set of contamination will be characterized by a shift in major ion chemistry and the initial monitoring parameters should include only major ions (Ca, Na, Cl2, and So4) and those parameters which geotechnical testing has shown to be present in the seepage solution.
The major constituents in most of the municipal solid waste (MSW) landfill leachates are the salts of Sodium, Potassium, Magnesium and calcium and are found to have low toxicity. To investigate the influence of type and concentration of each of these major constituents, isolated solutions of individual salts of known concentrations are adopted. The cations presents in the solutions include Sodium, Potassium, Magnesium and Calcium and most common anions present are the chlorides, carbonates, sulphates and Hydroxides. Thus the chosen four salt solutions consist of Sodium Chloride (NaCl), Calcium Chloride (CaCl2), Sodium sulphate (NaSo4), Calcium Sulphate (CaSo4). The chemicals were purchased from a single manufacturer, for uniformity and purity.
The solutions were prepared at room temperature by filling a 1000 ml volumetric flask with pure distilled water and adding a known mass of the salt. Corrections were made in the mass of the salts for the divalent cations to convert to equivalent monovalent concentrations. The flask was sealed and repeatedly inverted 3-5 minutes to ensure uniform mixing of the solution. The mixture was allowed to stand for at least 6 hours, occasionally inverting the flask so that uniform distribution of the solute is ensured. Potassium and Sodium have lower replace ability relative to heavy metals when contaminant transport occurs through the soils with montmorillonite, illite and kaolinite clay minerals.
Experimental Program
Determination of moisture content and dry density
1. B.C. soil with liquid limit 60% are chosen to cover wide range liquid limit.
2. Salts are used each of chloride compounds and sulphate compounds (NaCl, CaCl2 NaSo4 and CaSo4 ) with two Concentrations of 0.1N and 1.0N were dissolved in deionized water and mixed with B.C soil with liquid limit 60%.
3. The B.C soil with liquid limit 60% were soaked with four pore fluids of NaCl, CaCl2, NaSo4 and CaSo4 with two concentrations of 0.1N and 1.0N separately and kept in desiccators allowing the pore fluids to interact with the soil.
4. The modified proctor compaction test was carried out to determine the optimum moisture content and dry density according to ASTM (D 1557) for immediate day, first day, second day, third day and fifth day of soaking soils.
Analysis of test results: Experimental results have clearly demonstrated that the contaminants influence on compaction strength significantly. The relation between OMC with the soaking period and dry density with the soaking period for four Chloride compounds (NaC1, CaC12 NaSo4 and CaSo4,) with two concentrations of 0.1N and1.0N are plotted in Figures 1-8. The addition of salts to the soil increased the dry density and the optimum moisture content decreases. Similar results were reported by Wood [8] and Frydam [9]. They attributed this behavior to the fact that at low moisture content the soil structure (before compaction) tends to change from edge-to-face type of flocculation to face-to-face flocculation (salt flocculation) with the increase in salt concentration. Consequently under the influence of dynamic compaction, the clay particles become more oriented and the compacted dry unit weight increases with the increase in salt content. The decrease in the optimum moisture content as the salt content increased may be explained due to the higher the face-to-face flocculation the lower is the amount of water required for lubrication.
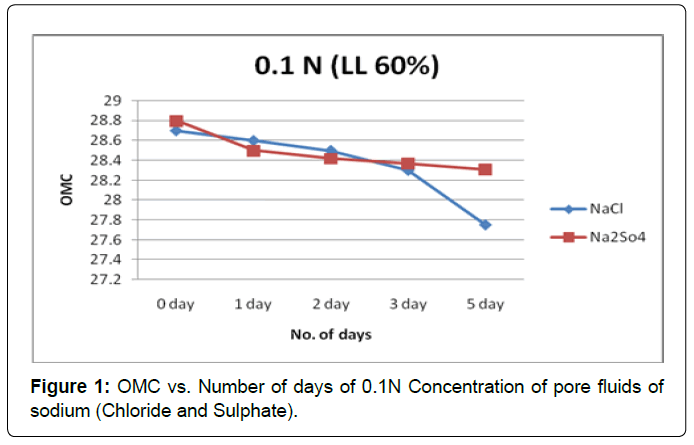
Figure 1 shows the plot of OMC with the soaking period for soil type-A with pore fluids of sodium chloride and sodium sulphate solutions of concentration 0.1N. It is observed that OMC decreases marginally in initial soaking period and in later soaking period i.e. after third day OMC decreases substantially in pore fluids of sodium chloride.
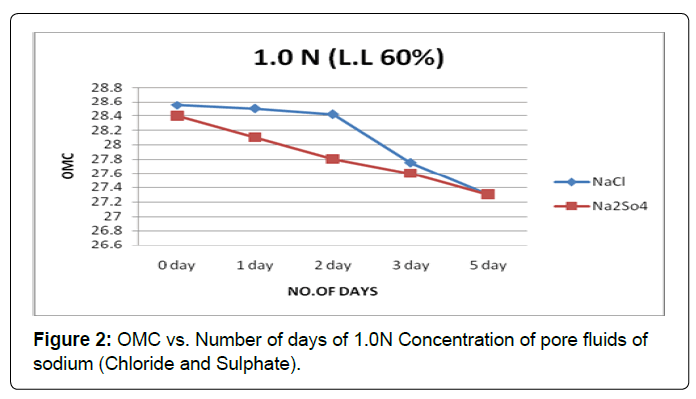
Figure 2 shows the plot of OMC with the soaking period for soil type-A with pore fluids of sodium chloride and sodium sulphate solutions of concentration 1.0N. It is observed that OMC decreases substantially in pore fluid of sodium sulphate, but in pore fluid of sodium chloride OMC decreases marginally in initial soaking period and then substantially in later soaking period.
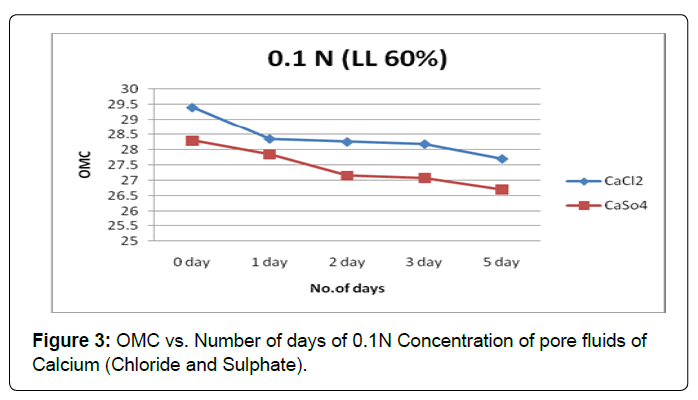
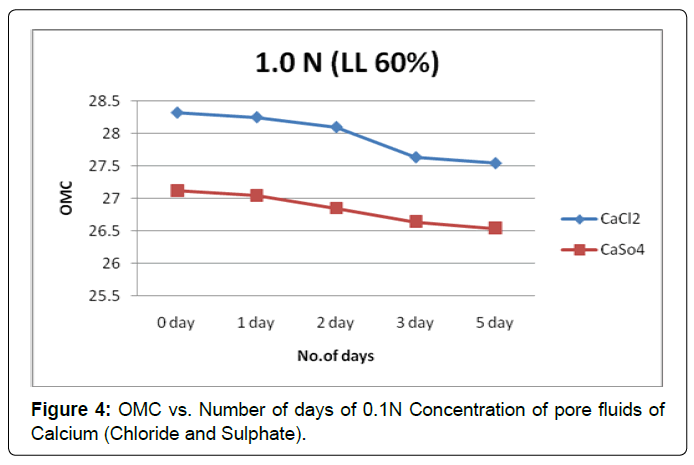
Figures 3 and 4 shows the plot of OMC verses the soaking period for soil type-A with pore fluids of chloride and sulphate of calcium solutions of both concentration 0.1N and1.0N. The increase in the contact period the OMC shows a gradual decreasing trend in both pore fluid of chloride and sulphate of calcium solutions of both concentrations.
Here sulphate anions are more influenced than the chloride anions to reduce clay activity in lower concentration of both pore fluids.
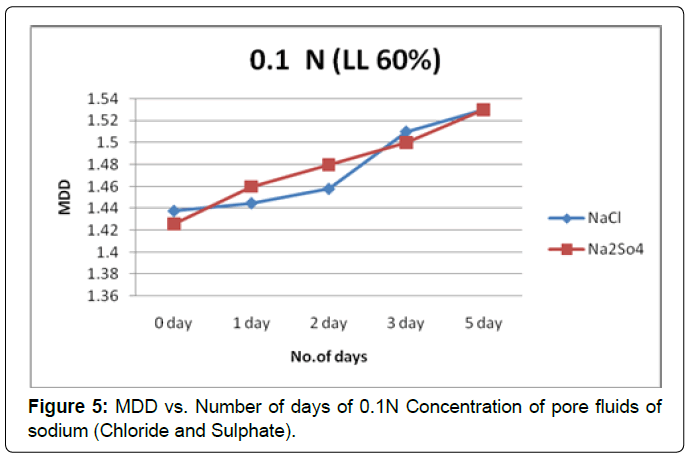
Figure 5 shows the plot of MDD verses the soaking period for soil type-A with pore fluids of chloride and sulphate of sodium solutions of concentration 0.1N. It is observed that MDD increase is substantially in both pore fluids; however the effect of monovalent and divalent cation i.e. sodium influence is same in lower concentration pore fluid.
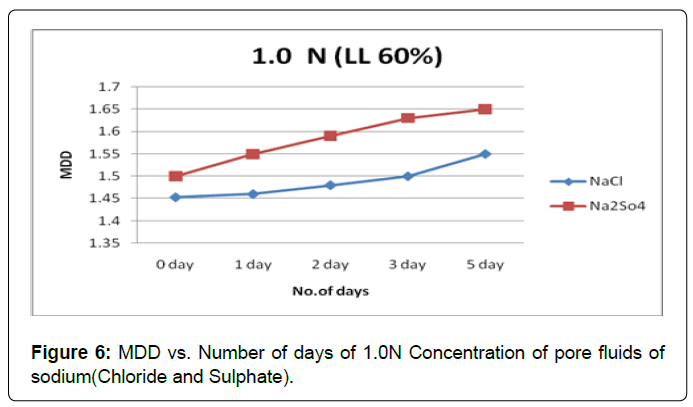
Figure 6 shows the plot of MDD verses the soaking period for soil type-A with pore fluids of chloride and sulphate of sodium solutions of concentration 1.0N. It is observed that MDD increases in both pore fluids of concentration 1.0N However the effect of pore fluid of sodium sulphate solution are more compared to the pore fluid of sodium chloride solution in increases MDD.
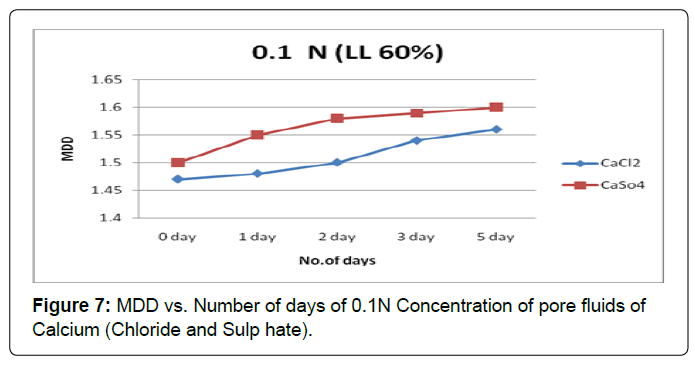
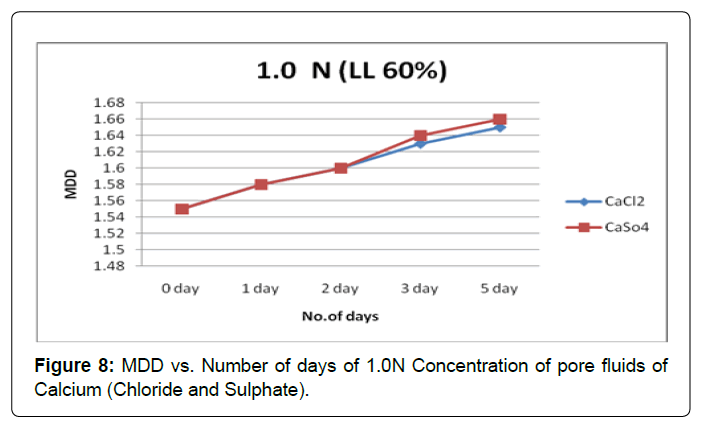
Figures 7 and 8 shows the variation in MDD with the soaking period for soil type-A with pore fluids of chloride and sulphate of Calcium solutions of concentrations 0.1N and1.0N. It is observed that MDD increase is substantially in both pore fluids of concentrations 0.1N and1.0N. However the effect of monovalent cation i.e. calcium influence is same in higher concentration pore fluids.
Conclusions
Earlier studies focused mainly on the study of Index properties such as liquid limit, plastic limit etc of higher activity clays when exposes to various contaminants, However very limited study focused on engineering properties like compaction characteristics has been published in literature. Hence in the present study extensive experimental work was done to understand compaction characteristics of B.C soil when subjected to different contaminants having different concentrations.
From the present study following conclusions can be drawn.
1. The OMC and MDD of black cotton soils having liquid limit of 60% irrespective of their clay fractions, are sensitive to the changes in the cations and anions present the pore fluids.
2. The OMC values reduced and MDD values increased exponentially for black cotton soils having liquid limit 60% the addition of pore fluids of NaCl, CaCl2 NaSo4 and CaSo4 with both concentration, decreases in OMC is more for lower concentration. For higher concentration of the pore fluids decreases in OMC is steady over increased period of the contact with the soils.
3. In black cotton soil having liquid limit 60% the addition of pore fluids of NaCl, CaCl2 NaSo4 and CaSo4, causes increases the MDD in low concentration. However at higher concentration of pore fluid CaCl2 MDD increases higher compare to than the other pore fluids.
4. Decrease in OMC and increase in MDD is more effective in sulphate anions compare to the chloride anions in both concentration of pore fluids.
References
- Mutalik Desai VG, Desai V, Rao DH(2009) Prediction of Compression Index using Artificial Neural Networks.
- Sridharan A,VenkatappaRao (1975)Mechanisms controlling liquid limit behaviour of clays. Proceedings ofIstambulconf on soil mechanics and Foundation Enggl: 75-84.
- Nelson JD, Miller DJ (1992)Expansive Soils: Problems and Practice inFoundation and Pavement Engineering.
- Schmitz RM, Van Passen LA (2003)Thedecay of liquid limit of clays with increasing salt concentration.IngeokringNewsletter 9: 10-14.
- Arasan S,Yetimoglu T (2008) Effect ofinorganic salt solutions on the consistency limits of two clays. TurkishJournal of Eng. Env Science 32: 107-115.
- Sivapullaiah PV,Sridharan A,VijayaBhaskarRajuK (1996) Index Properties of Soils Contaminated by Electrolytes.Proc of the 3rdInternational Symposium on Environmental Geotechnology 1: 198-207.
- Sivapullaiah PV,Savitha S (200) Indexproperties of Illite - Bentonite mixtures in electrolyte solutions. TechnicalNote, Geotechnical Testing Journal 22: 257-265.
- Wood KB (1971) Highway Engineering Hand Book. McGrawHill Book Company, New York, USA.
- Frydman IR,Ehrenreich T (1977)Stabilization of heavy clay with Potassium chloride. Journal of Geotechnical Engineering 8: 95-107.
Relevant Topics
- Architect
- Architectural Drawing
- Architectural Engineering
- Building design
- Building Information Modeling (BIM)
- Concrete
- Construction
- Construction Engineering
- Construction Estimating Software
- Engineering Drawing
- Fabric Formwork
- Interior Design
- Interior Designing
- Landscape Architecture
- Smart Buildings
- Sociology of Architecture
- Structural Analysis
- Sustainable Design
- Urban Design
- Urban Planner
Recommended Journals
Article Tools
Article Usage
- Total views: 14043
- [From(publication date):
March-2016 - Apr 21, 2025] - Breakdown by view type
- HTML page views : 13078
- PDF downloads : 965