Commentary to Quantitative PET/MRI Evaluation and Application in Neurology
Received: 01-Mar-2018 / Accepted Date: 10-Mar-2018 / Published Date: 17-Mar-2018 DOI: 10.4172/2161-0460.1000430
With the high spatial resolution of magnetic resonance imaging (MRI) particularly for the soft tissue such as in brain and non-ion radiation involved, the integrative positron emission tomography (PET)/ MRI system is expected to provide almost equivalent image quality compared with PET alone or PET/CT system, and simultaneous MRI information that is not conventionally available [1]. PET-MRI opens new horizons in multi-parametric neuroimaging for clinical research that allows simultaneous imaging of multiple parametric changes such as blood flow and metabolism at the same time. While MRI could provide superior structural information, applying MRI anatomical priors to reduce the PET partial volume effect could be achieved to improve spatial resolution of PET images for clinical and research usages. The PET/MRI scanner might help address these issues with advantages of reduced scan time and simultaneous nature of data acquisition. For the past few years, multiple applications of the integrated PET/MRI imaging including inflammation, functional metabolism and microstructure mapping have been reported [1,2]. Further applications in brain tumors and cancer response monitoring have reported complementary and valid information that this relatively new modality could achieve based on newly developed techniques. Newer and better MRI-based attenuation correction (AC) such as zero TE (ZTE) for more bone-related tissue signal detection and faster reconstruction compared to UTE/Dixon has been reported [3-6]. Advanced MRI-based motion correction for PET image reconstruction, newer PET time of flight reconstruction and incorporation with compressed sensing techniques have offered attractive superior temporal and spatial resolutions for disease diagnosis and prevention [7-9]. We briefly review the applications of PET/MRI in neurology with two examples in Alzheimer’s disease and brain tumor cases in this commentary.
Numerous imaging studies in Alzheimer’s disease (AD) have been performed to study characteristic brain alterations including brain atrophy with earlier involvement of medial temporal lobe [10], brain hypo- perfusion in the posterior cingulate and parietal regions [11], reduced functional and structural connectivity in the default mode network (DMN) based on MRI findings [12] and hypo-metabolism based on PET imaging findings [13]. A regional coupling between metabolism of glucose (via PET kinetic analysis) and cerebral blood flow (CBF) had been reported with reductions post-caffeine compared to precaffeine conditions in controls [14], and in demented patients [15] based on separate scans. An evaluation of the real-time coupling of these two key imaging parameters with simultaneous acquisitions had not been performed before. For example, FDG metabolism had been found to increase twice as the energy brain activation needs (i.e., larger change in metabolism than change in CBF), and at a later time the unmetabolited fraction of the tracer was cleared off so that the tissue concentration of the tracer could reflect the metabolic rate [16,17]. Neurovascular coupling between neurochemistry (e.g., dopamine receptor occupancy) and hemodynamic change (e.g., cerebral blood volume) using simultaneous PET/fMRI acquisitions had been reported recently with similar temporal profile and dose response in non-human primates [18]. Translational studies of neurovascular and neuro-metabolic coupling effects in human beings and patients remain challenging due to inter-subject variability and lack of control template. Despite these challenges, the integrated PET/MRI scanner gained popularity due to some advantages including intrinsic between-modality image registration, better interpretation of underlying co-existing pathophysiological events, and most importantly, less patient discomfort [19].
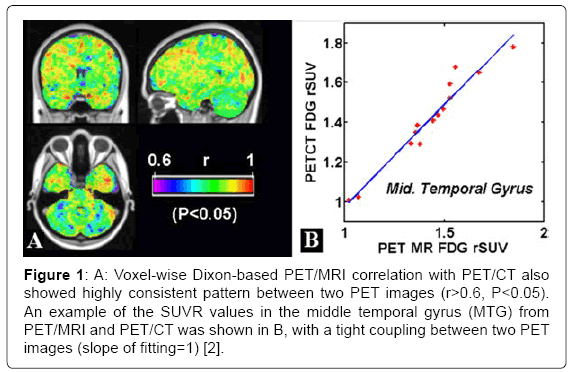
The article by Zhou [2] had evaluated the most up-to-date PET/MRI scanner performance with multiple functional and structural metrics together with application demonstrations. Voxel-wise analyses in the template space also showed the majority of brain voxels (>95%) with significant correlations (r>0.54, P<0.05) between PET/MRI with Dixon MR-based AC method and PET/CT based on SUVR (standard uptake value ratio, a quantitative metric of normalized PET tracer dosage uptake) choosing cerebellum as the reference region (Figure 1A). A tight coupling between PET/MRI PET image and PET/CT PET image with a slope of fitting close to 1 in the middle temporal gyrus (MTG) was demonstrated in (Figure 1B). Furthermore, the global mean difference based on SUVR between PET/MRI and PET/CT was small using Dixon MR-based AC and PET/CT (difference=4%), And our findings agree with currently accepted notion that the MRI-based AC could achieve comparable imaging quality to standard-CT AC, with ≤ 5% differences between the PET/MRI and PET/CT PET images. The integrated PET/ MRI images showed comparable image quality to stand-alone imaging modality (both MRI and PET) with the gains of simultaneous multiparametric acquisitions, reduced scan time and potential patient discomfort. Furthermore, tight correlation between blood flow and metabolism was found in several brain gray matter regions including the temporal lobe in patients [1,2].
Figure 1: A: Voxel-wise Dixon-based PET/MRI correlation with PET/CT also showed highly consistent pattern between two PET images (r>0.6, P<0.05). An example of the SUVR values in the middle temporal gyrus (MTG) from PET/MRI and PET/CT was shown in B, with a tight coupling between two PET images (slope of fitting=1) [2].
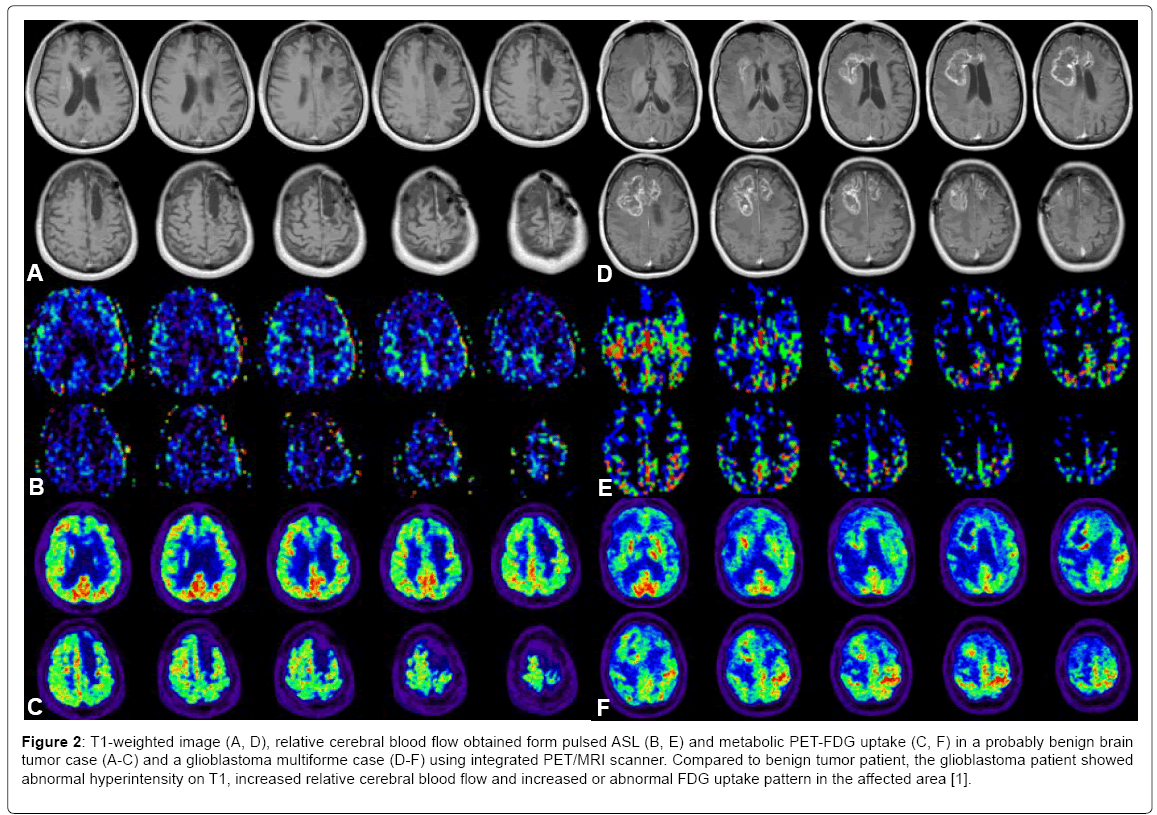
Tumor growth typically requires new blood vessel formation, causing changes in neurovascular and neurometabolic measures such as blood brain barrier (BBB) permeability, blood flow and metabolism [20]. Typical microstructure alteration of tumor characteristics include changes of diffusion of cell density of tumors and extracellular space narrowing and restricted diffusion properties compared to surrounding tissue types. For instance, using multiple imaging metrics obtained from the integrated PET/MRI scanner, we could differentiate images of MRI T1, CBF and PET- FDG uptake of a possible benign tumor case and a glioblastoma multiforme case. (Figure 2) demonstrated representative images of MRI T1, CBF and PET-FDG uptake of a possible benign tumor case (A-C) and a glioblastoma multiforme case (D-F) [1]. Compared to benign tumor patient, the glioblastoma patient showed abnormal hyperintensity on T1, hyperperfusion and increased FDG uptake in the affected area. Our study described the convenience and feasibility of using simultaneous MRI and PET modality to characterize structural, functional, and molecular signatures of brain tumor. We had demonstrated the abnormal increased metabolic PET and MRI hyperintensity and higher blood flow of glioblastoma compared to a benign tumor case. Further study with more subjects and an automatic multi-imaging feature classification to obtain an objective integrated score to predict disease survival rate and recurrence is warranted in the future [20] for the relatively new integrated PET/MRI scanner.
Figure 2: T1-weighted image (A, D), relative cerebral blood flow obtained form pulsed ASL (B, E) and metabolic PET-FDG uptake (C, F) in a probably benign brain tumor case (A-C) and a glioblastoma multiforme case (D-F) using integrated PET/MRI scanner. Compared to benign tumor patient, the glioblastoma patient showed abnormal hyperintensity on T1, increased relative cerebral blood flow and increased or abnormal FDG uptake pattern in the affected area [1].
References
- Zhou Y (2016) Functional neuroimaging with multiple modalities: Principles, device and applications. Nova Science Publishers.
- Zhou Y (2015) Quantitative mMR (PET/MRI) evaluation and application in dementia Jacobs J Med Diagn Med Imaging vol 1: 005.
- Larson PE, Han M, Krug R, Jakary A, Nelson SJ, et al. (2016) Ultrashort echo time and zero echo time MRI at 7T. MAGMA 29: 359-370.
- Delso G, Khalighi M, Voert E, Barbosa F, Sekine T, et al. (2016) Effect of time-of-flight information on PET/MR reconstruction artifacts: Comparison of free-breathing versus breath- hold MR-based attenuation correction. Radiology 282: 152509.
- Hitz S, Habekost C, Fürst S, Delso G, Förster S, et al. (2014) Systematic comparison of the performance of integrated whole-body PET/MR imaging to conventional PET/CT for (1)(8)F- FDG brain imaging in patients examined for suspected dementia. J Nucl Med 55: 923-931.
- Sekine T, Ter Voert EE, Warnock G, Buck A, Huellner M, et al. (2016) Clinical evaluation of ZTE attenuation correction for brain FDG-PET/MR imaging-comparison with atlas attenuation correction. J Nucl Med 57: 1927-1932.
- Bai B, Lin Y, Zhu W, Ren R, Li Q, et al. (2014) MAP reconstruction for Fourier rebinned TOF-PET data. Phys Med Biol 59: 925-949.
- Catana C (2015) Motion correction options in PET/MRI. Semin Nucl Med 45: 212-223.
- Dutta J, Huang C, Li Q, El Fakhri G (2015) Pulmonary imaging using respiratory motion compensated simultaneous PET/MR. Med Phys 42: 4227-4240.
- Rusinek H, Endo Y, De Santi S, Frid D, Tsui WH, et al. (2004) Atrophy rate in medial temporal lobe during progression of alzheimer disease. Neurology 63: 2354-2359.
- Chao LL, Buckley ST, Kornak J, Schuff N, Madison C, et al. (2010) ASL perfusion MRI predicts cognitive decline and conversion from MCI to dementia. Alzheimer Dis Assoc Disord 24: 19-27.
- Zhou Y, Dougherty JH Jr, Hubner KF, Bai B, Cannon RL, et al. (2008) Abnormal connectivity in the posterior cingulate and hippocampus in early Alzheimer's disease and mild cognitive impairment. Alzheimers Dement 4: 265-270.
- Silverman DH, Small GW, Phelps ME (1999) Clinical value of neuroimaging in the diagnosis of dementia. Sensitivity and specificity of regional cerebral metabolic and other parameters for early identification of alzheimer's disease. Clin Positron Imaging 2: 119-130.
- Wang DJ, Chen Y, Fernandez-Seara MA, Detre JA (2011) Potentials and challenges for arterial spin labeling in pharmacological magnetic resonance imaging. J Pharmacol Exp Ther 337: 359-366.
- Musiek ES, Chen Y, Korczykowski M, Saboury B, Martinez PM, et al. (2012) Direct comparison of fluorodeoxyglucose positron emission tomography and arterial spin labeling magnetic resonance imaging in Alzheimer's disease. Alzheimers Dement 8: 51-59.
- Buxton RB (2012) Dynamic models of BOLD contrast. Neuroimage 62: 953-961.
- Buxton RB, Frank LR, Wong EC, Siewert B, Warach S, et al. (1998) A general kinetic model for quantitative perfusion imaging with arterial spin labeling. Magn Reson Med 40: 383-396.
- Sander CY, Hooker JM, Catana C, Normandin MD, Alpert NM, et al. (2013) Neurovascular coupling to D2/D3 dopamine receptor occupancy using simultaneous PET/ functional MRI. Proc Natl Acad Sci U S A 110: 11169-11174.
- Bai B, Li Q, Leahy RM (2013) Magnetic resonance-guided positron emission tomography image reconstruction. Semin Nucl Med 43: 30-44.
- Hutterer M, Hattingen E, Palm C, Proescholdt MA, Hau P, et al. (2015) Current standards and new concepts in MRI and PET response assessment of antiangiogenic therapies in high-grade glioma patients. Neuro Oncol 17: 784-800.
Citation: Zhou Y (2018) Commentary to Quantitative PET/MRI Evaluation and Application in Neurology. J Alzheimers Dis Parkinsonism 8: 430. DOI: 10.4172/2161-0460.1000430
Copyright: © 2018 Zhou Y. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 5077
- [From(publication date): 0-2018 - Apr 02, 2025]
- Breakdown by view type
- HTML page views: 4240
- PDF downloads: 837