Combining Ability for Grain Yield, Agronomic Traits and Striga hermonthica Resistance of Yellow Endosperm Maize
Received: 30-Jun-2018 / Accepted Date: 07-Aug-2018 / Published Date: 17-Aug-2018
Keywords: Maize; Striga hermonthica; Combining ability; Resistance; Yield
Introduction
Maize is one of the most important food crops in the world and together with rice and wheat, provides at least 30% of the food calories to more than 4.5 billion people in 94 developing countries [1]. This crop is gaining momentum compared to other cereals, both in terms of productivity and use in human and animal food. In Mali, the area covered by maize is 803,136 ha with an average yield of 2.17 t/ha [2]. With this performance, Mali comes first in terms of productivity among the West African countries that produce maize [3]. However, this yield is low compared to Mexico 3.71 t/ha and the United States 10.96 t/ha [4]. This low yield is generally caused using open pollinated and local varieties, biotic and abiotic factors. One of the major biotic factors is Striga hermonthica. Since 1990, maize varieties production has been hampered by Striga. In 2005, a total of 25 African countries were infested by Striga as reported by De Groote et al. [5]. In Mali and other maize growing countries, the cultivated area infested by Striga ranged from 30% to 40% [6]. Farmers have reported losses between 20% and 80% and were eventually forced to abandon highly infested fields [7]. About 300 million people in Africa are affected by Striga damage [8,9] causing yield loss estimated at 10 million tons grain this loss can be economically estimated to $ US 7 billion [10,11].
There is need for high yielding hybrids with resistance to Striga hermonthica present on the farmer’s field.
The use of Striga resistant hybrids will increase maize production, productivity and lead to improved incomes and livelihoods of farmers as well as enhance the sustainability of the seed companies. Reports of genetic resistance to Striga have been reported for maize [12,13]. Inbreeds with stable resistance to Striga hermonthica could be useful as parents of hybrids for marketing in Striga hermonthica infested areas [14]. Striga resistant lines were introduced to Mali from IITA to develop high yielding hybrids with resistance to Striga hermonthica. These inbreeds must be evaluated for combining ability to develop hybrids which exhibit high heterosis. The combining ability is prerequisite for developing economically viable hybrid maize varieties. Genotypes that support reduced Striga hermonthica emergence can form an important basis for developing resistant hybrids. The broad objective of this study was to enhance the productivity of maize in Striga endemic areas. The specific objectives were (i) to identify parental lines for resistance to Striga and yield under Striga-infested and Striga-free conditions, (ii) to identify maize hybrids for resistance to Striga and yield under Striga-infested and Striga-free conditions and (iii) assess the general and specific combining ability of inbreed lines and their hybrids under Striga-infested and Striga-free conditions.
Material and Methods
Site description
The experiments under Striga-infested and Striga-free conditions were conducted at CRRA Sotuba (North-West) in central Mali at an altitude of 320 m and in Sanankoroba. Sotuba is situated in southern Mali at an altitude of 320 m, latitude 12°39’47’’ N, longitude 7°54’50’’ E and isohyet 600-1000 mm. The soil at this site is sandy with low water holding capacity, low inherent soil fertility and low organic matter content. Sanankoroba is situated in southern Mali at an altitude of 379 m (masl), latitude 12° 23’51.67’’ N, longitude 7°56’22.10’’ E. Sanankoroba is a Striga endemic zone in Mali and a preferred location for testing maize for responses to Striga hermonthica infestation. The experimental station of Sotuba has an infested field for evaluating genotypes response to Striga hermonthica infestation.
Planting materials
Fifteen Striga resistant maize inbreed lines and three testers with different reaction pattern to Striga hermonthica were crossed in line by tester fashion to generate 45 F1 hybrids in the Regional Agronomic Research Centre of Sotuba/ Mali. The inbreed lines and testers were obtained from the International Institute of Tropical Agriculture (IITA). The three testers were TZSTRI106, TZSTRI1207 and TZSTRI1033. They have different reaction to Striga hermonthica. Inbreed tester TZSTRI106 is a Striga resistant line derived from a backcross containing Zea diploperennis in its genome, TZSTRI1207 is a Striga tolerant line derived from a backcross containing a temperate inbreed line (B73) and TZSTRI1033 is a Striga susceptible line derived from a bi-parental cross between a temperate line (B73) and a line from Thailand (KI21).
Experimental design and field management
The hybrid trial was composed of 48 entries made up of 45 testcrosses obtained from a line by tester cross plus three hybrids checks. The checks included one tolerant hybrid, Mata (TZE-Y Pop DT STRC4 × TZEI 13) and two susceptible hybrids. Farako and Tieba. The 48 hybrids along with the 18 parents were evaluated in Sotuba and Sanankoroba during the growing season of 2014 and 2015 under Strigainfested and Striga-free conditions.
In each location, the 45 single cross hybrids and 3 checks were arranged in a 6 × 8 alpha lattice design with three replications and the parents were arranged in a RCBD with three replications. Hybrids and parents were randomized within each replicate. An experimental plot consisted of a 5m long single row with plants within a row spaced 0.25m apart and 0.75m distance between rows. The fields were planted with two seeds and later thinned to one plant per hill at two weeks after emergence to give a population density of 53,333 plants per hectare. A compound fertilizer at both Sotuba and Sanankoroba consisted of two applications. The first application was carried out 30 days after planting at the rate of 30 kg ha-1 each of N, P and K. Urea was used as top-dressing at the rate of 30 kg/ha-1 N two weeks later. Under Strigainfested environments weeds were manually controlled.
Artificial Striga infestation procedure
The artificial Striga infestation was carried as described by Kim [15] and Kim & Winslow [16]. Matured Striga plants were collected in infested maize field from previous season in Sanankoroba. Then the mature Striga plant were air dried for 7-9 days. After drying, the Striga plants were threshed and seed collected were stored for a minimum of six months to allow the conditioning of the seeds and breakage of dormancy. Germination test was conducted as described by Menkir [13] and germinable Striga seed were thoroughly mixed with finely sieved sand at the ratio 1:99 by weight. The sand served as the carrier and provided adequate volume for rapid and uniform infestation. For the field infestation, artificial inoculation with Striga seeds was carried out by digging small holes at the crop planting hill along the ridge and infesting with about 3000 germinable Striga seeds (8.5g sand/Striga mixture). Field infestation was done using by Menkir et al. [17] method. Apart from the Striga seed infestation, management practices were the same for both Striga-infested and non-infested plots.
Data Collection
Under both Striga-free and Striga-infested conditions, ten traits including grain yield (Yield), days to 50% silking (DYSK), days to 50% anthesis (DYTS), anthesis silking interval (ASI), ear aspect (EASP), ear height (EHT), ears per plant (EPP), plant aspect (PASP), plant height (PLHT) and husk tip cover (HUSK) were measured from each experiment at each location. Under Striga infestation, additional data were collected on Striga related traits such as Striga damage ratings (STRA) and Striga emergence count (STRC) at 8 and 10 weeks after planting (WAP). Striga damage rating was on a scale of 1-9 as described by Kim [18] where 1=Normal plant no visible symptoms growth, 2=Small and vague purplish- brown blotches visible leaf, 3=Mild leaf blotching with some purplish-brown necrotic spots, 4=Extensive blotching and mild wilting, slight but noticeable stunting and reduction in ear and tassel size, 5=Extensive leaf blotching wilting and some scorching moderate stunting; ear and tassel size reduction., 6=Extensive leaf scorching with mostly grey necrotic spots some stunting and reduction in stem diameter ear size and tassel size, 7=Definite leaf scorching with grey necrotic spots and leaf wilting and rolling severe stunting and reduction in stem diameter ear size and tassel size often causing stalk lodging brittleness and husk opening at a late growing stage, 8=Definite leaf scorching with extensive grey necrotic spots conspicuous stunting leaf wilting rolling severe stalk lodging and brittleness reduction in stem diameter ear size and tassel size and, 9=Complete scorching of all leaves causing premature death or collapse of host plant and no ear formation.
Ear aspect which is the assessment of the general appeal of the ears without the husks was rated on a scale of 1-9, where 1=excellent with no disease/insect damage, large cobs, uniform ears and fully filled grains, 2=very good with no disease/insect damage and fully filled grains, one or two irregularity in cob size, 3=good with no disease/insect damage and fully filled grains, one or two irregularity in cob size, 4=mild insect damage, no disease, fully filled grains, one or two irregularity in cob size poor, 5=mild disease/insect damage and fully filled grains, one or two irregularity in cob size, 6=severe disease/insect damage and fully filled grains, smaller cobs, non-uniform cob size, 7=severe disease/insect damage, scanty grain filling, few ears, non-uniformity of cobs, 8=severe disease/insect damage, scanty grain filling, very few ears and, 9=only one or no ears.
The factors considered included ear size; uniformity of size, color and texture; extent of grain filling and insect and disease damage.
Husk tip cover was rated on a scale of 1-5 where 1 indicates very tight husks extending beyond the tip and 5 indicates exposed ear tip.
Data Analysis
SAS was used to perform analysis of variance for alpha lattice design.
The analysis of combining ability was based on the model described by Kempthorne, Comstock & Robinson [19,20]. The general combining ability (GCA) and specific combining ability (SCA) effects were estimated for each environment and across environments.
The statistical model used for the combined analysis is as follows:
a. Model of combining ability for each environment
Yijk=μ+rk+fi+mj+ (f x m) ij+eijk
Yijk: The observed measurement for the kth replication of the ixjth progeny; μ: experimental mean; fi: is the effect of the ith line (GCAlinei); I=1, 2, 3….21; tj: is the effect of the jth tester (GCAtesterj); j=1, 2, 3; (f x m) ij: is the interaction effect of the ith line with the jth male (SCAij); rk: effect replication within environment; k=1, 2; eijk: is the error effect associated with the ijkth observation;
b. Model of combining ability for across environments
Yijkm=μ+rk+li+tj+ (l x m) ij + (f x s) im + (t x s) jm + (l x t x s) ijm+eijkm
Yijkm: The observed measurement for the kth replication at the mth environment of the ixjth progeny; (l x s) im: is the interaction effect of the ith line and mth environment; im=1….n; (t x s) jm: is the interaction effect of the jth tester and mth environment; jm=1……n; (l x t x s) ijm: is the interaction effect of the ith line and jth tester at the mth environment; ijm=1, n; rk: effect replication within environment; k=1,..n; eijkm: is the error effect associated with the ijkmth observation [21];
c. Estimation of GCA and SCA effects
GCA was computed as:
GCAl=Xl – μ and
GCAt=Xt – μ
Xl and Xt=Mean of female and male respectively
GCAl and GCAt=General combining ability of female and male respectively; μ = Overall mean of crosses in the trial
SCA will be computed as:
SCAij=Xi – Ej=SCAij=Cross (ij) mean – [GCAlinei+GCAtesterj+μ]
Xi=Observed mean value of the cross; Ej=Expected mean value of the cross based on the 2 GCAs of its parents;
Ij=crosses, ij=1…n
Grain yield under Striga-infested environments was calculated as follows [22]: GY=fwt × ((100-m))/85 × 10000/ ((Ȣ ×Φ)) ×0.8
Where, GY=grain yield (kg ha-1); Fwt=field weight of harvested ears per plot (kg); m=moisture content grain at harvest 10,000=land area per hectare (m2); Ȣ=area harvested per plot (0.75 m × 0.25 m × 18), 0.75 m is the larger of a row and 0.25 m is the distance between 2 holes and 18 is the number of inner plants from the 20 plants per plot which will be harvested. Φ=number of hills/plot (20) and 0.80=shelling percentage. 85=is the adjustment of grain yield at 15% moisture content
Results
Combining ability of lines × testers under Striga-infested and Striga-free conditions
The genotypes effects were significant (P ≤ 0.05) for most traits under Striga-infested and Striga-free conditions except ASI under Striga-free conditions.
Lines and tester mean square were significant for all traits under Striga-infested and Striga-free conditions except ASI of line under Striga-free conditions, STRA at 8 and 10 WAP and STRC at 8 and 10 WAP under Striga-infested, ASI of tester under Striga-free conditions, and Yield and ASI of tester under Striga-infested (Table 1).
| Striga-free conditions | Striga-infested conditions | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sources of variation | d.f | Grain Yield | ASI | PLHT | Grain Yield | ASI | PLHT | STRA 8 WAP | STRA 10 WAP | STRC 8 WAP | STRC 10 WAP |
| Site | 3 | 1049282.4ns | 6.13* | 99540.36** | 110066912.3** | 28.17** | 75382.52** | 30.83** | 13.90* | 4.31** | 0.95* |
| Year | 1 | 140153317.9** | 28.47** | 23522.62** | 7382736** | 8.07* | 298249.47** | 14.34** | 6.67ns | 2.46** | 0.41* |
| GCALine | 14 | 134252052.4** | 0.55ns | 1236.64** | 1349627.2** | 2.47* | 1152.40** | 1.30ns | 1.42ns | 0.05ns | 0.05ns |
| GCATester | 2 | 1566743.8* | 0.10ns | 5219.81** | 494986.6ns | 0.64ns | 3954.08** | 10.96* | 7.12* | 1.07** | 1.33** |
| Site x GCALine | 42 | 13644832.3** | 2.61* | 374.04ns | 584004.9ns | 2.25* | 783.23** | 1.25ns | 1.29ns | 0.12ns | 0.15ns |
| Year x GCALine | 14 | 716423.5ns | 0.77ns | 1102.84** | 2040459.2** | 1.83* | 693.28* | 1.28ns | 0.96ns | 0.04ns | 0.07ns |
| Site x GCATester | 6 | 1745432.1* | 4.23* | 2362.69** | 676138.2ns | 11.76** | 7967.86** | 0.74ns | 4.80ns | 0.06ns | 0.03ns |
| Year x GCATester | 2 | 3782049.3* | 1.64ns | 5036.05** | 597430.2ns | 1.05ns | 2611.05** | 4.10ns | 1.81ns | 0.48* | 0.90ns |
| SCA | 28 | 19451800.3** | 2.07* | 1194.59** | 1102226.8** | 2.00* | 529.23** | 1.69ns | 1.43ns | 0.12ns | 0.11ns |
| Year x SCA | 28 | 3841517.5** | 1.85* | 1054.69** | 1729755.2** | 1.67* | 351.06ns | 1.14ns | 1.15ns | 0.10ns | 0.11ns |
| Site x SCA | 84 | 4172841.8** | 3.05** | 741.97** | 828702.7** | 2.33** | 544.54* | 2.40* | 2.30ns | 0.10ns | 0.09ns |
| Year x Site x SCA | 135 | 1625563.5** | 2.93** | 2616.78** | 3842522** | 4.09** | 3204.97** | 2.81** | 2.68* | 0.36** | 0.23** |
| Pooled error | 718 | 6278365.6** | 1.20 | 283.66 | 389173.5 | 1.1 | 334.56 | 1.4 | 1.91 | 0.09 | 0.11 |
Table 1: Mean squares of grain yield and other traits of single cross hybrids under Striga-free and Striga-infested conditions. *Significant at P=0.05; **Significant at P=0.01; GY= grain yield; EPP=number of ears per plant; PLHT=plant height; ASI=anthesis-silking interval; EASP=ear aspect; EHT=ear height and PASP=plant aspect, STRA 8=Striga damage rating at 8 WAP; STRA 10=Striga damage rating at 10 WAP; STRC 8=Striga emergence count at 8 WAP; and STRC 10=Striga emergence count at 10 WAP; WAP=week after planting.
Line and tester by site interactions were significant for most traits under Striga-infested and Striga-free conditions except PLHT of line by site under Striga-free conditions, and yield and STRA and STRC at 8 and 10 WAP of line by site under Striga-infested conditions; yield and STRA and STRC at 8 and 10 WAP of tester by site under Striga-infested conditions.
Line and tester by year interactions were significant for most traits under Striga-infested and Striga-free conditions except yield and ASI of line by year under Striga-free conditions, and STRA and STRC at 8 and 10 WAP of line by year under Striga-infested conditions; yield and STRA and STRC at 8 and 10 WAP of tester by year under Striga-infested conditions, and ASI of tester by year under Striga-free conditions, and yield and STRA and STRC at 8 and 10 WAP under Striga-infested conditions. Line, tester, line by year and tester by year mean square were not significant for ASI under Striga-free conditions. Line × tester mean square was significant for all trait under Striga-free condition but was not significant for STRA and STRC at 8 and 10 WAP under Strigainfested conditions. Line x tester by site interactions were significant for all trait under Striga-free condition but was not significant for STRA at 10 WAP and STRC at 8 and 10 WAP under Striga-infested conditions. Line × tester by year interactions were significant for all trait under Striga-free condition but was not significant for PLHT, STRA at 8 and 10 WAP and STRC at 8 and 10 WAP under Striga-infested conditions.
SCA mean squares were larger than GCA mean squares for grain yield, days to silking, anthesis-silking interval, plant height, ear aspect, plant aspect and husk cover (Table 2).
| Genotypes | Striga-free conditions | Striga-infested conditions | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| GY | ASI | PLHT | GY | ASI | PLHT | STRA8 WAP | STRA10 WAP | STRC8 WAP | STRC10 WAP | |
| TZISTR110 | -327.82 | 0.57 | 1.08 | 265.00 | 0.04 | 1.38 | 0.10 | -0.07 | -0.27** | 0.04 |
| TZISTR112 | 158.03 | -0.43 | -2.49 | 59.25 | 0.40 | -1.68 | 0.02 | 0.24 | 0.01 | -0.02 |
| TZISTR113 | -237.15 | -0.16 | -3.00 | -152.26 | -0.16 | -6.98 | -0.26 | -0.10 | -0.1 | 0.07 |
| TZISTR1028 | 532.33** | -0.02 | 0.04 | -200.90 | -0.16 | -7.31 | -0.04 | 0.26 | 0.37** | -0.02 |
| TZISTR1211 | -506.22** | 0.34 | 0.71 | -307.74* | 0.04 | -6.07 | -0.01 | 0.04 | -0.21** | 0.05 |
| TZISTR1214 | 272.48 | -0.02 | -1.52 | 52.01 | -0.1 | -4.09 | -0.23 | -0.04 | 0.15* | 0.03 |
| TZISTR1218 | -87.76 | -0.24 | -3.80 | -28.69 | -0.16 | 9.42 | 0.13 | -0.04 | -0.18** | -0.05 |
| TZISTR1222 | 240.47 | 0.12 | 11.53** | 288.31 | -0.08 | 13.02** | 0.07 | 0.07 | 0.04 | 0.08 |
| TZISTR1223 | 647.59** | -0.16 | -0.4 | 302.45* | -0.21 | 1.09 | 0.07 | 0.04 | -0.35** | 0.06 |
| TZISTR1226 | 36.38 | -0.16 | 3.35 | -220.19 | -0.02 | -1.45 | -0.29 | -0.15 | 0.09 | 0.02 |
| TZISTR1227 | 156.24 | -0.02 | 1.96 | -97.21 | -0.1 | 6.96 | 0.10 | -0.04 | -0.32** | -0.08 |
| TZISTR1230 | -83.08 | 0.01 | -5.55 | -21.84 | 0.12 | -2.63 | 0.07 | 0.01 | 0.01 | -0.04 |
| TZISTR1235 | -121.56 | 0.07 | 1.63 | 6.11 | 0.34 | -2.5 | 0.24 | -0.04 | 0.43** | 0.00 |
| TZISTR1237 | -248.27 | -0.13 | -1.88 | -28.90 | -0.13 | 1.57 | -0.04 | -0.1 | 0.15* | -0.05 |
| TZISTR1238 | -431.65** | 0.21 | -1.65 | 84.60 | 0.20 | -0.74 | 0.07 | -0.07 | 0.18* | -0.09 |
| SE ± line | 192.74 | 0.37 | 4.40 | 174.02 | 0.34 | 6.37 | 0.25 | 0.26 | 0.08 | 0.09 |
| TZISTR1033 | -240.35 | -0.06 | 2.81 | -58.76 | -0.06 | -4.07 | -0.03 | 0.1 | 0.36** | 0.13** |
| TZISTR106 | 44.24 | 0.01 | -4.07 | 9.74 | 0.01 | 2.81 | -0.09 | -0.37* | -0.31** | -0.06** |
| TZISTR1207 | 196.11 | 0.06 | 1.26 | 49.02 | 0.06 | 1.26 | 0.12* | 0.27 | -0.05** | -0.07** |
| SE ± testers | 167.38 | 0.18 | 4.18 | 70.77 | 0.30 | 7.68 | 0.07 | 0.19 | 0.02 | 0.01 |
Table 2: General combining ability effects of lines and testers under Striga-free and Striga-infested conditions. *Significant at P=0.05; **Significant at P=0.01; GY=grain yield; ASI=anthesis-silking interval; PLHT=plant height; STRA 8=Striga damage rating at 8 WAP; STRA 10=Striga damage rating at 10 WAP; STRC 8=Striga emergence count at 8 WAP; and STRC 10=Striga emergence count at 10 WAP; WAP=week after planting.
Mode of gene action controlling measured traits
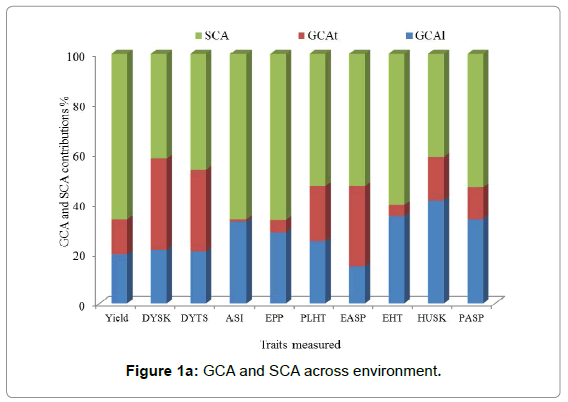
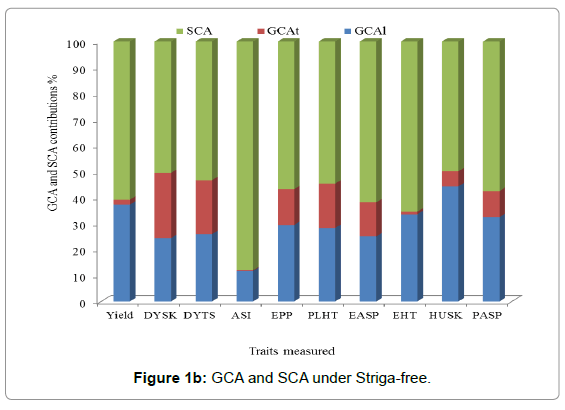
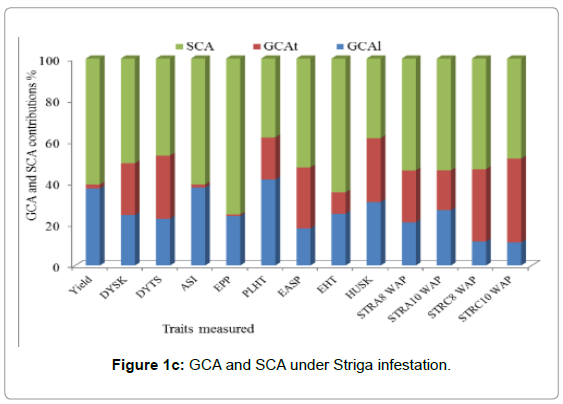
The proportion of the GCA over the total genetic effect of the sum of squares was used to determine the relative importance of GCA and SCA effects. The predictability based on GCA [23] is higher when the ratio is almost equal to one. Across environments the SCA percent contribution was greater than GCA line plus GCA tester percent contribution for most traits except DYSK, DYTS, and Husk. The SCA percent contribution varied from 67% (grain EPP) to 53% (PLHT and EASP). GCA line percent contribution varied from 41% (Husk) to 15% (EASP). The line percent contribution was the highest from Husk (41%) followed by EHT (35%), PASP (34%), ASI (33%), EPP (28%), PLHT (25%), and grain yield (20%), respectively. While the contribution of tester varied from 37% (DYSK) to 1% (ASI) (Figure 1a). Under Striga-free conditions, the relative contribution of SCA was greater than GCA (GCA line +GCA tester) for all traits measured. The highest SCA percent contribution was 87.93% (ASI) and the lowest percent contribution was 50.54% (DYSK). Lines percent contribution varied from 44% (Husk) to 12% (ASI), the lines contribution was greater than the testers contribution for all traits measured (Figure 1b) under Striga-free conditions. Under Striga-infested conditions the percent contribution of SCA was greater for grain yield and Striga related traits (Figure 1c). The lines and testers contributed similarly for husk tip cover. However, the relative contribution for lines was greater for GY, ASI, PLHT, EHT and STRA 10WAP.
GCA effects of line and testers for various traits under Strigainfested and Striga-free conditions. Among the lines, TZISTR112, TZISTR1214, TZISTR1222 and TZISTR1223 exhibited positive GCA effects for GY under Striga-infested and Striga-free conditions. Among the testers, TZISTR106 and TZISTR1207 exhibited positive GCA effects for GY under Striga-infested and Striga-free conditions. Parental line, TZISTR1223 and tester TZISTR1207 manifested desirable GCA effect for GY and STRC. Also, parental line, TZISTR1214 exhibited desirable GCA for STRA. Lines, TZISTR110, TZISTR113, TZISTR1218, TZISTR1227 and tester, TZISTR106 exhibited desirable GCA for STRA and STRC (Table 2).
Six crosses exhibited significant positive SCA effects for grain yield while seven had negative SCA effects under Striga-free conditions (Table 3). Cross TZISTR106/TZISTR1230 recorded the highest positive SCA effect for grains yield while the lowest was recorded by the cross TZISTR1207/TZISTR1222. Seven crosses displayed significant negative SCA effects for both DYSK and DYTS, four had negative and three positive SCA effects. Eight crosses showed significant SCA for ASI; four had negative and four positive SCA effects. For EPP, seven crosses displayed significant SCA effects, five had negative and two positive SCA effects. Six crosses showed significant SCA for PLHT; four had negative and two positive SCA effects.
| Genotypes | Striga-free conditions | Striga-infested conditions | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| GY | ASI | PLHT | GY | ASI | PLHT | STRA8 WAP |
STRA10 WAP |
STRC8 WAP |
STRC10 WAP |
|
| TZISTR1033/TZISTR1227 | 737.81** | 0.12ns | 2.48ns | 609.03** | 0.18ns | -5.75ns | -0.24ns | 0.03ns | -0.01ns | 0.02ns |
| TZISTR106/TZISTR1218 | 695.98** | 0.08ns | -5.15ns | 548.50** | 0.08ns | 0.63ns | 0.06ns | 0.06ns | -0.03ns | -0.10ns |
| TZISTR1207/TZISTR1214 | 557.29** | 0.10ns | 6.62* | 508.43** | 0.22ns | 4.68ns | 0.17ns | 0.13ns | -0.01ns | 0.01ns |
| TZISTR1033/TZISTR1235 | 458.76** | 0.79** | 4.04ns | 454.00** | 0.15ns | -7.08* | -0.15ns | -0.39ns | -0.02ns | -0.08ns |
| TZISTR1033/TZISTR1223 | 331.95* | 0.26ns | -7.65** | 302.44** | -0.05ns | -4.78ns | -0.24ns | -0.11ns | 0.04ns | 0.07ns |
| TZISTR1033/TZISTR1028 | 324.88** | 0.12ns | -2.66ns | 300.76** | -0.19ns | 5.37ns | 0.29ns | 0.33ns | -0.03ns | 0.03ns |
| TZISTR106/TZISTR1237 | 269.82ns | 0.06ns | -0.20ns | 299.03** | 0.30ns | 4.28ns | 0.04ns | -0.19ns | 0.00ns | 0.07ns |
| TZISTR1207/TZISTR113 | 269.49ns | -0.34ns | -2.82ns | 287.79* | 0.11ns | -3.04ns | 0.48* | 0.63* | 0.06ns | -0.04ns |
| TZISTR1207/TZISTR1226 | 264.72ns | -0.01ns | 3.56ns | 241.47* | 0.30ns | 4.61ns | -0.30ns | -0.14ns | -0.09ns | -0.08ns |
| TZISTR1033/TZISTR113 | 234.74ns | 0.26ns | 4.73ns | 195.27ns | -0.10ns | 11.37** | -0.26ns | -0.44* | 0.03ns | 0.07ns |
| TZISTR106/TZISTR1222 | 233.17ns | -0.11ns | 0.77ns | 194.00ns | -0.34ns | -1.90ns | 0.04ns | -0.33ns | -0.03ns | 0.00ns |
| TZISTR106/TZISTR1230 | 222.60ns | -0.17ns | 5.02ns | 191.09ns | 0.30ns | 2.73ns | 0.43* | 0.37ns | 0.01ns | 0.02ns |
| TZISTR106/TZISTR1226 | 200.56ns | 0.17ns | 6.59* | 183.54ns | -0.06ns | 5.47ns | 0.34ns | 0.45ns | 0.01ns | 0.07ns |
| TZISTR1207/TZISTR1218 | 171.60ns | -0.09ns | 3.18ns | 170.66ns | -0.14ns | -6.35* | -0.08ns | -0.12ns | 0.04ns | 0.10ns |
| TZISTR1033/TZISTR110 | 136.57ns | 0.37* | 1.54ns | 154.76ns | 0.12ns | -1.41ns | 0.96** | -0.03ns | -0.13* | -0.10ns |
| TZISTR1207/TZISTR112 | 108.78ns | 0.35ns | 5.46ns | 138.80ns | 0.22ns | -3.13ns | 0.23ns | -0.14ns | -0.09ns | -0.05ns |
| TZISTR1033/TZISTR1222 | 100.22ns | 0.15ns | 10.12** | 114.47ns | 0.15ns | 11.93** | -0.26ns | 0.58* | 0.05ns | 0.03ns |
| TZISTR1033/TZISTR112 | 65.69ns | -0.54** | -4.06ns | 99.53ns | 0.18ns | 1.69ns | -0.18ns | 0.11ns | 0.05ns | 0.07ns |
| TZISTR1207/TZISTR1237 | 56.32ns | 0.21ns | -3.91ns | 76.57ns | -0.17ns | 0.37ns | -0.44* | -0.37ns | -0.09ns | -0.07ns |
| TZISTR106/TZISTR1214 | 52.84ns | 0.11ns | -7.34* | 44.93ns | -0.40* | -7.43* | -0.19ns | 0.06ns | 0.04ns | 0.03ns |
| TZISTR1207/TZISTR1238 | 47.67ns | -0.29ns | 3.32ns | 41.45ns | 0.08ns | 3.46ns | 0.11ns | 0.02ns | -0.01ns | -0.04ns |
| TZISTR106/TZISTR1211 | 33.08ns | -0.08ns | 0.21ns | 41.09ns | 0.13ns | 1.71ns | -0.27ns | -0.16ns | -0.01ns | -0.07ns |
| TZISTR1033/TZISTR1211 | 24.75ns | -0.57** | 5.07ns | 21.71ns | -0.38* | -1.37ns | 0.10ns | 0.00ns | -0.07ns | -0.02ns |
| TZISTR106/TZISTR1238 | -12.73ns | 0.31ns | -1.56ns | 20.97ns | -0.45* | -7.73* | 0.34ns | -0.05ns | 0.01ns | 0.03ns |
| TZISTR1207/TZISTR1235 | -48.99ns | -0.48* | -5.40ns | -37.25ns | -0.31ns | 1.52ns | 0.00ns | 0.36ns | 0.06ns | 0.07ns |
| TZISTR106/TZISTR1223 | -80.76ns | -0.08ns | 3.78ns | -50.30ns | 0.13ns | 10.51** | -0.02ns | 0.39ns | -0.05ns | -0.07ns |
| TZISTR1033/TZISTR1238 | -87.58ns | -0.02ns | -1.76ns | -62.42ns | 0.37* | 4.27ns | -0.46* | 0.03ns | 0.00ns | 0.01ns |
| TZISTR1207/TZISTR1211 | -128.16ns | 0.49* | 3.48ns | -62.79ns | 0.25ns | -0.34ns | 0.17ns | 0.16ns | 0.08ns | 0.09ns |
| TZISTR1207/TZISTR110 | -153.85ns | 0.11ns | -5.19ns | -65.30ns | -0.09ns | 2.10ns | -0.55* | 0.47* | 0.18** | 0.13* |
| TZISTR1207/TZISTR1230 | -172.96ns | 0.03ns | 2.94ns | -74.67ns | 0.00ns | 5.24ns | -0.47* | -0.14ns | 0.02ns | 0.05ns |
| TZISTR106/TZISTR110 | -178.10ns | -0.32ns | -8.50** | -89.46ns | -0.04ns | -0.70ns | -0.41* | -0.44* | -0.05ns | -0.03ns |
| TZISTR106/TZISTR1028 | -196.72ns | -0.39* | 2.06ns | -109.02ns | 0.41* | -6.38* | -0.07ns | -0.41ns | 0.09ns | 0.03ns |
| TZISTR1033/TZISTR1230 | -225.31ns | -0.15ns | -0.28ns | -116.42ns | -0.30ns | -7.98* | 0.04ns | -0.22ns | -0.04ns | -0.08ns |
| TZISTR106/TZISTR1227 | -251.99ns | 0.19ns | -1.40ns | -136.23ns | 0.19ns | 0.14ns | -0.19ns | 0.37ns | 0.09ns | 0.06ns |
| TZISTR1207/TZISTR1028 | -280.38ns | -0.18ns | 3.86ns | -191.74ns | -0.22ns | 1.01ns | -0.22ns | 0.08ns | -0.06ns | -0.07ns |
| TZISTR106/TZISTR112 | -306.11ns | -0.04ns | -10.89** | -238.33* | -0.40* | 1.44ns | -0.05ns | 0.03ns | 0.05ns | -0.02ns |
| TZISTR1207/TZISTR1223 | -334.95* | -0.27ns | 4.11ns | -252.14* | -0.09ns | -5.73ns | 0.25ns | -0.28ns | 0.00ns | -0.01ns |
| TZISTR1207/TZISTR1222 | -349.75* | -0.31ns | 1.36ns | -308.47** | 0.19ns | -10.03** | 0.23ns | -0.26ns | -0.02ns | -0.03ns |
| TZISTR1033/TZISTR1237 | -388.07* | -0.16ns | -10.16** | -375.60** | -0.13ns | -4.64ns | 0.40* | 0.56* | 0.09ns | 0.00ns |
| TZISTR106/TZISTR1235 | -389.88* | 0.27ns | -4.55ns | -416.75** | 0.16ns | 5.56ns | 0.15ns | 0.03ns | -0.04ns | 0.01ns |
| TZISTR1033/TZISTR1226 | -455.76* | 0.08ns | -1.91ns | -425.01** | -0.24ns | -10.08** | -0.04ns | -0.31ns | 0.08ns | 0.01ns |
| TZISTR1207/TZISTR1227 | -622.97* | -0.21ns | 0.72ns | -472.80** | -0.36* | 5.61ns | 0.42* | -0.39ns | -0.08ns | -0.07ns |
| TZISTR106/TZISTR113 | -728.59** | 0.01ns | 1.97ns | -483.07** | -0.01ns | -8.34* | -0.21ns | -0.19ns | -0.09ns | -0.03ns |
| TZISTR1033/TZISTR1214 | -128.16ns | 0.49* | 3.48ns | -553.36** | 0.18ns | 2.75ns | 0.01ns | -0.19ns | -0.04ns | -0.04ns |
| TZISTR1033/TZISTR1218 | -153.85ns | 0.11ns | -5.19ns | -719.16** | 0.06ns | 5.72ns | 0.01ns | 0.06ns | -0.01ns | 0.00ns |
| SE± | 127.3403 | 0.22316 | 3.43793 | 293.15 | 0.49 | 7.51 | 0.50 | 0.49 | 0.10 | 0.10 |
Table 3: Specific combining ability of crosses for yield and other traits under Striga-free and Striga-infested conditions. *Significant at P=0.05; **Significant at P=0.01; GY, grain yield; DYSK=days to 50% silking; DYTS=days to 50% anthesis; EPP=number of ears per plant; PLHT=plant height; ASI=anthesis-silking interval; EASP=ear aspect; EHT=ear height; HUSK=husk tip cover; PASP=plant aspect; STRA 8=Striga damage rating at 8 WAP; STRA 10=Striga damage rating at 10 WAP; STRC 8=Striga emergence count at 8 WAP; and STRC 10=Striga emergence count at 10 WAP; WAP=week after planting.
Twenty-four hybrids showed significant SCA for EASP; eleven had negative and thirteen showed positive SCA effects. The entire crosses showed significant SCA for EHT; twenty had negative and twenty-four showed positive SCA effects. Twelve hybrids showed significant SCA for PASP; half had negative and the other half had positive SCA effects (Table 3).
Under Striga-infested condition; nineteen crosses exhibited significant SCA effects for grain yield; ten had negative and nine displayed positive SCA effects. Cross TZISTR1033/TZISTR1227 recorded the highest positive SCA effect for grains yield while the lowest was recorded by the cross TZISTR1207/TZISTR1226. Twelve crosses displayed significant negative SCA effects for both DYSK and DYTS, eight had negative and four positive SCA effects. Seven crosses showed significant SCA for ASI; five had negative and two positive SCA effects. For EPP, twelve crosses displayed significant SCA effects, seven had negative and five positive SCA effects. Twelve crosses showed significant SCA for PLHT; nine had negative and three positive SCA effects. Thirteen hybrids showed significant SCA for EASP; six had negative and seven showed positive SCA effects. Except TZISTR1207/ TZISTR1214 and TZISTR106/TZISTR110, all the other crosses showed significant SCA for EHT; eighteen had negative and twenty-five showed positive SCA effects. Twenty-five hybrids showed significant SCA for PASP; thirteen had negative and the twelve had positive SCA effects. Crosses TZISTR1207/TZISTR113 and TZISTR1033/TZISTR1237 showed significant positive SCA effects negative for Striga damage ratings at 8 and 10 WAP, while TZISTR106/TZISTR110 showed significant negative SCA effects. Sixteen crosses showed negative SCA effect for Striga emergence counts at 8 and 10 WAP (Table 3).
Discussion
In the present study a desirable line and tester for resistance to Striga would show negative GCA effects for Striga damage ratings and Striga emergence counts and positive GCA effects for grain yield under Striga-infested conditions.
There were significant environmental effects for all the parameters measured. The significant environmental variation for all traits under both Striga-infested and Striga-free conditions indicates that each environment was unique and highly variable, emphasizing the need for testing in more than one environment over several years. Similarly, the significant genotype x environment interactions detected for grain yield and most other traits is an indication that the inbred lines should be tested in several environments to identify stable, Striga resistant inbred lines for hybrid production. Similar results were reported by Menkir et al., Badu-Apraku et al. and Ifie et al. [14,24-27].
GCA lines and GCA testers mean squares were significant for all traits except ears per plant. Both GCA for inbreed and SCA effects for hybrids were significant (P<0.05) for yield under Striga-infested and Striga-free conditions, indicating the importance of additive and nonadditive effects for controlling grain yield. This finding corroborates with Menkir [13] who reported that Striga resistance is controlled by non- additive gene action. GCA tester was greater than GCA line for some traits except for grain yield and ASI, under both conditions. This indicates that the major contribution of additive variance for grain yield and ASI was due to the line. This finding disagrees with Duarte et al. [28] who suggested that the improvement of grain yield is under the higher frequency of favorable alleles for testers than lines.
Parental lines TZISTR112, TZISTR1028, TZISTR1214, TZISTR1222, TZISTR1223, TZISTR1226 and TZISTR1227 were the best general combiners for grain yield under Striga-free condition. These lines have favorable alleles for yield and can be used in maize breeding programs to develop high yielding hybrid maize for farmers. Lines TZISTR113, TZISTR1028, TZISTR1214, TZISTR1218, TZISTR1223, TZISTR1226, TZISTR1227, TZISTR1237 and tester TZISTR1033 had negative value of ASI under both Striga-infested and Striga-free conditions. The highly significant line x tester means squares for grain yield indicate that nonadditive gene effects must be considered if maximum improvement of yield is to be achieved. These results are similar to those of Gethi and Smith, Yallou et al. and Badu-Apraku et al. [29-31] who showed that SCA effects is more important than GCA effects for host plant damage inheritance. The significant GCA line and GCA tester and SCA for grain yield and other traits under Striga infestation indicates that there were differences in the performance of the inbred lines as parents in hybrid combinations. The non-significant GCA tester x site and GCA line × site interactions for most traits under Striga infestation indicate that the performance of crosses between parental lines were stable across the Striga environments. This suggests that the selection of superior Striga resistant hybrid is better across different Striga environments. This finding disagrees with the finding of Makumbi et al. [32]. Striga emergence had low SCA effects for all the hybrids which indicate good resistance to Striga emergence under the infestation conditions. This agrees with Adeosun et al. [33] who reported that tolerant plants have little Striga emergence. While Kim [18] recommended Striga damage ratings for assessing crop genotypes for tolerance to Striga infestation. Furthermore, Rodenburg and Bastiaans, Badu-Apraku and Lum [34,35] concluded that resistant maize cultivars should be able to support few emerged parasites and sustain low STRA reduced emergence, resulting from effective host-plant resistance, which is a good strategy for longterm control of Striga in Africa.
Expression of genetic variability for traits associated with resistance to Striga hermonthica in maize, including grain yield under Striga infestation, host plant damage symptom rating and number of emerged Striga plants, is largely dependent on the presence of severe infection with the parasite. In this study, there were significant GCA lines and SCA mean squares for all traits except ears per plant, Striga damage rating and Striga emergence count. This indicates the presence of genotypic variability among inbred lines used as female parents. This finding is in disaccord with finding of Gethi and Smith [29] who reported significant GCA mean squares for Striga emergence counts and non-significant GCA mean squares for Striga damage rating. The proportion of the SCA mean squares over GCA for grain yield and most other traits under Striga infestation indicates that non-additive as well as additive effects are important and that non-additive genetic effects were more important than additive effects. This is consistent with the findings of Badu-Apraku et al. and Choukan [36,37] that GCA and SCA are mostly used to identify inbred line with good characters. Lines, TZISTR1214, TZISTR1226 and TZISTR1237 exhibited desirable negative GCA effects for Striga damage rating. However, lines TZISTR110, TZISTR113, TZISTR1218, TZISTR1227 and tester, TZISTR106 exhibited desirable negative GCA effects for Striga damage rating and Striga emergence count making them good combiners for maize Striga resistance traits and can be used to improve maize for Striga resistance. Lines TZISTR1214, TZISTR1223, tester TZISTR106 and TZISTR1207 had significant positive GCA effect for grain yield and negative effect for Striga damage rating and Striga emergence count. These lines and testers are good combiners for grain yield and maize Striga resistance traits. Testers TZISTR106 and TZISTR1207 resistant and tolerant to Striga respectively, had significant negative GCA effect for Striga emergence count while the susceptible Tester TZISTR1033 had significant positive GCA effect for STRC. This is in disagreement with Rodenburg and Bastiaans [34] who suggested that Striga emergence count would not be a sufficient criterion to point out genetic control of Striga tolerance of maize.
Lines TZISTR113, TZISTR1218 and TZISTR1227 had significant negative effect for grain yield and negative effect for Striga counts, they can be utilized as source of Striga resistance in maize breeding. Significant negative GCA for ASI indicates that the silk and pollen shed are done together ensuring good synchronization. Line TZISTR112, testers TZISTR106 and TZISTR1207 had significant positive effect for grain yield and negative effect for ASI, these line and testers had pollen grain and silking appearing at the same time which ensures good synchronization under Striga infestation despite the fact that the parasitic weed can delay flowering period. They are therefore suitable for hybrid seed production. Testers TZISTR106 and TZISTR1207 had positive GCA effect for grain yield this finding is in agreement with finding of Karaya et al. [38]. Lines TZISTR1222, TZISTR1223, testers TZISTR106 and TZISTR1207 had positive GCA for grain yield and negative effects for plant height indicating that they can resist to plant height reduction due to Striga effect on plant.
Reduced emergence, resulting from effective host-plant resistance, is a good strategy for long-term control of Striga in Africa. Host plant damage rating refers to the general appearance of a host plant caused by Striga [18]. The specific combining ability results indicated that hybrids TZISTR1033/TZISTR1235, TZISTR1207/TZISTR1226, TZISTR1033/ TZISTR110, TZISTR1207/TZISTR112, TZISTR1207/TZISTR1237 and TZISTR106/TZISTR1211 are good specific combiners for grain yield and maize Striga related traits. TZISTR106/TZISTR110 showed significant negative SCA effects for Striga damage rating. In this study, hybrids resistant to Striga hermonthica were developed from resistant tester (TZISTR106) × resistant lines, tolerant tester (TZISTR1207) × resistant line and susceptible tester (TZISTR1033) × resistant line. TZISTR1033/TZISTR1227, TZISTR106/TZISTR1218, TZISTR1207/ TZISTR1214, TZISTR1033/TZISTR1235, TZISTR1033/TZISTR1223 and TZISTR1033/TZISTR1028 had significant positive SCA effect for grain yield under Striga-free and Striga-infested conditions.
Both GCA for inbred and SCA effects for hybrids were significant (P<0.05) for grain yield under Striga-free conditions, indicating the importance of additive and non-additive effects for controlling grain yield. This finding agrees with Derera et al. [39] on maize hybrids yield under drought conditions.
In this study, testers were used to evaluate the combining abilities of lines; therefore, negative GCA estimates for these testers would be more interesting because the better expression of the favorable alleles from different lines depends on the frequency of unfavorable alleles from the testers as reported by Barata and Carena [40]. While crosses including TZISTR1033/TZISTR1214, TZISTR1207/TZISTR1222, TZISTR1207/ TZISTR1235, TZISTR106/TZISTR1208, TZISTR106/TZISTR1223 and TZISTR106/TZISTR1230 were the best specific combiners for grain yield. Among them, TZISTR1207/TZISTR1222, TZISTR1207/ TZISTR1235 and TZISTR106/TZISTR1230 were the best specific combiners for resistance to stalk lodging. TZISTR106/TZISTR1230 was the best specific combiners for earliness.
Conclusion
For the 18 inbred lines studied, SCA was greater than GCA line and GCA tester. Non-additive gene action plays a predominant role in the inheritance of grain yield and most traits under Striga infestation. GCA line effects were more important for grain yield, anthesis-silking interval and plant and ear height than GCA tester effects under Striga infestation. Inbred parents TZISTR112, TZISTR1214, TZISTR1222, TZISTR1223, tester TZISTR106 and TZISTR1207 were the best general combiners for grain yield under both Striga-infested and Striga-free conditions. These lines could be used for heterosis breeding in maize. Lines TZISTR113, TZISTR1214, TZISTR1226 and TZISTR1237 were identified as best combiners for Striga damage ratings at 8 and 10 WAP. Inbred line TZISTR1218 and TZISTR1227 were the best general combiner for Striga emergence count at 8 and 10 WAP. These lines could be exploited in maize breeding programs as they have beneficial alleles for resistance to Striga. Hybrids with yield higher than the check were identified.
Acknowledgement
The authors wish to acknowledge Alliance for Green Revolution in Africa (AGRA) for funding this work. The authors also acknowledge IITA (International Institute for Tropical Agriculture) Ibadan, Nigeria for supplying germplasm for this work, WACCI (West Africa Centre for Crop Improvement) University of Ghana and IER (Institut d’Economie Rurale) my home institution.
References
- Shiferaw B, Prasanna BM, Hellin J Bänziger M (2011) Crops that feed the world 6. Past successes and future challenges to the role played by maize in global food security. Food Security 3: 307-327
- Faostat (2016) Statistical Yearbook 2016 World Food and Agriculture. FAO Food Agric. Organziation UN Rome Italy.
- Abate T (2015) A Quarterly Bulletin of the Drought Tolerant Maize for Africa Project. Nairobi, Kenya. 4: 1-4.
- Faostat (2014). Statistical Yearbook 2014: World Food and Agriculture. FAO Food Agric. Organziation UN Rome Italy.
- De Groote H, Wangare L Kanampiu F (2007) Evaluating the use of herbicide-coated imidazolinone-resistant (IR) maize seeds to control Striga in farmers’ fields in Kenya. Crop Protection. 26: 1496-1506.
- De Groote H, Wangare L, Kanampiu F et al. (2008). The potential of herbicide resistant maize technology for Striga control in Africa. 97: 83-94.
- Atera EA, Itoh K (2011) Evaluation of ecologies and severity of Striga weed on rice in sub-Saharan Africa. Agriculture and Biology Journal of North America. 2: 752-760.
- Aliyu L, Lagoke STO, Carsky RI, Kling J, Omotayo O, et al. (2004). Technical and economic evaluation of some Striga control packages in maize in the northern Guinea savanna 66.
- Ejeta G (2007). The Striga scourge in Africa: A growing pandemic. In G. Ejeta & J. Gressel (Eds.), Integrating New Technologies for Striga Control. 3-16.
- Khan ZR, Pickett JA, Wadhams LJ, Hassanali A, Midega CAO (2006) Combined control of Striga hermonthica and stemborers by maize-Desmodium spp. intercrops. Crop Protection 25: 989-995.
- Venne J, Beed F, Avocanh A, Watson A (2009) Integrating Fusarium oxysporum f.sp strigae into cereal cropping systems in Africa. Pest Management Scie 65: 572-580.
- Adetimirin VO, Kim SK, Aken'Ova ME (2000) Expression of mature plant resistance to Striga hermonthica in maize. Euphytica 115: 149-158.
- Menkir A (2006) Assessment of reactions of diverse maize inbred lines to Striga hermonthica (Del.) Benth. Plant Breeding 125: 131-139.
- Menkir A, Franco J, Adepoju A, Bossey B (2012) Evaluating consistency of resistance reactions of open-pollinated maize cultivars (Del.) Benth under artificial infestation. Crop Sci 52: 1051-1060.
- Kim SK (1991) Breeding maize for Striga tolerance and the development of a field infestation technique. 96-108.
- Kim SK, Winslow MD (1991) Progress in breeding maize for Striga tolerance/resistance at IITA In: Proceedings of the 5th International Symposium of Parasitic Weeds, Nairobi, Kenya. 494-499.
- Menkir A, Kling JG (2007) Response to recurrent selection for resistance to Striga hermonthica (Del.) Benth in a tropical maize population. Crop Sci 47: 674-682.
- Kim SK (1994) Genetics of maize tolerance of Striga hermonthica. Crop Sci 34: 900-907
- Kempthorne O (1957) An introduction to genetic statistics. John Wiley and Sons, Inc. New York, USA. 468-473.
- Comstock RE, Robinson HF (1948) The components of genetic variance in populations of biparental progenies and their use in estimating the average degree of dominance. Biometrics 4: 254-266.
- Hede AR, Srinnivasan G, Stolen O, Vasal SK (1999) Identification of heterotic pattern in tropical inbred maize lines using broad-based synthetic testers. Maydica 44: 325-331.
- Ifie BE (2013) Genetic analysis of Striga resistance and low soil nitrogen tolerance in early maturing maize (Zea mays L.) Inbred Lines University of Ghana, Accra, Ghana. 191.
- Menkir A, Badu-Apraku B, The C, Adepoju A (2003) Evaluation of heterotic patterns of utas lowland white maize inbred lines. Maydica 48: 161-170.
- Badu-Apraku B, Akinwale RO, Franco J, Oyekunle M (2012) Assessment of reliability of secondary traits in selecting for improved grain yield in drought and low-nitrogen environments. Crop Sci 52: 2050-2062.
- Badu-Apraku B, Oyekunle M, Akinwale RO, Wangare L, Kanampiu F, et al. (2013a) Combining ability and genetic diversity of extra-early white maize inbreds under stress and non-stress environments. Crop Sci 53: 9-26.
- Ifie BE, Badu-A, Praku B, Gracen V, Danquah EY (2015) Genetic analysis of grain yield of IITA and CIMMYT early-maturing maize inbreds under Striga-infested and low soil nitrogen environments. Crop Sci 55: 610-623.
- Duarte IA, Ferreira JM, Wangare L, Kanampiu F, Nuss CN (2003) Potencial discriminatório de três testadores em "topcrosses" de milho. Pesquisa Agropecuária Brasileira 38: 365-372.
- Gethi JG, Smith ME (2004) Genetic responses of single crosses of maize to Striga hermonthica (Del.) Benth and Striga asiatica (L.) Kuntze. Crop Science 44: 2068-2077.
- Yallou CG, Menkir A, Adetimirin VO, Kling JG (2009) Combining ability of maize inbred lines containing genes from Zea diploperennis for resistance to Striga hermonthica (Del.) Benth. Plant breeding, 128: 143-148.
- Badu-Apraku B, Fakorede MAB, Lum AF (2007) Evaluation of experimental varieties from recurrent selection for Striga resistance in two extra-early maize populations in the savannas of West and Central Africa. Experimental Agriculture, 43: 183-200.
- Makumbi D, Betrán JF, Bänziger M, Ribaut JM (2011) Combining ability, heterosis and genetic diversity in tropical maize (Zea mays L.) under stress and non-stress conditions. Euphytica 180: 143-162
- Adeosun JO, Gbadegesin RA, Shabeyan JY, Aba DA, Idris PO et al. (2001) Evaluation of sorghum cultivars for their resistance to Striga hermonthica. Moor Journal of Agricultural Research 2: 25-30.
- Rodenburg J, Bastiaans L (2011) Host plant defence against Striga spp.: reconsidering the role of tolerance. Weed Research 51: 438-441.
- Badu-Apraku B, Fakorede MAB, Lum AF (2007a) Evaluation of experimental varieties from recurrent selection for Striga resistance in two extra-early maize populations in the savannas of West and Central Africa. Experimental Agriculture 43: 183-200
- Badu-Apraku B, Oyekunle M, Fakorede MAB, Vroh I, Akinwale RO et al. (2013b) Combining ability, heterotic patterns and genetic diversity of extra-early yellow inbreds under contrasting environments. Euphytica, 192: 413-433.
- Choukan R (2008) Methods of genetical analysis of quantitative traits in plant breeding. 1st edn., agricultural extension, education and research organization 270.
- Karaya H, Kiarie N, Mugo S, Kanampiu F, Ariga E et al. (2012) Identification of new maize inbred lines with resistance to Striga hermonthica (Del.) Benth. Journal Crop Protection, 1: 131-142
- Derera J, Tongoona P, Vivek BS, Wangare L, Kanampiu F et al. (2008) Gene action controlling grain yield and secondary traits in southern African maize hybrids under drought and non-drought environments. Euphytica 162: 411-422.
- Barata C, Carena MJ (2006) Classification of North Dakota maize inbred lines into heterotic groups based on molecular and testcross data. Euphytica 151: 339-349.
Citation: Sangaré S, Menkir A, Ofori K, Gracen V (2018) Combining Ability for Grain Yield, Agronomic Traits and Striga hermonthica Resistance of Yellow Endosperm Maize. J Plant Genet Breed 2: 107
Copyright: © 2018 Sangaré S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Usage
- Total views: 4499
- [From(publication date): 0-2018 - Nov 27, 2025]
- Breakdown by view type
- HTML page views: 3472
- PDF downloads: 1027