Commentary Open Access
Community Cardiovascular Screening to Identify Middle School Children at Risk of Sudden Cardiac Death: The Houston Early Age Risk Testing and Screening (HEARTS) Study
John P Higgins1*, Susan T Laing1, Alejandra Rodriguez1, Asif Ali1, Monica B Patel1, Troy D Tuttle1, Ashley N Callaghan1, Ijeoma E Ananaba1, Joshua A Samuels1, Ketan N Patel1, Gurur Biliciler-Denktas2, J Jason3, Khashayar K Vahdat4, Satish J Bankuru4, David M Filsoof4, William J McKee4, Nan Li4, Robert A McGuffey4, Joshua D Stokes5, Evelyn Henry RN5, Benjamin Yang5, David D McPherson5 and Amol Rajmane6
1Department of Cardiology, The University of Texas Health Science Center at Houston and Renal Diseases and Hypertension, Texas, USA
2Department of Internal Medicine, and the Division of Pediatric Cardiology, Department of Pediatrics, Texas, USA
3The University of Texas at Houston Medical School, Houston, TX; Bend Memorial Clinic, Bend, Texas, USA
4The University of Texas Medical School at Houston, Houston, Texas, USA
5The Clinton School of Public Service, Little Rock, AK, Texas, USA
6Memorial Hermann-Texas Medical Center, Houston, TX and Health and Medical Services-Houston Independent School District, Houston, Texas, USA
- *Corresponding Author:
- John P Higgins
The University of Texas Health Science Center at Houston (UTHealth)
Director of Exercise Physiology
Memorial Hermann Ironman Sports Medicine Institute Chief of Cardiology
Lyndon B. Johnson General Hospital, Harris Health System, 5656 Kelley St
UT Annex 104, Houston, Texas, 77026, USA
Tel: 713-500-6549
Fax: 713-500-6556
E-mail: John.P.Higgins@uth.tmc.edu
Received Date: February 23, 2015 Accepted Date: June 08, 2015 Published Date: June 13, 2015
Citation: Berman H (2015) Community Cardiovascular Screening to Identify Middle School Children at Risk of Sudden Cardiac Death: The Houston Early Age Risk Testing and Screening (HEARTS) Study. J Child Adolesc Behav 3:217. doi:10.4172/2375-4494.1000217
Copyright: © 2015 Pedersen M et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Child and Adolescent Behavior
Abstract
Purpose: Sudden cardiac arrest in school children is often associated with an underlying cardiac abnormality and can be triggered by athletic activity. We tested the feasibility and efficacy of an onsite four-point 15-minute preparticipation cardiac screening of school children to improve detection of cardiac abnormalities. Methods: Two hundred and fifty four sixth-grade students were screened at their schools for cardiac abnormalities by onsite focused history, focal cardiovascular physical exam, 12-lead electrocardiogram, and limited echocardiogram. Results: Subjects were primarily African American and Hispanic; 54.7% were girls. We identified 103 (40.6%) subjects with abnormalities on history and physical exam, 50 (19.7%) with hypertension, 80 (31.5%) with electrocardiographic abnormalities, and 32 (13.0%) with echocardiographic abnormalities. Based on these findings, 25 subjects (9.8%) were advised not to participate in rigorous exercise pending further evaluation. The ability to detect abnormalities increased 36.0% with addition of electrocardiograms and 40.0% with addition of echocardiograms. Conclusion: Our onsite four-point screening system is feasible and effective in detecting undiagnosed cardiac abnormalities and identifying false-positive results. Both athletic and non-athletic children had undiagnosed cardiac abnormalities, which suggests the utility of screening all schoolchildren for cardiac abnormalities.
Keywords
Echocardiography; Electrocardiology; Screening; Sudden cardiac death; Athletes
Introduction
Sudden cardiac death (SCD) in young athletes is unexpected and tragic, and is usually caused by occult cardiovascular disease [1,2]. Numerous structural, electrical, and acquired cardiovascular abnormalities are capable of causing SCD [3]. Identifying cardiac disease at an early age may allow early risk stratification and/or lifestyle modifications, pharmacotherapy, and device therapy to reduce subsequent SCD [3-6]. Such cardiac abnormalities placing an athlete at sports participation risk have been observed by electrocardiogram and/or echocardiogram in up to 5% of athletes screened [2,7-10]. Presently, there are no universally accepted standards for preparticipation cardiovascular screening (PPCS) of young athletes or standardized testing or credentialing for healthcare professionals performing such screenings in the United States [11,12]. PPCS by history and physical examination alone is not sufficient to guarantee detection of many critical cardiovascular abnormalities in young children [13,14].
Adding a 12-lead electrocardiogram (ECG) to the usual history and physical examination has been recommended by large organizations worldwide including the European Society of Cardiology, International Olympic Committee, Israel Ministry of Health, Japanese Ministry of Health, and Fédération Internationale de Football Association (FIFA) [7,15-17].
Interestingly, the National Basketball Association (NBA) requires an annual standardized ECG screening as well as rest and stress echocardiogram for every player prior to training. Those interpreting ECGs should be aware of normal variations in young athletes from physiologic training effects [18-22]. Two-dimensional echocardiography is the principal diagnostic tool for clinical recognition of hypertrophic cardiomyopathy (commonest cause of athlete SCD in United States), as it can reveal otherwise unexplained asymmetric left ventricular wall thickening; [23] it is also the procedure of choice for differentiating physiological hypertrophy from hypertrophic cardiomyopathy [24-26].
Echocardiography can also detect other relevant abnormalities associated with SCD in young athletes, such as valvular heart disease, aortic root dilatation, ventricular dysfunction, atrial septal defects, coronary anomalies, and patent ductus arteriosus [2,27]. Although PPCS has generally been limited to athletes due to reported higher incidence of SCD, the risk of SCD in the non-athletic population may be similar and deserves further evaluation [1,13,28-30].
The purpose of the Houston Early Age Risk Testing and Screening (HEARTS) Study was to test the feasibility and efficacy of onsite cardiovascular screening of 12-year-old athletic and non-athletic school children, determine the sensitivity and specificity of the modalities to detect abnormalities, and to reduce SCD in this population by managing abnormal findings with medicine or surgery and/or proscribing certain activities.
Materials and Methods
Study design and population
Between May 2009 and March 2010, we screened 254 sixth graders from three middle schools in the Greater Houston area, chosen because they were in medically underserved areas. These screenings took place at the school gyms and were conducted by a group of cardiologists, internists, and technologists with portable equipment, as described below. The screening consisted of a medical history, a focused physical exam, 12-lead electrocardiography, and limited 2- dimensional echocardiography. The study was approved by the institutional review board of The University of Texas Health Science Center at Houston. This study meets the ethical standards of the journal [31].
Screening
A single-page, 10-item questionnaire was given to the subjects to take home and was completed by them and their parents prior to the screening, and checked by the study personal on the day of screening. This questionnaire was modified from the Preparticipation Physical Evaluation Form (designed to promote the health and safety of the athlete in training and competition) developed by the American Academy of Family Physicians and Pediatrics, American College and American Osteopathic Academy of Sports Medicine, American Heart Association, American Medical Society and American Osteopathic Society for Sports Medicine [7].
Because most children in sixth grade are not involved competitively in athletics, and to better define which subjects were exercising regularly, we defined “athletes” per the CDC guidelines as those performing at least 1 hour of physical activity per day [32]. This was determined by self-report, and physical activity was defined as a planned, structured movement of the body designed to enhance physical fitness [33].
Focused and standardized physical examinations targeting findings specific to underlying heart conditions known to be associated with SCD in children were performed and documented [5]. This included physical stigmata of Marfan syndrome, auscultation for heart murmurs (supine, standing, with Valsalva maneuver), femoral pulses, and brachial artery blood pressure.
A pathological murmur include a holosystolic or diastolic murmur, grade 3 or higher murmur, harsh quality, an abnormal S2, maximal murmur intensity at the upper left sternal border, a systolic click, or increased intensity when the patient stands [34-36]. Casual Blood Pressure: all of the casual BPs were measured by auscultation using an aneroid sphygmomanometer (Mabis MedicKit 5, Mabis Healthcare, Waukegan, IL). Aneroid calibration and recertification in auscultatory BP measurement technique occurred annually for all of the equipment and personnel. Standardized methods for obtaining casual BP have been described previously [37].
Study personnel measured auscultatory BP 3 times at the screening study visit, aIn this analysis, participants' casual BP obtained within 30 days of ABPM placement were used to classify their ambulatory BP category (see below). The 2010 CDC body-mass-index-for-age charts for children and adolescents were used to categorize subjects weight [39]. A portable ECG machine (GE Mac 5500, GE Healthcare, Milwaukee, WI) was used to perform standard 12-lead ECG scans. Each ECG was evaluated on site by a study cardiologist using the European Society of Cardiology criteria [40].nd the mean was used as the participant's casual BP for the present analysis. Each participant's casual BP was classified according to the National High Blood Pressure Education Program Fourth Report on the diagnosis, evaluation, and treatment of high BP in children and adolescents: [38] normotensive (<90th percentile and < 120/80 mmHg), prehypertensive (≥ 90th and <95th percentiles or <90th percentile and >120/80 mmHg), and hypertensive (≥ 95th percentile).
In this analysis, participants' casual BP obtained within 30 days of ABPM placement were used to classify their ambulatory BP category (see below). The 2010 CDC body-mass-index-for-age charts for children and adolescents were used to categorize subjects weight [39]. A portable ECG machine (GE Mac 5500, GE Healthcare, Milwaukee, WI) was used to perform standard 12-lead ECG scans. Each ECG was evaluated on site by a study cardiologist using the European Society of Cardiology criteria [40].
For example, the presence of T-wave inversion 2 mm in two or more adjacent leads in an athlete is considered abnormal [40]. If a significant ECG finding was noted based on these criteria, further work-up including complete echocardiograms, stress tests, magnetic resonance imaging and consultation with a pediatric cardiologist was advised [40,41]. Screening two-dimensional Doppler echocardiograms were performed on all subjects by experienced sonographers using two portable ultrasound units, an Acuson P50 (Siemens Medical Solutions, Concord, CA) and a GE Vivid I (GE Healthcare). On-site pediatric cardiologists with expertise in echocardiography used a check list of findings so that each echocardiograph (two-dimensional, pulsed Doppler, and color flow mapping) was evaluated in a standardized manner.
The limited echocardiogram is completed in about 7 minutes and includes the following views, designed to capture the more common cardiac abnormalities in children associated with SCD and included parasternal long- and short-axis views; apical two-, four-, and fivechamber views; and suprasternal notch views. The major items checked off by the Cardiac echocardiology physician included: Left and Right ventricular systolic function and chamber size and wall thickness, Left and right atrial size, Interventricular and Interatrial Septum (2D and color doppler), Aortic-Mitral-Pulmonary-Tricuspid valve, Aorta and Pulmonary artery, Aortic root/arch (2D and color doppler), Left and Right coronary ostia (visualized and in correct location). Definitions of significant abnormalities were based on standard echocardiographic diagnostic criteria [42].
For example, the right venticle was defined as dilated if measurement of right ventricular outflow tract dimensions at the proximal/subvalvular level in the parasternal long-axis anterior portion view was>3.3 cm [43]. When a significant abnormality was detected, the subject was advised to have a follow-up complete twodimensional Doppler echocardiogram exam. Each subject with normal findings was given a Clearance Form allowing participation in school athletics for 2 years, based upon the Lausanne Recommendations [44]. If the subject had a major finding detected by history, physical, ECG, or echocardiogram, he or she was referred for additional screening and to the UTHSC-H pediatric cardiology subspecialty clinic for further evaluation.
Statistical analysis
The data were analyzed by S.J.B. All the continuous variables were tested for normal distribution. Depending on the type of distribution, a two-sided t-test, Fischer’s exact test (parametric variables), or Mann- Whitney test (non-parametric variables) was used to assess for differences between the means. Prevalence percentages were used to present the various cardiac abnormalities detected. SAS v 9.1.3 (SAS Version - Cary, NC: SAS Institute, 2003) was used for data management and statistical analyses. A p-value of less than 0.05 was considered significant.
Results
Demographic characteristics
Of the 361 sixth-graders in the three middle schools, 259 subjects agreed to participate in the study, and informed consent was obtained from their parents. Of the 259, one subject was excluded because of paraplegia and four others were absent on the screening day, making a total of 254 participants. Demographic information on the subjects is summarized in Table 1. One hundred thirty nine (54.7%) were female. The study population was predominantly African-American (139, 54.7%), followed by Hispanic (83, 32.7%), non-Hispanic white (27, 10.6%), and Asian (4, 1.6%). The mean age was 11.9 ± 0.7 years. Sixtyone percent of the study participants (154) were classified as athletes. Athletes did not differ significantly from nonathletes by age, ethnicity, or body mass index.
| Characteristics | Male (n=115) | Female (n=139) | Total(N=254) | ||
|---|---|---|---|---|---|
| Athletes (n=72) |
Non-athletes (n=43) |
Athletes (n=82) |
Non-athletes (n=57) |
||
| Mean age (SD) | 11.9 (±0.7) | 11.9 (±0.7) | 11.8 (±0.7) | 11.8 (±0.6) | 11.9 (±0.7) |
| Ethnicity, n (%) | |||||
| African American | 36 (50.0) | 25 (58.1) | 48 (58.5) | 30 (52.6) | 139 (54.7) |
| Hispanic | 22 (30.6) | 16 (37.2) | 22 (26.8) | 23 (40.4) | 83 (32.7) |
| non-Hispanic white | 14 (19.4) | 1 (2.3) | 9 (11.0) | 3 (5.3) | 27 (10.6) |
| Asian | 0 (0.0) | 1 (2.3) | 3 (3.7) | 0 (0.0) | 4 (1.6) |
| Other | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.8) | 1 (0.4) |
| Mean body mass index (SD) | 21.3 (±5.4) | 22.2 (±5.7) | 21.9 (±6.0) | 23.1 (±5.4) | 22.0 (±5.7) |
| Body mass index category, n (%) | |||||
| Underweight | 3 (4.2) | 0 (0.0) | 3 (3.7) | 1 (1.8) | 7 (2.8) |
| Normal weight | 37 (51.4) | 21 (48.8) | 47 (57.3) | 23 (40.4) | 128 (50.4) |
| Overweight | 14 (19.4) | 10 (23.3) | 11 (13.4) | 14 (24.6) | 49 (19.3) |
| Obese | 18 (25.0) | 12 (27.9) | 21 (25.6) | 16 (28.1) | 67 (26.4) |
| Mean hours of exercise per week (SD) | 13.4 (±7.0) | 2.3 (±2.0) | 13.3 (±6.3) | 2.1 (±2.0) | 9.1 (±7.6) |
Table 1: Demographic characteristics of the study population.
History and cardiovascular physical exam
Before the study, 16 (6.3%) of the 254 subjects had been restricted from physical activity or participation in sports on the basis of history (Table 2). Thirty-nine (15.4%) subjects had an ongoing medical condition, 10 (3.9%) subjects complained of having passed out or nearly passed out during or after exercise, and 84 (33.1%) subjects had a family history of heart disease.
| History findings | n (%) | Physical exam findings | n (%) |
|---|---|---|---|
| Restriction from sports before study | 16 (6.3) | Arm to height ratio> 1.05 | 54 (21.3) |
| Ongoing medical condition | 39 (15.4) | Hypertension* | 50 (19.7) |
| Syncope/presyncope during exercise | 10 (3.9) | Heart murmurs | 13 (5.1) |
| Chest pain during exercise | 38 (15.0) | Precordial heave/pulsation | 4 (1.6) |
| Palpitations during exercise | 37 (14.6) | Marfan’s thumb/wrist sign | 3 (1.2) |
| Prior testing for heart disease | 18 (7.1) | Arachnodactyly | 4 (1.6) |
| Sudden death in family | 17 (6.7) | Fixed split second heart sound | 3 (1.2) |
| Heart disease in family | 84 (33.1) | Radio-femoral delay | 1 (0.4) |
* Stage 1 hypertension in 21 (8.3%) subjects, Stage 2 hypertension in 29 (11.4%) subjects
Table 2: History and physical exam findings.
The cardiovascular physical exams revealed 103 (40.6%) subjects with abnormal physical findings, the commonest a positive Marfan’s arm span (54, 21.3%), abnormal blood pressure (50, 19.7%), and heart murmurs (Table 2). We restricted for further evaluation six of 25 (24.0%) subjects on the basis of abnormal medical history or physical exam findings alone, with no other documented abnormal ECG or echocardiogram findings.
12-Lead ECG Findings
Eighty of our subjects (31.5%) had positive ECG findings; some subjects had more than one abnormality (Table 3). The addition of ECG to the standard screening revealed nine additional subjects (35.0%) with significant cardiovascular abnormalities but yielded a high percentage of false-positive findings. ECG identified 42 subjects (16.5%) with left ventricular hypertrophy, but only 6 of those subjects had abnormal findings that could lead to detrimental health effects triggered by physical exertion (i.e., the subjects were not cleared for active sports pending further evaluation), and only three were confirmed to have increased left/right ventricular thickness. Electrocardiogram had a sensitivity of 50.0%, a specificity of 84.3%, a negative predictive value of 98.6%, and a low positive predictive value of 7.1% in identifying left ventricular hypertrophy. The second commonest abnormality on ECG was early repolarization/isolated Jpoint elevation (40, 15.7%).
| Abnormal ECG findings, n (%) | Athletes(n=154) | Non-athletes(n=100) | Total (n=254) |
|---|---|---|---|
| Left ventricular hypertrophy | 30 (19.5) | 12 (12.0) | 42 (16.5) |
| Early repolarization | 26 (16.9) | 14 (14.0) | 40 (15.7) |
| T-wave inversion | 5 (3.2) | 3 (3.0) | 8 (3.1) |
| Incomplete right bundle branch block |
4 (2.6) | 3 (3.0) | 7 (2.8) |
| Long QTc | 0 (0.0) | 5 (5.0) | 5 (2.0) |
| Right ventricular hypertrophy | 3 (1.9) | 1 (1.0) | 4 (1.6) |
| Left axis deviation | 3 (1.9) | 0 (0.0) | 3 (1.2) |
| Right atrial enlargement | 0 (0.0) | 2 (2.0) | 2 (0.8) |
| Pre-excitation | 1 (0.6) | 0 (0.0) | 1 (0.4) |
| Left atrial enlargement | 0 (0.0) | 1 (1.0) | 1 (0.4) |
| Right axis deviation | 1 (0.6) | 0 (0.0) | 1 (0.4) |
| Left anterior fascicular block | 1 (0.6) | 0 (0.0) | 1 (0.4) |
| Total ECG Abnormalities | 49 (19.3) | 31 (12.2) | 80 (31.5) |
| Echo findings, n (%) | Athletes(n=154) | Non-athletes(n=100) | Total (n=254) |
| Atrial septal defect/ patent foramen ovale |
4 (2.6) | 6 (6.0) | 10 (3.9) |
| Left ventricular hypertrophy | 1 (0.6) | 5 (5.0) | 6 (2.4) |
| Tricuspid regurgitation | 4 (2.6) | 1 (1.0) | 5 (2.0) |
| Patent ductus arteriosus | 3 (2.0) | 2 (2.0) | 5 (2.0) |
| Pulmonary artery dilation | 1 (0.6) | 2 (2.0) | 3 (1.2) |
| Right ventricular dilation | 3 (2.0) | 0 (0.0) | 3 (1.2) |
| Aortic stenosis/incompetence | 2 (1.3) | 1 (1.0) | 3 (1.2) |
| Mitral valve prolapse/bowing | 0 (0.0) | 2 (2.0) | 2 (0.8) |
| Ventricular septal defect | 1 (0.6) | 0 (0.0) | 1 (0.4) |
| Aortic root dilation | 0 (0.0) | 1 (1.0) | 1 (0.4) |
| Abnormal aortic arch flow | 1 (0.6) | 0 (0.0) | 1 (0.4) |
| Pulmonary valve insufficiency | 0 (0.0) | 1 (1.0) | 1 (0.4) |
| Right atrial dilation | 1 (0.6) | 0 (0.0) | 1 (0.4) |
| Right coronary ostium, not visualized |
1 (0.7) | 0 (0.0) | 1 (0.4) |
| Total Echo abnormalities | 17 (6.7) | 15 (5.9) | 32 (12.6) |
Table 3: Prevalence of electrocardiogram (ECG) and echocardiogram (Echo) abnormalities.
Two-Dimensional DOPPLER echocardiogram findings
Interatrial septal defect (10, 3.9%) was the most common abnormal finding, followed by increased left ventricular wall thickness (six, 2.4%), abnormal tricuspid valve regurgitation (five, 2.0%), and patent ductus arteriosus (five, 2.0%) (Table 3). Additional findings included pulmonary artery dilation, increased right ventricle chamber size, aortic stenosis/regurgitation, mitral valve prolapse, ventricular septal defect, patent ductus arteriosus, aortic root dilation, and increased right atrial size.
Adding ECG and echocardiogram
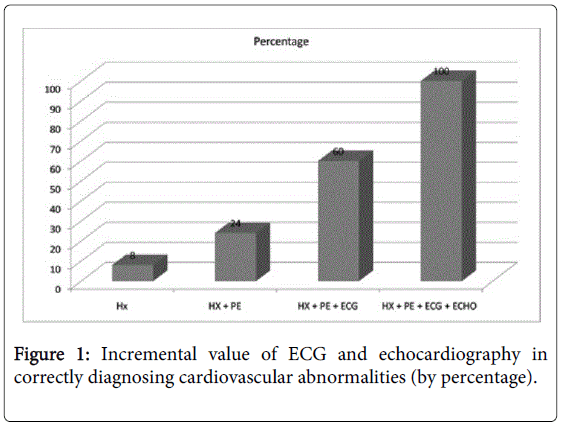
Figure 1 shows the incremental value of adding ECG and echocardiography to history and physical examination: they detected more subjects with left ventricular hypertrophy, enlarged chambers, valvular abnormalities, preexcitation, long QTc, interatrial or interventricular septal defects, and aortic root abnormalities. Adding an ECG revealed seven (36.0%) more children with abnormalities, and adding an echocardiogram an additional 10 (40.0%) more. The echocardiograms were also valuable in reclassifying false-positive findings (by history, physical and ECG) results in 86.3% of cases, especially left ventricular hypertrophy and early repolarization, thus minimizing further workup.
Sex differences
The average of diastolic blood pressures of males (70.5 ± 7.9) was higher compared to that of females (69.0 ± 7.8) (p=0.0129). Males also had more positive abnormal ECG findings (53.0% vs. 13.7%, p< 0.0001). No differences by sex were noted in physical exam, mean systolic blood pressure, or echocardiography.
Weight groups
The percentages of underweight, normal weight, overweight, and obese subjects are shown in Table 1. Overweight and obese subjects had a higher prevalence of hypertension than the underweight and normal groups (30.2% vs. 11.1%; p-value=0.0002), and were more likely to have an abnormality detected on physical examination (50.9% vs. 32.6%; p-value=0.0034). There was however no significant differences in weight groups regarding abnormal ECG or echocardiogram findings.
Athletic status
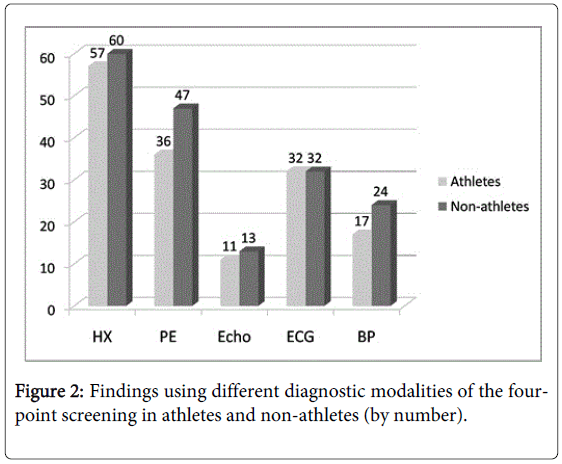
No significant differences in the electrocardiogram, echocardiogram, or blood pressure findings of athletes and nonathletes were found (Figure 2).
Clearance status and progress reports
After carefully evaluating the history, physical exam, ECG, and echocardiography findings, we divided the subjects into three groups. One-hundred and ninety-one (75.2%) had normal findings and were cleared with no restriction for active sports for 2 years. Thirty-eight (15.0%) subjects had findings not associated with SCD but needed further follow-up; they were still cleared with no restriction. Twentyfive (9.8%) (Table 4) had abnormal findings that could lead to detrimental health effects triggered by physical exertion and so were not cleared for active sports pending further evaluation.
| Subject No. | Athletes (n=11) | Follow up outcome |
|---|---|---|
| 0028 | Right ventricular hypertrophy , right ventricular dilation, Ventricular septal defect | Mild increase in LV size with preserved systolic function- stress test planned –No show |
| 0121 | Severe palpitations, Sinus tachycardia | Follow up in process |
| 0138 | Chest pain, Patent ductus arteriosus | Patent ductus arteriosus closed by Amplatzer occluder |
| 0152 | Stage 2 hypertension, Atrial septal defect (Ostium secundum) | Lost to follow up |
| 0164 | Incomplete Right bundle branch block, Abnormal aortic flow | Follow up in process |
| 0167 | Right ventricular dilation | Complete echocardiogram normal. |
| 0179 | Patent ductus arteriosus | Lost to follow up |
| 0185 | Stage 2 hypertension, Premature atrial complexes, Patent foramen ovale | Follow up in process |
| 0198 | Long QTc, Patent ductus arteriosus | Lost to follow up |
| 0203 | Chest pain/ syncope, palpitations | Follow up in process |
| 0219 | Wolff-Parkinson-White Syndrome | Patient being evaluation for possible ablation of accessory pathway. |
| Non-athletes (n=14) | Follow up outcome | |
| 0001 | Atrial septal defect/Patent foramen ovale | Transcatheter atrial septal defect closure. |
| 0041 | Stage 2 hypertension, Left ventricular hypertrophy, Coarctation of aorta | Cardiac catheterization with balloon angioplasty/stent placement of coarctation of aorta |
| 0066 | Stage 2 hypertension | Treated with lisinopril |
| 0074 | Chest pain, Aortic root dilation | Lost to follow up |
| 0077 | Stage 2 hypertension, Left ventricular hypertrophy, Patent foramen ovale/Atrial septal defect | Full echocardiogram revealed Small patient foramen ovale with minor left to Right shunt, annual follow up. |
| 0079 | Stage 2 hypertension, Patent foramen ovale, Interatrial Aneurysm | Follow up in process |
| 0088 | Chest pain, palpitations, Atrial septal defect | Follow up in process |
| 0097 | Stage 2 hypertension, Left ventricular hypertrophy | Lost to follow up |
| 0104 | Aortic arch dilation, Patent ductus arteriosus | Lost to follow up |
| 0146 | Syncope, Stage 2 hypertension, Left ventricular hypertrophy | Lost to follow up |
| 0148 | Stage 2 hypertension, Atrial septal defect/Patent foramen ovale | Follow up in process |
| 0151 | Stage 2 hypertension, Long QTc, Left ventricular hypertrophy | Follow up in process |
| 0154 | Long QTc | Follow up in process |
| 0166 | Stage 2 hypertension, Sinus tachycardia | Lost to follow up |
Table 4: Screening findings of the restricted subjects and follow up.
We referred the 11 (4.3%) athletic subjects and 14 (5.5%) nonathletic subjects with abnormal findings for additional screening. The most frequent of these abnormal findings were hypertension (44.0%), vascular abnormalities (16.0%, patent ductus arteriosus and ventricular septal defect), ventricular hypertrophy (16.0%, left ventricular and RV hypertrophy), rhythm conduction disturbances (16.0%, long QTc and Wolff-Parkinson-White syndrome.), and aortic root abnormalities (12%, aortic root dilation and coarctation).
Follow up
There were several cases in which a major abnormal finding resulted in significant action being taken (Table 4). Specifically, one coarctation of the aorta required stenting, a large atrial septal defect underwent transcatheter closure, and one patent ductus arteriosis was closed. Also, several children with Stage 2 hypertension underwent lifestyle changes, were started on medication, and/or are now being followed in a hypertension clinic. One child with Wolff-Parkinson- White syndrome was referred to a pediatric electrophysiologist for ablation. In several cases, a complete echocardiogram with a bubble study revealed the screening finding was a false positive. Many of the other children are currently enrolled in a pediatric clinic and are receiving follow up for their abnormal screening tests. Unfortunately, a significant number of children were lost to follow up (left the school or state).
Discussion
This prospective study suggests that our onsite four-point cardiovascular screening tool for athletic and non-athletic sixth graders is feasible and effective. ECG and echocardiography incrementally improved screening in this population (36.0% and 40.0%, respectively) with a small increase in assessment time, showing that the method is feasible in a population-based, community setting. The small percentage of subjects with cardiovascular abnormalities detected by history and physical exam suggests these are an ineffective screening tool [2,45,46]. The inclusion of ECG increased combined sensitivity by identifying potential accessory pathways and ion channelopathies. On its own, echocardiography identified four (16.0%) of the 25 participants with abnormal findings that were not identified by medical/physical and ECG findings.
Cardiovascular findings ranged from clinically relevant and associated with sudden cardiac death (e.g. coarctation of the aorta) to clinically relevant (e.g. atrial septal defect) to normal variants (e.g. physiological hypertrophy). Subjects with a normal pediatric finding such as mild pulmonary or tricuspid valve regurgitation were not classified as “abnormal findings” but rather as normal variants.
Cardiovascular abnormalities were more common (9.8%) than previously reported [2,7,9,10,47]. However, we included nonathletic children and screened medically underserved, minority communities, which may have higher prevalence’s of undiagnosed abnormalities, obesity and hypertension. While our population was predominantly African American and Hispanic, a recent report of similar screening of 400 healthy predominantly white children (mean age 11.8 years) noted undiagnosed cardiac abnormalities in 5.8%, serious cardiac conditions in 2.5%, and hypertension in 5%[8]. We are now screening a larger number of children from a broader sociodemographic pool in Houston to determine whether this screening procedure leads to early detection across all ethnicities.
Our results also show the importance of screening non-athletes (Table 4 and Figure 2). The case for restricting the screening to just athletes is economic. For instance, in one large study, PPCS detected hypertrophic cardiomyopathy in only 22 of 33,735 young athletes (0.07%), yet caused only 1 sudden death among the athletes (2.0 %) but 16 sudden deaths among the non-athletes (7.3 %), suggesting the need for screening both groups [48]. Screening non-athletes may lower the incident rate of SCD by capturing subjects that might become athletic later [49]. Our slightly higher rates of cardiovascular abnormalities in non-athletes may be due to a self-selection bias, the non-athletes avoiding active sports because of underlying cardiovascular abnormalities.
Twenty-five participants (9.8%) were not cleared for sports participation (Table 4); most of these subjects had both positive medical history/physical exam findings such as chest pain and stage 2 hypertension, abnormal ECG (e.g. Wolff-Parkinson-White Syndrome), or a significant structural abnormality on echocardiography (dilated aortic root, coarctation of the aorta). The long QTc cases were mostly false positives, or may have been due to temporary electrolyte abnormalities or medication effects, as most were only hundredths of seconds prolonged (e.g. 480 milliseconds) and were normal on subsequent visits. Several are currently being followed up by electrophysiology; however several were lost to follow up. The impact of sidelining 10% of school children from physical activity is high, given the rates of obesity and diabetes in this group. Thus, a screening program would need to consider this aspect and strive to get this 10% worked up and managed appropriately to allow them to return to physical activity if possible based on their final diagnosis and disposition.
It is not surprising that 37 subjects reported palpitations during exercise, as palpitations are common in children and usually well tolerated [50]. However, palpations with chest pain or shortness of breath may indicate a possible underlying cardiac condition associated with SCD [50].
As seen in Table 2, more subjects had an arm-to-height ratio>1.05 than had a positive Marfan’s thumb/wrist sign. One child diagnosed with these findings had an echocardiography abnormality (a dilated aortic root). These findings suggest that these physical examination findings may not be specific for the diagnosis of Marfan’s Syndrome in this young population, which is important because of associated heart problems in Marfan’s Syndrome. The diagnosis of Marfan syndrome relies on defined clinical criteria (revised Ghent nosology), comprising a set of major and minor manifestations in different body systems, have proven to work well in adults; however, concerns with the current nosology are that some of the diagnostic criteria have not been sufficiently validated, are not applicable in children or necessitate expensive and specialised investigations [51].
It is also important that the final diagnosis in a number of the children who screened abnormal would not have ultimately prevented their participation in sports (e.g., patent foramen ovale, stage 2 hypertension). However, several children did have conditions that might have resulted in SCD had they not been identified and treated, including coarctation of the aorta, Wolff-Parkinson-White Syndrome, and aortic arch dilation. In addition, we identified many children who were obese (referred to primary physician for counseling) and/or had hypertension (referred to pediatric hypertension clinic and treated) that if left unchecked, would likely lead to premature coronary artery disease in adulthood.
Finally, we may not have detected all abnormalities, as our screening methodology has a lower ability to detect certain abnormalities than other methods e.g., anomalous coronary arteries are better detected by computed tomographic angiography imaging [52].
Interestingly, we did not identify any children with hypertrophic cardiomyopathy, perhaps because we did not screen enough children; [41] or because overt cardiac hypertrophy may not develop until adolescence or later [53]. As detection of hypertrophic cardiomyopathy is a major goal of any childhood screening program, our screening clearance is for only 2 years, so that those with the genotype but not expressing the phenotype, can be detected at later required screenings (i.e., young children predisposed to the phenotype of hypertrophic cardiomyopathy may not show evidence of left ventricular hypertrophy).
Isolated congenital coronary artery anomalies of wrong sinus origin are another rare but well-described cause of SCD, and symptoms occur in less than half of people [54]. While we did not detect any congenital coronary artery anomalies in our study, our screening echocardiograms were able to identify the origins of the coronary vessels in all but one case, consistent with others [55].
We do not know whether our screening program is cost effective. Screening all our subjects took two cardiologists (one pediatric cardiologist), two cardiology fellows, two medicine residents, two medical students, two ECG and echocardiography technologists, and two coordinating staff members 8 hours on site at the schools and necessitated use of two portable echocardiography and two ECG machines and eight tables and chairs. Most of our staff were volunteers, and the equipment was loaned, so costs were nominal. It would be possible to have fewer staff involved, but screening time would increase. In addition, while the HEARTS screening in this population did detect abnormalities and led to correction of some conditions associated with SCD, a definitive diagnosis by complete follow up was not possible for many of the children. It is likely that the low socioeconomic status of the screened population contributed to difficulties in follow up as well as relocation of some of the children. We did attempt to ascertain location of patients who had relocated from the school nurse but in many cases this information was not available.
Despite these possible shortcomings, our study demonstrated the feasibility and efficacy of administering our 15-minute standardized onsite four-point screening test to sixth-grade children to identify cardiac abnormalities. We were able to reclassifying as normal most of the subjects with abnormal ECG findings. These preliminary data suggest that many sixth-grade athletic and non-athletic children have cardiac abnormalities. Others have confirmed that a community-based ECHO in athletes is feasible and is associated with a high rate of technically adequate imaging [56]. On the basis of our encouraging results, the Texas state legislature is considering mandating that insurance companies reimburse for such preparticipation screening for all schoolchildren [57]. We are examining the feasibility of extending this program to other parts of the United States. However, cost issues at this time render. Further research needs to be conducted into better screening methods that are cost-effective so that early identification of high-risk persons may help prevent SCD. Importantly, screening methods not only need to be sensitive, but also limit the number of false-positives (unnecessary workup) and falsenegatives (missed cases). A screening program must be able to distinguish normal physiological athlete variants from abnormal pathology, otherwise many unnecessary and costly additional procedures will occur [6]. It may turn out that a combination of genetic testing as well as phenotype testing (physical examination, ECG, ECHO) may be the best approach to identify these at-risk persons [58].
Conclusion
Cardiovascular screening of athletes should detect underlying cardiac disease associated with sudden cardiac death and thus allow physicians to manage and minimize athletes risk via medical/surgical management and activity proscription.
A new upgraded screening approach, which adds an ECG and limited echocardiogram to the existing history and physical examination appears to better identify those young children with underlying cardiovascular conditions that puts them at higher risk of sudden cardiac death. Unfortunately, the current recommendations for preparticipation screening of cardiovascular diseases by the AHA fail to identify many of these at risk persons.
There are pros and cons to an advanced screening approach like HEARTS. The pros are that there is a window of opportunity to save a life if an ‘at risk’ individual is identified early on prevent further deaths of athletes, and that technology exists that may help identify those at risk before the SCD event. The cons are that the cost to screen a large population is very high; also, the extra costs of workup for those who may have a false-positive screen (normal variant) and the emotional costs of being restricted from a team competition may exceed the benefits. We encourage further physician/provider education and research on improved screening of this young population, and hope for a cost effective solution in the future that will save young lives.
Acknowledgements
We thank the schools, students, school nurses, teachers, and parents for their participation in this study and Maureen Goode, PhD, ELS, for substantive editing of the manuscript. We thank Leslie L. Alexander, Thaddeus B. Brown, Sarah Joseph, and the Houston Rockets for their support of our research. We thank Gayle M. Kinnie and Ileana V. Treviño from the Memorial Hermann Foundation for their support of time and effort. We thank Beverly Smulevitz, B.S., RDCS, Division of Cardiology, Department of Internal Medicine, The University of Texas at Houston Medical School, for her assistance with echocardiography quality control. We thank Judy Stokes, RN, for her assistance with screening.
Funding Sources
This work was supported in part by a non-restricted grant through the Memorial Hermann Foundation and The University of Texas Health Science Center at Houston Center for Clinical and Translational Sciences (CTSA UL1 RR024148).
Conflict of Interest Statement
John P. Higgins, MD, MBA, MPhil; Susan T. Laing, MD, MPH; J. Jason West, MD; Gurur Biliciler-Denktas, MD; Khashayar K. Vahdat, MD; Joshua A. Samuels, MD; Amol Rajmane, MBBS, MS; Asif Ali, MD; Satish J. Bankuru, MD; Monica B. Patel, MD; Ketan N. Patel, MD; Troy D. Tuttle, MS; David M. Filsoof, MD; William J. McKee, MD; Nan Li, MD; Robert A. McGuffey, MD; Joshua D. Stokes MPS; Alejandra Rodriguez, MBA; Ashley N. Callaghan, MA; Evelyn Henry RN, MSN; Ijeoma E. Ananaba, MD; Benjamin Yang, MD; and David D. McPherson, MD disclose no conflicts of interest.
Disclosures
The authors have no financial or other interest in the products or distributor of the products in the manuscript. In addition, the authors have no other kinds of associations, such as consultancies, stock ownership, or other equity interests or patent-licensing arrangements, with any of the products in the manuscript.
References
- Harmon KG, Asif IM, Klossner D, Drezner JA (2011) Incidence of sudden cardiac death in National Collegiate Athletic Association athletes.Circulation 123: 1594-1600.
- Baggish AL, Hutter AM Jr, Wang F, Yared K, Weiner RB, et al. (2010) Cardiovascular screening in college athletes with and without electrocardiography: A cross-sectional study.Ann Intern Med 152: 269-275.
- Chandra N, Bastiaenen R, Papadakis M, Sharma S (2013) Sudden cardiac death in young athletes: practical challenges and diagnostic dilemmas.J Am Coll Cardiol 61: 1027-1040.
- Mayer F, Bonaventura K, Cassel M, Mueller S, Weber J, et al. (2012) Medical results of preparticipation examination in adolescent athletes.Br J Sports Med 46: 524-530.
- Maron BJ, Thompson PD, Ackerman MJ (2007) Recommendations and considerations related to preparticipation screening for cardiovascular abnormalities in competitive athletesupdate: a scientific statement from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism: endorsed by the American College of Cardiology Foundation. Circulation 115:1643-1455.
- Asif IM, Rao AL, Drezner JA (2013) Sudden cardiac death in young athletes: what is the role of screening?Curr Opin Cardiol 28: 55-62.
- Chandra N, Papadakis M, Sharma S (2010) Preparticipation screening of young competitive athletes for cardiovascular disorders.Phys Sportsmed 38: 54-63.
- Vetter VL, Dugan N, Guo R, Mercer-Rosa L, Gleason M, et al. (2011) A pilot study of the feasibility of heart screening for sudden cardiac arrest in healthy children.Am Heart J 161: 1000-1006.
- Pelliccia A, Maron BJ, Culasso F, Di Paolo FM, Spataro A, et al. (2000) Clinical significance of abnormal electrocardiographic patterns in trained athletes.Circulation 102: 278-284.
- Fuller CM, McNulty CM, Spring DA, Arger KM, Bruce SS, et al. (1997) Prospective screening of 5,615 high school athletes for risk of sudden cardiac death.Med Sci Sports Exerc 29: 1131-1138.
- Lawless CE1 (2009) Return-to-play decisions in athletes with cardiac conditions.Phys Sportsmed 37: 80-91.
- Siddiqui S, Patel DR (2010) Cardiovascular screening of adolescent athletes.Pediatr Clin North Am 57: 635-647.
- Lawless CE, Best TM (2008) Electrocardiograms in athletes: interpretation and diagnostic accuracy.Med Sci Sports Exerc 40: 787-798.
- Wilson MG, Basavarajaiah S, Whyte GP, Cox S, Loosemore M, et al. (2008) Efficacy of personal symptom and family history questionnaires when screening for inherited cardiac pathologies: the role of electrocardiography.Br J Sports Med 42: 207-211.
- Pelliccia A, Zipes DP, Maron BJ (2008) Bethesda Conference #36 and the European Society of Cardiology Consensus Recommendations revisited a comparison of U.S. and European criteria for eligibility and disqualification of competitive athletes with cardiovascular abnormalities. J Am Coll Cardiol 52:1990-1996.
- Hevia AC, Fernández MM, Palacio JM, Martín EH, Castro MG, et al. (2011) ECG as a part of the preparticipation screening programme: an old and still present international dilemma.Br J Sports Med 45: 776-779.
- Maron BJ (2010) National electrocardiography screening for competitive athletes: Feasible in the United States?Ann Intern Med 152: 324-326.
- Higgins JP (2008) Normal resting electrocardiographic variants in young athletes.Phys Sportsmed 36: 69-75.
- Rawlins J, Carre F, Kervio G (2010) Ethnic differences in physiological cardiac adaptation to intense physical exercise in highly trained female athletes. Circulation 1211078-1085.
- Basavarajaiah S, Boraita A, Whyte G (2008) Ethnic differences in left ventricular remodeling in highly-trained athletes relevance to differentiating physiologic left ventricular hypertrophy from hypertrophic cardiomyopathy. J Am Coll Cardiol 51:2256-2262.
- Corrado D, Biffi A, Basso C, Pelliccia A, Thiene G (2009) 12-lead ECG in the athlete: physiological versus pathological abnormalities.Br J Sports Med 43: 669-676.
- Chandra N, Papadakis M, Sharma S (2012) Cardiac adaptation in athletes of black ethnicity: differentiating pathology from physiology.Heart 98: 1194-1200.
- Maron BJ (2007) Hypertrophic cardiomyopathy and other causes of sudden cardiac death in young competitive athletes, with considerations for preparticipation screening and criteria for disqualification. Cardiol Clin25:399-414.
- Lauschke J, Maisch B (2009) Athlete's heart or hypertrophic cardiomyopathy?Clin Res Cardiol 98: 80-88.
- Maron BJ (2009) Distinguishing hypertrophic cardiomyopathy from athlete's heart physiological remodelling: clinical significance, diagnostic strategies and implications for preparticipation screening. Br J Sports Med 43:649-656.
- Pelliccia A, Di Paolo FM, De Blasiis E, Quattrini FM, Pisicchio C, et al. (2010) Prevalence and clinical significance of aortic root dilation in highly trained competitive athletes.Circulation 122: 698-706, 3 p following 706.
- Corrado D, Basso C, Pavei A, Michieli P, Schiavon M, et al. (2006) Trends in sudden cardiovascular death in young competitive athletes after implementation of a preparticipation screening program.JAMA 296: 1593-1601.
- Burke AP, Farb A, Virmani R, Goodin J, Smialek JE (1991) Sports-related and non-sports-related sudden cardiac death in young adults.Am Heart J 121: 568-575.
- Louie EK, Edwards LC 3rd (1994) Hypertrophic cardiomyopathy.Prog Cardiovasc Dis 36: 275-308.
- Heron M, Tejada-Vera B (2009) Deaths: leading causes for 2005.Natl Vital Stat Rep 58: 1-97.
- Harriss DJ, Atkinson G (2011) Update--Ethical standards in sport and exercise science research.Int J Sports Med 32: 819-821.
- CDC (2012) How much physical activity do children need?
- Haskell WL, Lee IM, Pate RR (2007) Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation 116:1081-1093.
- Frank JE, Jacobe KM (2011) Evaluation and management of heart murmurs in children.Am Fam Physician 84: 793-800.
- McCrindle BW, Shaffer KM, Kan JS, Zahka KG, Rowe SA, et al. (1996) Cardinal clinical signs in the differentiation of heart murmurs in children.Arch Pediatr Adolesc Med 150: 169-174.
- Poddar B, Basu S (2004) Approach to a child with a heart murmur.Indian J Pediatr 71: 63-66.
- Flynn JT, Mitsnefes M, Pierce C, Cole SR, Parekh RS, et al. (2008) Blood pressure in children with chronic kidney disease: a report from the Chronic Kidney Disease in Children study.Hypertension 52: 631-637.
- National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents (2004) The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents.Pediatrics 114: 555-576.
- CDC (2012) Clinical Growth Charts: BMI-for-age.
- Corrado D, Pelliccia A, Heidbuchel H, Sharma S, Link M, et al. (2010) Recommendations for interpretation of 12-lead electrocardiogram in the athlete.Eur Heart J 31: 243-259.
- Maron BJ, Gardin JM, Flack JM (1995) Prevalence of hypertrophic cardiomyopathy in a general population of young adults. Echocardiographic analysis of 4111 subjects in the CARDIA Study. Coronary Artery Risk Development in (Young) Adults. Circulation92:785-789.
- Lopez L, Colan SD, Frommelt PC (2010) Recommendations for quantification methods during the performance of a pediatric echocardiogram: a report from the Pediatric Measurements Writing Group of the American Society of Echocardiography Pediatric and Congenital Heart Disease Council. J Am Soc Echocardiogr 23:465-495.
- Rudski LG, Lai WW, Afilalo J (2010) Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 23: 685-713.
- Bille K, Figueiras D, Schamasch P, Kappenberger L, Brenner JI, et al. (2006) Sudden cardiac death in athletes: the Lausanne Recommendations.Eur J Cardiovasc Prev Rehabil 13: 859-875.
- Pelliccia A, Corrado D (2010) Electrocardiography and preparticipation screening of competitive high school athletes. Ann Intern Med 153:132-133.
- Thompson PD, Levine BD (2006) Protecting athletes from sudden cardiac death.JAMA 296: 1648-1650.
- Zeltser I, Cannon B, Silvana L, Fenrich A, George J, et al. (2012) Lessons learned from preparticipation cardiovascular screening in a state funded program.Am J Cardiol 110: 902-908.
- Corrado D, Basso C, Schiavon M, Thiene G (1998) Screening for hypertrophic cardiomyopathy in young athletes.N Engl J Med 339: 364-369.
- Douglas PS1 (2008) Saving athletes' lives a reason to find common ground?J Am Coll Cardiol 52: 1997-1999.
- Hanash CR, Crosson JE (2010) Emergency diagnosis and management of pediatric arrhythmias.J Emerg Trauma Shock 3: 251-260.
- Loeys BL, Dietz HC, Braverman AC, Callewaert BL, De Backer J, et al. (2010) The revised Ghent nosology for the Marfan syndrome.J Med Genet 47: 476-485.
- Sundaram B, Kreml R, Patel S (2010) Imaging of coronary artery anomalies.Radiol Clin North Am 48: 711-727.
- Ho CY1 (2009) Hypertrophic cardiomyopathy: preclinical and early phenotype.J Cardiovasc Transl Res 2: 462-470.
- Frommelt PC1 (2009) Congenital coronary artery abnormalities predisposing to sudden cardiac death.Pacing Clin Electrophysiol 32 Suppl 2: S63-66.
- Frommelt PC, Frommelt MA (2004) Congenital coronary artery anomalies.Pediatr Clin North Am 51: 1273-1288.
- Weiner RB, Wang F, Hutter AM Jr, Wood MJ, Berkstresser B, et al. (2012) The feasibility, diagnostic yield, and learning curve of portable echocardiography for out-of-hospital cardiovascular disease screening. J Am Soc Echocardiogr 25: 568-575.
- https://www.capitol.state.tx.us/tlodocs/82R/billtext/html/HB01644I.htm.
- Higgins JP, Ananaba IE, Higgins CL (2013) Sudden cardiac death in young athletes: preparticipation screening for underlying cardiovascular abnormalities and approaches to prevention.Phys Sportsmed 41: 81-93.
Relevant Topics
- Adolescent Anxiety
- Adult Psychology
- Adult Sexual Behavior
- Anger Management
- Autism
- Behaviour
- Child Anxiety
- Child Health
- Child Mental Health
- Child Psychology
- Children Behavior
- Children Development
- Counselling
- Depression Disorders
- Digital Media Impact
- Eating disorder
- Mental Health Interventions
- Neuroscience
- Obeys Children
- Parental Care
- Risky Behavior
- Social-Emotional Learning (SEL)
- Societal Influence
- Trauma-Informed Care
Recommended Journals
Article Tools
Article Usage
- Total views: 15686
- [From(publication date):
June-2015 - Apr 05, 2025] - Breakdown by view type
- HTML page views : 11014
- PDF downloads : 4672