Commentary: Not Aricept but Donepezil Should not Permit for Lewy Body Disease without Proper Clinical Trials
Received: 22-Aug-2018 / Accepted Date: 24-Aug-2018 / Published Date: 31-Aug-2018 DOI: 10.4172/2161-0460.1000447
Keywords: Alzheimer’s Disease (AD); Aricept (original medicine of Donepezil); Effective range; Lewy Body Disease (LBD)
Abbreviations
AA: Anticholinergic Activity; Ach: Acetylcholine; AD: Alzheimer’s Disease; SAA: Serum Anticholinergic Activity; S: Schizophrenia
Commentary
In Japan, only Aricept (original medicine of Donepezil) is permitted for treatment of Lewy Body Disease (LBD) [1]. Other generic medicines of donepezil are not allowed for treatment of LBD because of patent for treatment of LBD. Since Nov in 2018, other generic medicines will be allowed for treatment of LBD because of ending of patent of Aricept for treatment for LBD.
However, we consider that not Aricept but generic medicines of donepezil should not permit for Lewy body disease without proper clinical trials mainly for two reasons. One is that the interactions between main component, i.e., donepezil, and other components in order to aggregate and to use as a pill form. The main component is same between original medicine, i.e., Aricept, and other generic medicines. However, other components are different from those of original medicine. Therefore, ingestion, absorption, distribution, transfer to the central nervous system and the degree of action to central nervous system and peripheral tissues are different for each medicines.
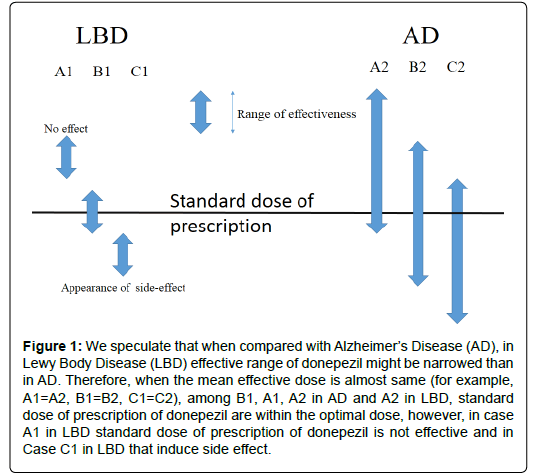
The other reasons are that LBD is sensitive against psychotropic medicines such as antipsychotics, however, even Aricept induces side effects such as sedations. We speculate that when compared with Alzheimer’s Disease (AD), in LBD effective range of donepezil might be narrowed. Therefore, when the mean effective dose is almost same for example, A1=A2, B1=B2, C1=C2, in (Figure 1), among B1, A1, A2 in AD and A2 in LBD, standard dose of prescription of donepezil are within the optimal dose, however, in case A1 in LBD standard dose of prescription of donepezil is not effective and in Case C1 in LBD that induce side effect (Figure 1).
Figure 1: We speculate that when compared with Alzheimer’s Disease (AD), in Lewy Body Disease (LBD) effective range of donepezil might be narrowed than in AD. Therefore, when the mean effective dose is almost same (for example, A1=A2, B1=B2, C1=C2), among B1, A1, A2 in AD and A2 in LBD, standard dose of prescription of donepezil are within the optimal dose, however, in case A1 in LBD standard dose of prescription of donepezil is not effective and in Case C1 in LBD that induce side effect.
From these two reasons, generic medicines of donepezil should not be permitted for LBD without proper clinical trials.
Conflict of Interest
Koji Hori received lecture fees from Eisai Co. Ltd., Pfizer Japan Inc., Novartis Pharma KK, Daiichi Sankyo Inc., Ono Pharmaceutical Co. Ltd., Janssen Pharmaceutical KK, Yoshitomi Yakuhin Co. Meiji Seika Pharma Co. Ltd., and Mitsubishi Tanabe Pharma Co. Mitsugu Hachisu received lecture fees from Meiji Seika Pharma Co. Ltd. and Mitsubishi Tanabe Pharma Co. Mchiho Sodenaga received lecture fees from Eisai Co. Ltd. However, the sponsors had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author Contributions
Koji Hori mainly coordinates the study regarding to AA or SAA. Michiho Sodenaga, approve the concepts of this article, gave advise and checked the article.
References
- McKeith IG, Boeve BF, Dickson DW, Halliday G, Taylor JP, et al. (2017) Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium. Neurology 89: 88-100.
Citation: Hori K, Sodenaga M (2018) Commentary: Not Aricept but Donepezil Should not Permit for Lewy Body Disease without Proper Clinical Trials. J Alzheimers Dis Parkinsonism 8: 447. DOI: 10.4172/2161-0460.1000447
Copyright: © 2018 Hori K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 3593
- [From(publication date): 0-2018 - Apr 25, 2025]
- Breakdown by view type
- HTML page views: 2803
- PDF downloads: 790