Combined Oxidative and Bio Method for Removal, Degradation or Disposal of Chlorinated Persistent Organic Pollutants
DOI: 10.4172/2155-6199.1000466
Abstract
Modern environmental technologies face massive challenges in dealing with persistent organic pollutants (POPs). The storage and disposal of POPs is now strictly controlled in most countries. However, sustainable techniques for POPs disposal are still lacking. This article discusses new approaches in hazardous waste management.
The performed degradation tests focus on chlorinated hydrocarbons, more precisely on polychlorinated biphenyls (PCBs) and dichlorodiphenyltrichloroethane (DDT), pollutants that are prevalent worldwide. Both compounds are extremely toxic and bioaccumulating substances. Production and use of PCBs are strictly prohibited in most countries, while DDT is still produced and used in some states. In regards to land restoration of contaminated soils a remediation method is needed that both sustainably degrades the pollutants and at the same time promotes the selfregeneration of the ecosystem.
The experiments on the decontamination of PCB-loaded substrates were carried out in a close-loop reactor using the patented chemical oxidation method Arvox. The original contamination could be degraded by more than 90%, while conventional methods usually do not reach more than 70%.
Degradation tests with with DDT-contaminated soil were carried out in situ. Chemical oxidation using Arvox was also applied, followed by a three-month biological post-treatment. In this case, 97% of the DDT compounds were degraded. Analyses of the treated soil showed a pH normalization and a high microflora activity in the substrate. The remediation process can be considered sustainable according to the current state of remediation practices.
The innovative approach of this research is to combine effective on-site chemical oxidation techniques with biological treatment as a new method for the efficient remediation of soils contaminated with halogenated POPs. In the experiments described below, the ecological, economic and social dimensions are taken into account as essential factors for sustainable remediation. Keywords: Degradation; Remediation; Organic pollutants
Keywords: Degradation, Remediation, Organic pollutants
Keywords
Degradation; Remediation; Organic poutants
Introduction
Soils contaminated with persistent organic pollutants (POPs) are a worldwide problem. POPs are a broad class of organic compounds, most of which are chlorinated hydrocarbons (CHCs) that are resistant to natural degradation processes. Because of their persistence, POPs bioaccumulate with potential significant impacts on human health and the environment. The international community published the Stockholm Convention (SC) on Persistent Organic Pollutants in 2001 with the aim of stopping or significantly reducing their production. Since then, the list of POPs [1] has been considerably extended. According to the authors, POPs could now be defined as a substance of organic origin that nature cannot eliminate within a certain period of time.
POPs may be part of some natural substances, eg. some crude oil fractions may be considered POPs. They may result as a by-product of a chemical reaction or may be deliberately produced for specific purposes such as herbicides/pesticides or transformer oil additives. Due to their extreme stability, various combustion technologies are used (and have been shown to be effective) to decompose POPs. However, they have always been strongly criticized and are no longer recommended as they can cause secondary environmental damage. For example, the SC promotes the environmentally sound management and disposal of POPs. The technologies used for disposal should therefore not be based on incineration and should not generate toxic or hazardous waste streams. The prevention of additional waste in the treatment of contaminated materials must be an integral part of the overall treatment process [1].
Thus, on the one hand, combustion technologies will be given the lowest priority (although such method appears to be the most effective for the POPs disposal) and might be used only for the post-treatment/ residual waste, which cannot be safely disposed by any other noncombustion technology.
However, on the other hand, chemical and biochemical oxidation of the pollutants can be considered a safe alternative to the combustion (although these processes are much less intensive than open combustion), for one because it does not generate harmful chlorinated organic compounds formed by oxidation/combustion of POPs at high temperatures. After all, it is worth recognizing that from the chemical point of view combustion itself is oxidation, just one occurring at a high temperature.
Lower intensity means longer disposal: Despite the fact that biodestruction powered by bacterial-produced free radicals and some special enzymes demonstrates higher oxidation efficiency, it is the most time consuming method. In-situ chemical oxidation is a wellknown method in which a contaminated substrate is saturated with a chemical oxidizer (hydrogen peroxide, potassium permanganate or sodium persulfate), resulting in the decomposition of organic pollutants. Currently, both of these oxidizing techniques can be used with some success for the remediation of CHC-contaminated natural environments [2-5].
It should be pointed out that all existing approaches for noncombustion POPs disposal could be hardly named “environmentally sound” as they unfortunately embody the principle of waste-to-waste transformation. It means that hazardous waste disposal results in another waste but of lower hazard class. This approach is appropriate and acceptable, eg. in case of landfill management: Sometimes processing of the landfilled wastes is economically beneficial, as in result one can transfer the resulting wastes to another landfill that is less expensive to maintain, due to the local regulations. Furthermore, most beneficial situation for the case is when the contaminant concentration becomes lower than the maximum allowable concentration (MAC) prescribed by local laws, thus processed substrate could be placed on some non-agricultural lands, without fees for landfilling.
However, it is common that, when lowering hazard class of the contaminant we are still not able to escape expensive storage for the resulting products. It is obvious that providing waste-to-waste contaminant disposal enforces the industry to landfill the newly produced wastes, and we should in advance plan the storage and processing schedule for the after-reaction leftovers. Therefore, it’s one of the advantages of sustainable remediation: for sure, the further track of the processed waste could be planned, and when landfilled, it would be properly decomposed by microflora until becoming hazard-free. This is a significant difference between regular contaminant destruction, aiming to simply decompose a target substance, and sustainable remediation, which provides complex disposal until the smallest potentially hazardous leftovers and reaction products are digested by microflora and the substrate is returned into the environment. Therefore, complex procedures seem to be more beneficial for the overall process.
The main stage of successful POPs decomposition is substitution or removal of the halogen atoms (eg. de-chlorination or de-bromination) where nonreactive C-Cl bonds are responsible for extremely stability of this kind of substances. Our numerous laboratory experiments have shown that the Arvox oxidation method (Arva Greentech AG ’ s patented formula and technology) is well suited for the inactivation (dehalogenation with subsequent destruction) of various halogenated hydrocarbons. We assumed that under certain conditions we could also successfully dechlorinate POPs molecules.
Therefore, in the first part of our study we tested the very possibility (as well as completeness) of the destruction of C-CI bonds by means of Arvox method-the experiment on PCB destruction in a laboratory closed-loop type chemical reactor was set for this purpose.
Providing remediation solutions for the industry, authors have gained experience in development and implementation of oxidative contaminant destruction, and in combining different disposal techniques to obtain the maximal cost/performance effectiveness within a short time frame. Our previous investigation shows that the combination of chemical and bio oxidizing techniques prove highly efficient in the treatment of crude oil-contaminated soils [6]. Following the global trend for sustainable remediation of disturbed ecosystems in the subsequent (field) experiment, we tested the performance of complex rehabilitation techniques (Arvox oxidation followed by bioremediation) for detoxification and subsequent complete restoration of the soil contaminated with DDT. This time the main goal has been not only to reduce contaminant content to the pursued MAC but also to create a sustainable ecosystem capable of prolonged selfrestoration.
For decades, DDT is used as a pesticide for prevention of malaria outbreaks and in agriculture [7-10]. The main issue of DDT pollution is that if PCB were prohibited by the Stockholm convention and have not been applied afterwards, DDT is still in use in developing countries. The reason behind this is that environmental pollution caused by DDT is considered a lesser evil compared to potential outbreaks of insect-carried diseases, from which developing countries have been suffering for ages. These days DDT is manufactured and used in North Korea, China and India, where India leads the field [11]. In such conditions, it is obvious that the problem of DDT contamination will stay acute for decades, and the most terrible cases are those where DDTs persist in soil.
There is a small market share of DDT waste management when the substance is stored in barrels, produced but not used, where the decontamination conditions are very close to PCB destruction processes: it is appropriate to use close loop chemical reactors to avoid any spreading into environment. The majority of DDT decontamination cases is on-site cleaning, where it should be destroyed in the soil remediation process. Specificity of such cases is that the speed of DDT (as well as PCB) turnover in environment is very high. Their expansion is caused by both physical and biological processes as they are highly fat-soluble and could be rapidly accumulated in every living cell. Furthermore, DDT is able to bioaccumulate and concentrates while moving up through the food chain, and as humans are on the top of it, we are the final target for DDT pollution. In addition, DDT is very resistant to bacterial decomposition [12-15].
Taking this fact into account we proposed on-site DDT disposal technology as a two-stage combined process:
• Rapid chemical oxidation/de-chlorination immobilizes DDT molecules, lowering their permeability and makes them more bioavailable for microorganisms-bio destructors;
• Following bioprocessing breaks down leftovers into non-hazardous compounds - leads to DDT mineralization and soil ecosystem restoration [15].
Materials and Methods
The product Arvox, developed and patented by the Swiss company Arva Greentech AG, is used on an industrial scale for the oxidative treatment of various substrates and types of contamination. The underlying reaction is a hydrogen peroxide-based solution with catalysts and pH regulators. In the microbiological stage the products Fermenstart and Biomax of the Azerbaijani company AgriBioEkoTex were applied. The research provided is continuing in cooperation with several scientific institutions in Germany (Technical Universities of Berlin and Munich), Ukraine (i/VN Karazin Kharkiv National University, ii/National Scientific Center «Institute for soil science and agrochemistry research named after ON Sokolovsky, Kharkiv) and Azerbaijan (i/A Guliyev Institute of Chemistry of Additives, ii/Institute of Soil Science and Agro Chemistry, Azerbaijan National Academy of Science, Baku).
Close-loop reactor experiments were performed to define whether the Arvox technology is able to destroy PCBs in a single mode in comparison with conventional disposal method. A pseudo-closed glass vessel is used without oxygen access from the outside but dropping an overpressure greater than 0.1; mixing was carried out using a heating magnetic stirrer. Conventional method of PCBs contaminated transformer oil de-chlorinating with sodium hydroxide (NaOH) and dimethyl sulfoxide (DMSO) has been chosen as a comparison model. Two samples of artificially contaminated transformer oil were prepared for the experiment. Later on, one of them was treated with Arvox and the other with the comparison method. The PCB standard sample obtained from Supelco (a Sigma-Aldrich Corporation subsidiary) was applied both for artificial contaminated sample preparation and analytical GC-ECD (Gas Chromatography-Electron Capture Detector) method establishment.
Both experiments were held according to the same scheme
Contaminated transformer oil (100 ml, PCB - 200 ppm) and added reagents were placed in 150 ml model glass reactor while constantly stirred (the mixing speed was adjusted to obtain maximum homogeneity of the solution) and thermostat heated at 85°C with a temperature regime of +/- 5°C. Upon reaching the temperature, the total reaction time was 4 hours. A pseudo-closed vessel is used without oxygen access from the outside but dropping an overpressure greater than 0.1 atm. After 4 hours, stirring stopped and heat was turned off. The phases of the reaction medium separated gradually through the natural cooling of the reactor over 4 hours. The aqueous phase was separated from the oil in a separating funnel.
The residual content of PCB in the oil was measured with GC-ECD at the certified state laboratory (Ukrmetrteststandart, Kiev city).
On-site experiments were held at High Technologies Park (Baku, Azerbaijan) under supervision and with the participation of the experts from the Institute of Soil Science and Agricultural Chemistry of the National Academy of Sciences of the Republic of Azerbaijan.
Site preparation 2 lots 1.5 × 2.5 meters, where
• 0.1% DDT contaminated substrate (1 ton),
• control area
Experiment was divided into 2 steps:
At the first stage of the experiment the contaminated substrate was oxidized with Arvox reagents which were applied directly (sprayed) in the form of aqueous solution over the contaminated soil area. Arvox technology was implemented in two cycles - the area under test was treated with an oxidizing agent twice with a one-week exposure between the cycles. A week later, the biological treatment stage was carried out.
Microbiological remediation was tested at the second stage of the experiment, which lasted for 3 months. Biological preparations were introduced into the substrate pretreated with Arvox. One characteristic of the Arvox technology application is that the treated substrate usually demonstrates alkaline pH indexes over 9. Such alkaline conditions are disadvantageous for some soil microflora, which is why we applied a special technique. Contaminated soil was mixed with straw (5%) and biocompost (up to 20%) on each test site, then Fermenstart reagent (previously dissolved in water in a ratio of 1:100) was added to prepare the substrate for introduction of bacteria. As the next step each test site was treated with an aqueous solution of Biomax reagent (ratio 1:100) containing active strains of microorganismsbiodestructors. The total amount of Biomax solution used in the experiment was 150 liters. The initial estimate microorganism concentration was at 3*1012 CFU (colony-forming units). The solution was introduced once a week in equal amounts of 37.5 liters for 4 weeks in a row. The experimental plots were plowed and watered.
Sampling was carried out on the basis of the methodology stipulated by GOST 17.4.4.02-84 "Methods of sampling and preparation of samples for chemical, bacteriological, helminthological analysis" [7].
GС-MS (Gas chromatography-mass spectrometry) measurements of the samples were performed by the analytical laboratory of the Ministry of Healthcare of Azerbaijan Republic, as well as by the analytical laboratory of the University of Giessen, Germany.
Results and Discussion
Considering the fact we are operating through our experiments with different modes of oxidation, we attempted to provide one methodology for the sustainable process of waste management for two different case types: in a closed loop chemical reactor and on-site. First step-universal for both cases high-intensity chemical oxidation with Arvox reagent, demonstrates high performance but leaves some reaction leftovers and oxidized contaminant particles.
PCB decontamination
The objective of the first leg of experimental work was to compare Arvox oxidative technique performance with the conventional noncombustion method of PCBs contaminated transformer oil dechlorination with sodium hydroxide (NaOH). In order to simulate actual conditions of the PCBs polluted oil decontamination projects both methods were tested under the same procedure in a close loop chemical reactor.
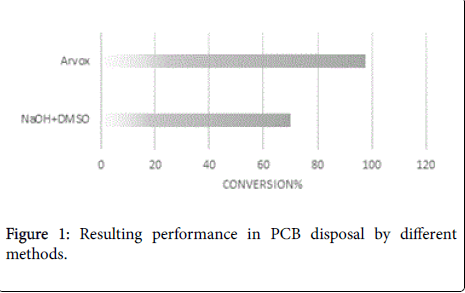
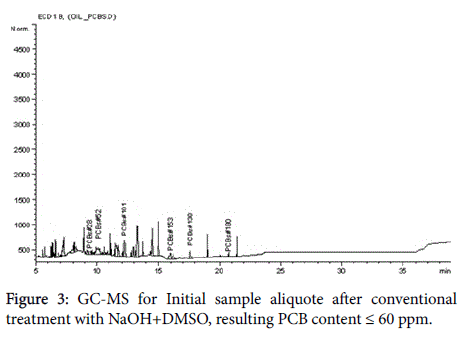
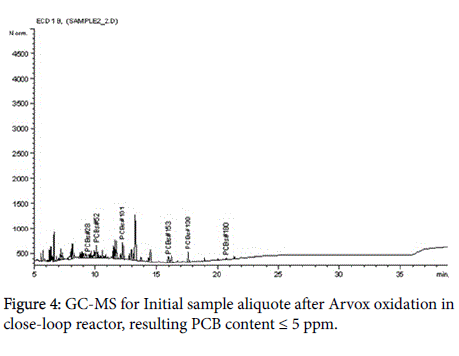
The analytical measurements demonstrate that Arvox application significantly reduced PCB content by almost 100% in one treatment cycle (Figure 1). Our method provided conversion of more than 90%: from 200 ppm in the initial sample downto 5 ppm after Arvox. NaOH +DMSO method demonstrated about 70% of PCBs conversion (resulting PCB content ≤ 60 ppm) in comparison with Arvox. This is fully consistent with the efficiency of PCB treatment with conventional technologies: conventional methods (non-combustion) show no more than 70%-80% PCB conversion per treatment cycle. This allows to reduce the concentration of PCB down to 50 ppm in the sample in one treatment cycle, but it does not allow to reach 2-5 ppm threshold - a safe level required under the legislation of most countries.
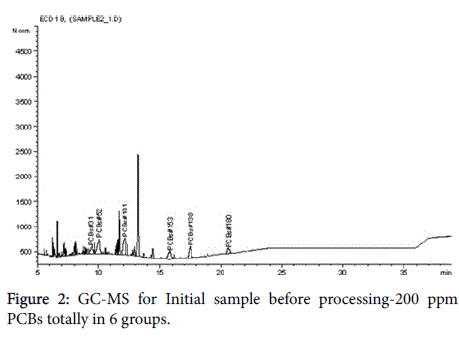
The chromatograms below represent the stages of PCB destruction process (Figures 2-4).
We believe that the outstanding efficiency of our oxidation method, as shown in the experiment described above, can be considered as a promising trend, even if not fully proven for industrial application as an independent POPs disposal technology. To the best of authors' knowledge, 90% or higher conversion rate could be reached when upscaling the Arvox technology. However, the experiment was mostly carried to demonstrate high performance potential of the Arvox chemical oxidation in chlorinated POPs destruction because rapid and powerful chemical oxidation is crucial not only for contaminant destruction but also for boosting whole remediation and disposal process and cutting down its costs. This becomes obvious taking into account the fact that most of the POP-contaminated wastes are disturbed land. They contain POPs in low concentrations (≤ 1000 ppm), making it ineffective to dispose of them by means of special treatment facility based on a closed-loop type chemical reactor.
DDT remediation on site
As mentioned above, the Arvox reaction provides a supportive environment for different post-treatment procedures, where chemical oxidation and bioprocessing stages could be carried out repeatedly if need be. Taking into account successful PCB de-chlorination trial we assumed that acting with Arvox reagents directly on a POPs contaminated substrate may enable us not only to destroy pollutants, but also to prepare reaction products for subsequent remediation processes. Thus, within the next leg of our work we performed upscaled onsite experiment to investigate:
• Compatibility of Arvox oxidative technology with Bioremediation techniques,
• Arvox+Bio method applicability for DDT-contaminated soil rehabilitation projects.
As shown by the experimental data (Table 1), the pollutant content in the soil decreased (in comparison with the control/untreated area) at both stages of the experiment:
| Sample/Type of pollutant | Initial contamination (untreated control area-start of the Pilot) | After arvox treatment phase (one week after start) | After bio treatment (three months after Arvox phase) |
|---|---|---|---|
| DDT total, ppm | 820 | 670 | 17 |
Table 1: Degradation of DDTs by Arvox+Bio combined technology.
• For the area after Arvox oxidative treatment: by 18% for POPs (DDT total);
• For the area treated with the Arvox+Bio technology: by 98% for POPs (DDT total).
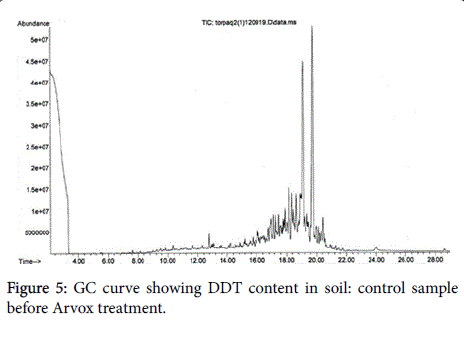
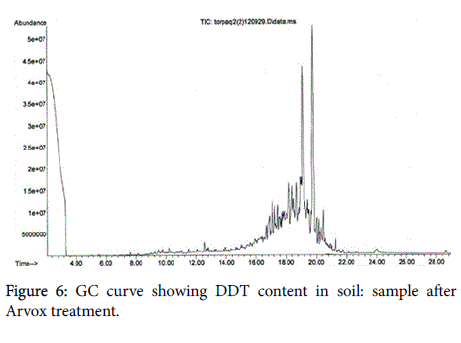
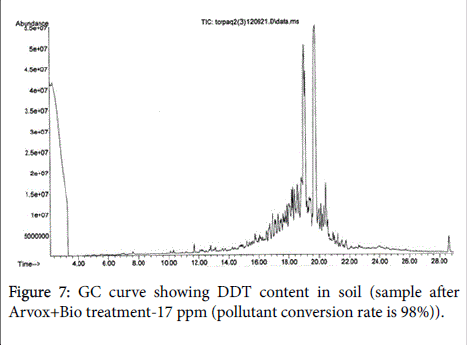
The chromatograms below show DDT decomposition through both stages (Figures 5-7).
We should note that, open field Arvox treatment appears less effective in contrast with POPs disposal technique performed in the close-loop reactor. Nevertheless, we would advise against interpreting these results literally, as GC method applied depicts not only initial contaminants content, but also substances structurally close to them. This means that by the end of Arvox treatment stage, a large number of initial DDT molecules were still present in the substrate, but in oxidized form. That makes a key difference, as oxidized DDT is less permeable and more bioavailable for destructors [16,17]. Consequently, it means that most of DDT molecules identified here by GC are ready to “soft” oxidation within the biological stage.
In addition, it is worth noting that chemical oxidation enriches the soil with oxygen and free radicals, which give a boost to the development and activation of aerobic microflora. It is a well-known fact that aerobic reactions are more beneficial than anaerobic due to their higher metabolic rates in the most of the bioprocessing spheres of application. Therefore, every oxygen enrichment of the substrate gives additional boost to the contaminant oxidation. Thus, here we can see another benefit of our combined technology - oxidative stage gives bacteria ability to respire.
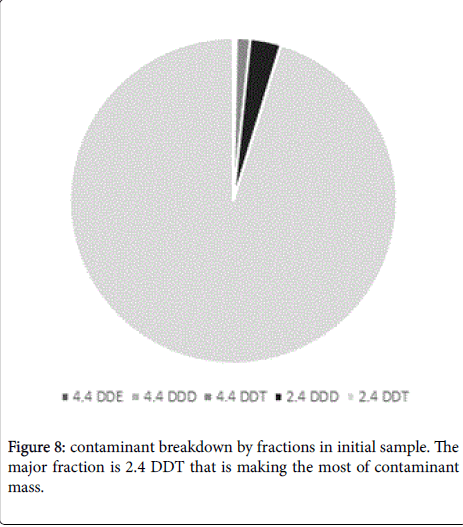
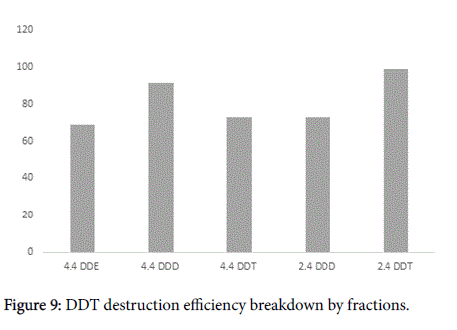
It should be noted that final conversion rates could be different depending on specific DDT type (Figures 8 and 9). The major contaminant fractions were destroyed almost completely, while the conversion rate for some minor fractions was lower. For example 4,4- DDE was decomposed only with 69% efficiency while the average conversion rate for the mixed DDT contamination was about 97% (Figure 9). Nevertheless, this result is only referring minor fractions of DDT contamination and in the case could be corresponded to low mixing intensity, that did not allow reaching every contaminant particle, especially on conglomerates. Conglomerates formation in soil is inevitable and moreover, Arvox treatment may sometimes lead to it as the chemical reaction produces sodium salts and may cause minor mineralization. This may bring about a lower bioavailability for minor fractions, still in our opinion this process should not be generalized for the substrate bulk [18].
During the experiment, the soil pH was monitored. Prior to the start of the Biophase of the experiment (after Arvox treatment), the pH value in processed soil was 9.5, a week after the start of the AgriBioEkoTex Bio composition exposure, pH was 8.4, two weeks later pH=7.2, and a month later pH=6.5. The data obtained demonstrate that the applied bacterial composition has successfully adapted to a high pH level. Moreover, further decrease of this indicator (to the values of fertile soil) proves successful restoration of soil ecosystem properties.
The whole remediation process took about three months, which is an extremely short time for a full bioremediation treatment of DDTs contaminated soil, and the reason behind that is the initial chemical boosting provided with Arvox oxidation reaction.
Below we tried to evaluate the results of DDT contaminated soil remediation in view of overall process sustainability and competitiveness regarding to the today’s state of the art situation.
Sustainability
We're rather confident, that the remediation process applied and presented here is not only efficient, but is also in compliance with principles of sustainability. The last decades demonstrated prevalence of sustainable remediation methods by three main points: economic, social and ecological, sustainable methodology illustrates a clear advantage implementing all three factors, each of almost equal importance (Figure 10). Any solution should be cost effective, socially acceptable (in compliance with the global and local legislation and guidelines) and environmentally friendly. The latest point is the most interesting, as environmental benefit does not always correspond directly to remediation activities and the total environmental impact is calculated as the total of positives and negatives evaluated for a specific site [19].
In spite of the fact that sustainable remediation is nowadays better practice than the strictly required one we consider that the situation will change in the near future for the following reason. At first glance, landfilling is the most conventional and cheapest way of disposal of hazardous and special wastes (including POPs such as DDT). As it is, low income developing countries offer low costs for such landfill disposal, but the situation is reversed for the developed economies. Nowadays cost of hazardous (special) waste landfilling could easily reach 150-200$ per ton. For example, King county/Seattle, US, provides special waste landfill disposal at “gate fee” of 175$ per ton without transporting [20,21]. Furthermore, it should be mentioned here that DDT under Basel Convention is classified as a hazardous waste [22], thus cost for its landfill disposal could raise up to about 500$ per ton (for example in Australia, where in 2012 average hazardous waste disposal cost was estimated at about 332$ per ton) [23]. This stands in stark contrast to the costs in the developing countries, but there is no chance in the modern world to transport hazardous waste cross border to the areas with low-cost landfilling without high transportation fees that renders the whole procedure pointless. Sometimes, even these high costs become insufficient to dispose of hazardous wastes, eg. in Spenser, Iowa, in spite of high disposal fees it is restricted for businesses to store more than 2,200 pounds [24] of hazardous waste on-site at any time.
Thus, we should take the situation as it is: landfilling that for a long time was the only approach to deal with wastes is becoming more and more expensive. Moreover, according to the forecasts, landfilling prices are rapidly growing: 5%-10% a year [25]. Due to the above stated trends and local legislation requirements it is more cost efficient to remediate a contaminated site than to have an unresolved issue (Figure 10).
Therefore, with an estimated treatment cost at about 179$ per ton our procedure is turned out cheaper than conventional average landfill disposal and its cost is comparable with the costs of standard bioremediation procedures for treatment of hydrocarbon pollution, eg. oil spills. However, bioremediation of CHCs is still not widespread because of their high toxicity. What is the technology advantage comparing with other remediation techniques? Here we should mention that hazardous wastes, persistent pollutants and other classes of ecological concern stipulated by international and local regulations are subject to continuous change. Taking into account the periodicity of updating UN Sustainable Development Goals targets [26] the lists of hazardous substances and their byproducts grow and MACs lower every 5 years. Thus, a currently acceptable solution can easily be revised and renounced in the nearest future. Under such conditions, it is extremely advantageous to provide solutions that would be able to progress in time and improve the remediation results even after treatment. The only known remedy to this is bioremediation, where the sustainable cenosis of microorganisms progressively destroys contaminants until their complete mineralization [27].
Obviously, for bioprocessing speediness and time effectiveness are one of the main advantages: the faster soil is processed and returned into the environment, the cheaper the process is, as the bioremediation is in fact a sort of landfilling and often requires infrastructure solutions [28]. Therefore, three-month period investigated in this research seems short enough, furthermore it is optimal in view of seasonal changes: usually bioprocesses can be optimally performed in warm and wet conditions, so the process that lasts more than 6 months could be naturally slowed down by unfavorable weather, eg. in winter. Such a short time frame allows to carry out the whole process during one most favorable season and to cut disposal costs al as the result.
Conclusions
• PCBs can be successfully treated (over 97% effectiveness in one application) with Arvox oxidation technology under certain conditions (close chemical reactor under high temperature and overpressure).
• Arvox efficiency in PCB treatment has surpassed the performance of the conventional chemical method with NaOH and reached similar results as the most effective (non-combustion) dechlorination method with sodium dispersion (NaD).
• Partially oxidized compounds may be generated within the course of oxidation of large molecules of POPs. These by-products of Arvox reaction are highly bioavailable and biodegradable.
• Arvox technique application enables us to safely combine it with the other soil detoxification methods and to significantly increase the chlorinated POPs degradation rates, as well as to reduce costs on the overall soil remediation procedure.
• When scaled-up, the Arvox oxidative method-based soil remediation technology demonstrated an efficiency comparable to that described for laboratory experiments, thus confirming its applicability for soil rehabilitation projects.
References
- UNO (2019) Protecting human health and the environment from persistent organic pollutants.
- Zinoviev S, Fornasiero P, Lodolo A, Miertus S (2007) Non-combustion Technologies for POPs Destruction, Review and Evaluation. United nations industrial development organization.
- Maas T (2003) Remediation options suitable for inner city re-development. ADEME presse, France.
- Foght J, April T, Biggar K, Aislabie J (2001) Bioremediation of DDT-contaminated soils: A review. Bioremed J 5: 225-246.
- Fang H, Dong B, Yan H, Tang F, Yu Y (2010) Characterization of a bacterial strain capable of degrading DDT congeners and its use in bioremediation of contaminated soil. J Hazard Mater 184: 281-289.
- Babayev ER, Ali-zade AM, Babayev VA, Harkavenko VV, Seryy SS (2019) The New combined chemical-biological remediation method for oil-polluted soils: Oxidative disposal and establishing sustainable soil cenosis. J Bioremediat Biodegrad 10: 464.
- RUNORM (2017) Nature protection. Soils. Methods for sampling and preparation of soil for chemical, bacteriological and helmintoglogical analysis.
- Megharaj M, Kantachote D, Singleton I, Naidua R (2000) Effects of long-term contamination of DDT on soil microflora with special reference to soil algae and algal transformation of DDT. Environ Pollut 109: 35-42.
- van den Berg H (2009) Global status of DDT and its alternatives for use in vector control to prevent disease. Environ Health Perspect 117: 1656-1663.
- FAO (1997) Prevention and disposal of obsolete and unwanted pesticide stocks in Africa and the near east.
- van den Berg H,Manuweera G, Konradsen F (2017) Global trends in the production and use of DDT for control of malaria and other vector-borne diseases. Malar J 16: 401.
- Byzov BA (1989) Microbiological aspects of soil contamination with pesticides. Microorganisms and protection of soil.
- Golovleva LA (1980) Degradation of pesticides with microorganisms: Potential, limitations and practical perspectives. Proceedings of the Institute of microbiology and virology of the Academy of Sciences of Kazakhstan SSR.
- Golovleva LA (1982) Role of microorganisms in decomposition of pesticides in the environment. Results of scientific studies for the agricultural practice.
- Sayles GD (1997) DDT, DDD, and DDE dechlorination by zerovalent iron. Environ Sci Technol 31: 3448-3454.
- Purnomo AS, Mori T, Takagi K, Kondo R (2019) Bioremediation of DDT contaminated soil using brown-rot fungi. Int Biodeterior Biodegradation 65: 619-695.
- Veeh RH, Inskeep WP, Camper AK (1996) Soil depth and temperature effects on microbial degradation of the 2,4-D. Environ Qual 25: 5-12.
- Warner SD, Hadley PW (2012) Sustainable Remediation: Integrating Risk, Science, and sustainability principles.
- King C (2019) Disposal Fees - solid waste, recycling, unsecured loads, and Cleanup LIFT discount.
- Basel Convention (1995) The control of transboundary movements of hazarduous wastes and their disposal.
- BDA Group Economics and Environment (2009) The full cost of landfill disposal in Australia. Department of the Environment, Water, Heritage and the Arts.
- Galantino C (2018) No End in sight to us landfill cost increases- pacific region to experience highest growth.
- Sustainable Development (2020) About the sustainable development goals.
- Shen R (2007) Cleaning of soil contaminated with mixture of organic pollutants arrived with the wastewater sludges in the region of yangtze river delta. J Agro-Environ Sci 4: 1501-1505.
- Cetkauskaite A, Grigonis U, Berzinskiene J (1998) Biodegradation: Selection of suitable model. Ecotoxicol Environ Safety 40: 19-28.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 5001
- [From(publication date): 0-2020 - Nov 14, 2025]
- Breakdown by view type
- HTML page views: 4053
- PDF downloads: 948