Combined Evaluation of FDG-PET/CT and CT Imaging Characteristics of Cervical Lymph Nodes to Increase the Interpretation Accuracy for Nodal Metastatic Involvement in Head and Neck Cancer
Received: 08-Sep-2015 / Accepted Date: 02-Oct-2015 / Published Date: 10-Oct-2015 DOI: 10.4172/2161-119X.1000213
Abstract
Rationale and objectives: Accurate staging of regional lymph nodes in patient with head and neck squamous cell carcinoma (HNSCC) has important therapeutic and prognostic implications. The objective of this study was to assess qualitative and quantitative imaging characteristics of cervical lymph nodes on FDG PET/CT and contrast enhanced CT (CECT), either alone or in cross-modality combinations, for more accurate diagnosis of nodal metastatic involvement in HNSCC patients who had hypermetabolic cervical lymph nodes on PET/CT imaging.
Materials and methods: Lymph nodes were evaluated using quantitative PET/CT parameters (SUVmax, SUVmean, metabolic tumor volume, total lesion glycolysis) and qualitative and quantitative CT parameters (shape, margin, focal hypoattenuation, fatty hilum, short axis, long axis, volume, eccentricity factor). A subset analysis was performed on FDG-avid lymph nodes that lacked imaging findings suggestive of extracapsular spread or necrosis. Statistical outcomes included sensitivity, specificity, accuracy, positive likelihood ratio, and odds ratio.
Results: In the full cohort (174 nodes), the most malignancy specific parameters were ill-defined margin and focal hypoattenuation, and the most accurate variables included SUVmax, SUVmean, ill-defined margin, short axis, and focal hypoattenuation. On multivariate analysis, ill-defined margin, short axis measurement, and SUVmax independently predicted nodal metastasis. In the subgroup of smaller lymph nodes without ill-defined margin or focal hypoattenuation (100 nodes), round shape was among the most accurate and specific criteria for malignancy. Cross-modality combination criteria with SUVmax and shape, eccentricity factor, or long to short axis ratio resulted in statistically significant improvements in specificity and accuracy for lymph node metastasis.
Conclusion: A combined evaluation strategy using individual PET/CT and CECT characteristics in cross modality combinations, particularly SUVmax with round shape on axial imaging or with loss of eccentricity, can improve diagnostic accuracy of malignant nodal involvement in the evaluation of indeterminate cervical lymph nodes for malignancy.
Keywords: Head and neck squamous cell carcinoma, FDG PET/CT, Malignant lymphadenopathy, CT.
251054Introduction
In patients with head and neck cancer, cervical lymph node metastasis is one of the most important predictors of poor prognosis thus, significantly impacts management decisions and ultimately clinical outcomes [1,2]. Given the significant morbidity and cost of unwarranted treatment of histologically negative necks, it is imperative for clinicians to accurately diagnose malignant nodal involvement. Alternatively, undertreating involved lymph nodes can lead to disease progression and shortened survival.
With the recognition of importance of modern imaging modalities, the American Joint Committee on Cancer recommends clinical staging including both physical examination and imaging modalities [3]. The National Comprehensive Cancer Network (NCCN) recommends CT and/or MRI with contrast as part of the initial workup of newly diagnosed head and neck cancer. In the NCCN guidelines PET/CT imaging should be considered in the more advanced stages of certain head and neck primary tumors [1].
Basic CECT or contrast enhanced MRI, are used to evaluate lymph nodes based on size, contrast enhancement patterns, morphological characteristics (including shape and margins) and distribution [4]. Central necrosis, suggested by focal hypoattenuation on CECT or by T2 hyperintensity on MRI,and extracapsular spread, suggested by irregular margins, are the most accurate and specific markers for metastatic involvement, and affected nodes are generally considered to harbor metastasis irrespective of size [4-6]. Size and abnormalities in nodal architecture such as round shape, clustering, and absence of fatty hilum, are considered less reliable criteria in clinical practice [6,7]. Advanced imaging techniques including diffusion weighted MRI and CT or MRI dynamic perfusion imaging are less used on clinical practice.
PET/CT assesses both metabolic and anatomic parameters of the lymph nodes. PET/CT has gained acceptance in the evaluation of cervical nodes by providing improved sensitivity and accuracy compared to other imaging modalities including US, CT and MRI [8-11]. However, concerns remain regarding the non-specific nature of FDG uptake, which results in false positive readings. Recent publications comparing hybrid PET/magnetic resonance imaging (PET/MR) and PET/CT demonstrated equivalent or slight superiority of PET/MR in the imaging workup of patients with head and neck cancer, however additional data is needed to establish the role of PET/MR as preferred imaging modality or problem solving tool [12,13].
The diagnostic benefit of interpretation the PET/CT in correlation with the CECT, is well established; however, it remains challenging for imaging experts to differentiate between benign reactive changes and metastatic disease in FDG-avid lymph nodes that lack one or more highly specific criteria for malignancy [2,14]. Research is now directed toward finding optimal diagnostic algorithms for these indeterminate nodes. Initial reports of diagnostic gains when analyzing PET/CT and CECT examinations together are promising [8,9]; however, there remains a need for the development of a reliable combination analysis for distinguishing reactive hyperplasia from metastatic disease in equivocal FDG-avid cervical lymph nodes.
We hypothesized that nodal assessment of imaging characteristics and measurable parameters in cross-modality combinations, would improve reader specificity and diagnostic accuracy for lymph node metastasis, particularly for those lymph nodes which did not have highly specific imaging features of metastatic involvement. Our objective was to describe clinically useful metrics derived from PET/CT and CECT, used alone or in combinations, for more accurate diagnosis of the histopathological nature of cervical lymph nodes in patients with HNSCC and FDG-avid cervical lymph nodes.
Methods
Patients
The Institutional Review Board approved this study and patient informed consent was waived based on the retrospective design in compliance with the Health Insurance Portability and Accountability Act. The objective of this study was to assess qualitative and quantitative imaging characteristics of cervical lymph nodes on FDG PET/CT and CECT, either alone or in cross-modality combinations, for more accurate diagnosis of nodal metastatic involvement in HNSCC patients who had hypermetabolic cervical lymph nodes on PET/CT imaging.
From a database of 1,744 patients who underwent both PET/CT and CECT between March 2006 and November 2012, 131 patients with HNSCC fulfilled the eligibility criteria either at staging or restaging (Table 1). Eligibility criteria included diagnosis of HNSCC either at staging or restaging, minimum 18 years of age, whole body PET/CT and CECT of the neck obtained simultaneously or within 4 weeks of each other with no intervening therapy, and the availability of either histopathology or long-term follow up of at least 12 months for confirmation of imaging findings. The interval between the last treatment and imaging was at least 3 months in the restaging group. Exclusion criteria included known granulomatous disease, HIV+ serology, active infection, diabetes with blood glucose level over 220 mg/dl, and patients on oral prednisone.
Imaging protocols
The PET/CT protocol included a whole-body FDG PET⁄CT obtained 60 min after injection of 10-15 mCi 18-FDG. The associated CT of the PET/CT study was acquired either with or without intravenous iopamidol (8-detector, 120 kVp, auto mAs of up to 380).
The dedicated multidetector CECT of the neck was acquired with the following protocol: 90 mL of intravenous iopamidol (Isovue-370); 30 seconds delay; 140 kVp, automatic current-time product (auto mAs) of up to 380 mAs. The term PET/CT is used for all PET/CT studies, with or without intravenous contrast.
Image interpretation
PET/CT and neck CECT were evaluated independently by a nuclear medicine physician (LK) and a neuroradiologist (PS), respectively, each reader with more than 30 years experience. The readers were blinded to each other’s interpretation and to other clinical information except the patient’s primary diagnosis and the time of imaging with respect to therapy.
Qualitative analyses
For CECT studies, lymph nodes were evaluated for round shape (defined as a loss of the reniform nodal shape) (yes/no), margin irregularity (defined as ill-defined contour or infiltration of adjacent tissues) (yes/no), focal hypoattenuation (yes/no), and fatty hilum (yes/no).
For PET/CT studies, a positive lesion was defined as increased FDG uptake incompatible with normal anatomical or physiological variants compared to a contralateral normal appearing site or adjacent background tissue when the contralateral site was involved by disease.
For statistical evaluation of reader performance, both the PET/CT and CECT readers evaluated nodes for metastasis using a five-point qualitative scale of reader confidence (1=Benign, 2=Probably Benign, 3=Equivocal, 4=Probably Malignant, 5=Malignant). Data were subsequently dichotomized as negative (scores 1-2) and positive (3-5) for end-point analyses.
Quantitative analyses
CECT quantitative parameters included short axis (SA) (mm), long axis (LA) (mm), short / long axis ratio, eccentricity factor (EF), and volume. CECT images were processed using Onco Quant imaging software via PACS reading platform (GE Healthcare, Wisconsin, USA) which used a semi-automated three-dimensional technique to calculate volumetric measurements. After the longest diameter of each cervical lymph node was manually measured on a transverse plane, the software automatically separated the node from surrounding anatomic structures and defined a region of interest that tightly enclosed the node. Once segmentation was completed, nodes were revaluated by the neuro radiologist and, if needed, manual corrections were made. Subsequently, computer software analyzed each axial image and produced unidimensional and volumetric measurements. The EF (EF = √1-(SA/LA)2) was calculated using the resulting parameters [15].
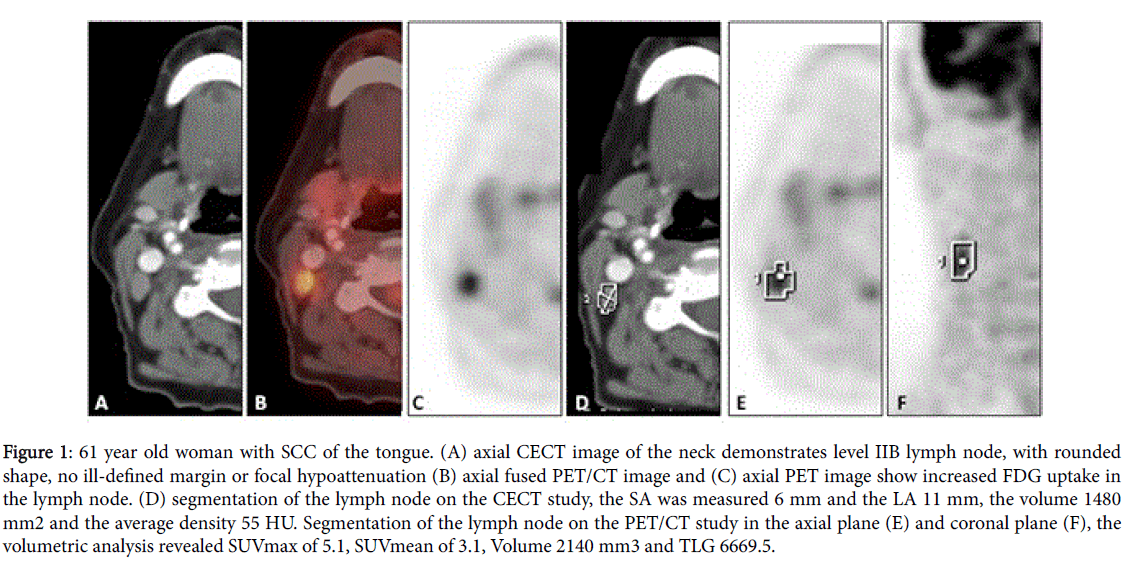
PET/ CT quantitative parameters included maximum standardized uptake value (SUVmax)(g/mL), SUV mean (g/mL), metabolic tumor volume (MTV)(mL), and total lesion glycolysis (TLG)(g)(TLG = MTV*SUVmean). Each lymph node was also segmented in three-dimensions using PETVCAR software via AW workstation platform (GE Healthcare, Wisconsin, USA). The automated segmentation technique defined nodal borders using a fixed threshold of 42% of maximal activity within the tumor (SUVmax) [16]. All tissue with activity greater than that percentage was included within the tumor volume. The 3D segmentation of each node was reviewed by a radiologist, and if needed, minor manual adjustments were made to completely avoid physiological sites (Figure 1).
Figure 1: 61 year old woman with SCC of the tongue. (A) axial CECT image of the neck demonstrates level IIB lymph node, with rounded shape, no ill-defined margin or focal hypoattenuation (B) axial fused PET/CT image and (C) axial PET image show increased FDG uptake in the lymph node. (D) segmentation of the lymph node on the CECT study, the SA was measured 6 mm and the LA 11 mm, the volume 1480 mm2 and the average density 55 HU. Segmentation of the lymph node on the PET/CT study in the axial plane (E) and coronal plane (F), the volumetric analysis revealed SUVmax of 5.1, SUVmean of 3.1, Volume 2140 mm3 and TLG 6669.5.
Surgery and histopathology
All positive findings on either PET/CT or CECT were confirmed by biopsy within 6 weeks of initial positive imaging result (n=137) or serial clinical and imaging follow up of ≥ 12 months (n=37) (median 17 months) as reference standard.
In patients without histopathology data, final diagnosis was considered benign if the node was stable for ≥ 12 months of clinical and radiographic follow-up when there was no therapy administration, or if the node decreased in size in the absence of intervening therapy. For image-histopathology correlation, images were analyzed on a level-by-level basis based on 6 cervical node levels, bilaterally.
Statistical analysis
Statistics were analyzed using IBM SPSS statistical software (version 20; SPSS, Inc. Chicago, Ill). Receiver operator curves (ROC) were generated, and area under the curve (AUC) and optimal cutoffs by Youden criteria were compared using nonparametric methods [17].
2x2 tables were created against our reference standard to calculate sensitivity, specificity, accuracy, positive likelihood ratio, and odds ratio, and significance was determined using chi-squared analyses (p<0.05, 2-sided). Multivariate analyses were performed using binomial logistic regression.
These analyses were performed on the full data set, as well as on a subset of lymph nodes without findings suggestive of extracapsular spread or necrosis (ill-defined margins or central hypoattenuation, respectively) in order to characterize the most discriminative characteristics for malignancy in difficult to evaluate nodes without these highly specific criteria. The PET/CT and the CECT derived variables were combined sequentially to maximize specificity, and these combinations were evaluated using relative positive likelihood ratios (rLR+) as described by Macaskill et al. [18]. If the rLR+ was greater than 1 and its 95% confidence interval did not contain 1, the combined test was considered significantly more accurate in identifying metastasis.
Results
A total of 174 lymph nodes from 114 patients were analyzed. Patient and lymph node characteristics are summarized in Table 1.
| Variable | n | (%) |
|---|---|---|
| Patient: | ||
| Count | 114 | |
| Age, years | ||
| Median (Mean, Range) | 61 | (62, 27 - 90) |
| Sex, M/F | 71/43 | |
| Primary Site | ||
| Oral Cavity | 52 | -46 |
| Pharynx | 40 | -35 |
| Larynx | 15 | -13 |
| Hypopharynx | 1 | -1 |
| Unknown | 6 | -5 |
| Lymph Node: | ||
| Count (Malignant/Total) | 87/174 | -50 |
| Level | ||
| I | 33 | -19 |
| II | 104 | -60 |
| III | 27 | -16 |
| IV | 6 | -3 |
| V | 4 | -2 |
| Lymph Node Identified at | ||
| Staging | 114 | -66 |
| Restaging | 60 | -34 |
Table 1: Patient, primary cancer, and lymph node characteristics.
Evaluation of FDG -avid lymph nodes in the entire cohort
Specificity, sensitivity and accuracy data at optimal cutoffs are summarized in Table 2. In univariate analysis, the variables most specific for malignancy were ill-defined margin (97%) and focal hypoattenuation (94%), and the variable most sensitive for malignancy was the absence of fatty hilum (95%). The most accurate tests included in a descending order were SUVmax, SUVmean, ill-defined margin, focal hypoattenuation, and short axis. On multivariate analysis, only ill-defined margin, short axis, and SUVmax were found to independently predict nodal metastasis (Table 2).
| Detection of Malignant Lymph Node by Individual Criteria | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Optimal Cutoffs | All Cutoffs | ||||||||
| All Nodes | Sens. | Spec. | Acc | ||||||
| Cutoff | (%) | (%) | (%) | O.R. | 95% CI | p-value | AUC | p-value | |
| Univariate: | |||||||||
| PET/CT: | |||||||||
| PET/CT Reader | - | 94 | 43 | 68 | 12.1 | 4.5 - 32.9 | < 0.001 | - | - |
| SUVmax | 4.1 | 87 | 72 | 79 | 17.1 | 7.8 - 37.8 | < 0.001 | 0.87 | <0.001 |
| SUVmean | 2.4 | 89 | 70 | 79 | 19.2 | 8.4 - 44.1 | < 0.001 | 0.87 | <0.001 |
| MTV | 3,405 | 43 | 91 | 67 | 7.3 | 3.1 - 17.0 | < 0.001 | 0.64 | <0.001 |
| TLG | 6,928 | 67 | 84 | 75 | 10.3 | 5.0 - 21.4 | < 0.001 | 0.81 | <0.001 |
| CECT: | |||||||||
| CT Reader | - | 87 | 84 | 86 | 36 | 15.4 - 84.5 | < 0.001 | - | - |
| Shape (round) | - | 66 | 77 | 72 | 6.6 | 3.3 - 12.9 | < 0.001 | - | - |
| Focal Hypoattenuation | - | 61 | 94 | 78 | 25.6 | 9.4 - 69.5 | < 0.001 | - | - |
| Ill-Defined Margin | - | 61 | 97 | 79 | 43.6 | 12.8 - 149.3 | < 0.001 | - | - |
| Absence of Fatty Hilum | - | 95 | 21 | 58 | 5.4 | 1.7 - 16.7 | 0.001 | - | - |
| Long Axis | 14.5 | 61 | 84 | 72 | 8.1 | 4.0 - 16.6 | < 0.001 | 0.78 | <0.001 |
| Short Axis | 8.5 | 71 | 82 | 76 | 11 | 5.4 - 22.5 | < 0.001 | 0.85 | <0.001 |
| Long / Short Axis | 1.6 | 82 | 63 | 72 | 7.6 | 3.8 - 15.3 | < 0.001 | 0.75 | <0.001 |
| EF | 0.8 | 82 | 63 | 72 | 7.6 | 3.8 - 15.3 | < 0.001 | 0.75 | <0.001 |
| Volume | 854 | 76 | 69 | 72 | 7 | 3.6 - 13.8 | < 0.001 | 0.79 | <0.001 |
| Multivariate: | |||||||||
| Ill-Defined Margin | - | - | - | - | 22.5 | 4.6-110.7 | <0.001 | - | - |
| Short Axis | - | - | - | - | 1.6 | 1.2-2.2 | 0.001 | - | - |
| SUVmax | - | - | - | - | 1.4 | 1.1-1.8 | 0.019 | - | - |
Table 2: Detection of malignant lymph node by individual criteria.
Across all possible cutoffs, the best performing quantitative PET/CT and CECT variables by ROC analysis (AUC) were SUVmax (0.87), SUVmean (0.87), and short axis (0.85). Short axis performed significantly greater in the detection of nodal metastasis than long axis (0.78) (p=0.02; 95% CI 0.009 – 0.124), but did not perform statistically greater than volume (p=0.081; 95% CI -0.006 – 0.105). SUVmax performed significantly greater in the detection of nodal metastasis than MTV (0.64) (p<0.001; 95% CI 0.13–0.34).
Evaluation of lymph nodes without focal hypoattenuation or ill-defined margin
In the subgroup analysis including lymph nodes without imaging findings suggestive of necrosis or extracapsular spread (n=100, 20 malignant), the individual criteria found to have the greatest overall accuracy at optimal cutoffs in univariate analysis included in descending order were round shape, SUVmean (cutoff=2.4), and SUVmax (cutoff=3.9) (Table 3). EF (cutoff=0.8) and long/short axis ratio (cutoff=1.6), performed identically on this data set. On multivariate binomial logistic regression of significant univariate, nonderivative variables, only SUVmax (>3.9), and short axis measurement independently predicted nodal metastasis.
| Detection of Malignant Lymph Node by Individual Criteria | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Excluding LN with focal hypoattenuation or ill-defined margin | |||||||||||
| Cutoff | Sens. | Spec. | Acc | 95% CI | All Cutoffs | ||||||
| (%) | (%) | LR+ | LR- | (%) | O.R. | of O.R. | p-value | AUC | p-value | ||
| Univariate: | |||||||||||
| PET/CT: | |||||||||||
| PET/CT Reader | - | 85 | 45 | 1.55 | 0.33 | 53 | 4.6 | 1.3 - 17.1 | 0.014 | - | - |
| SUVmax | 3.9 | 85 | 73 | 3.09 | 0.21 | 75 | 14.9 | 4.0 - 56.0 | < 0.001 | 0.83 | <0.001 |
| SUVmean | 2.4 | 85 | 74 | 3.24 | 0.2 | 76 | 15.9 | 4.2 - 59.9 | < 0.001 | 0.83 | <0.001 |
| MTV | 3,435 | 20 | 90 | 2 | 0.89 | 76 | 2.3 | 0.6 - 8.4 | 0.218 | 0.45 | 0.467 |
| TLG | 3,417 | 90 | 44 | 1.6 | 0.23 | 53 | 7 | 1.5 - 32.2 | 0.005 | 0.68 | 0.014 |
| CECT: | |||||||||||
| CT Reader | - | 45 | 90 | 4.5 | 0.61 | 81 | 7.4 | 2.3 - 23.1 | < 0.001 | - | - |
| Shape(round) | - | 45 | 83 | 2.57 | 0.67 | 75 | 3.9 | 1.3 - 11.1 | < 0.001 | - | - |
| Absence of Fatty Hilum | - | 85 | 23 | 1.1 | 0.67 | 35 | 1.6 | 0.4 - 6.3 | 0.461 | - | - |
| Long Axis | 14.5 | 30 | 86 | 2.18 | 0.81 | 75 | 2.7 | 0.9 - 8.5 | 0.084 | 0.6 | 0.173 |
| Short Axis | 6.5 | 90 | 59 | 2.18 | 0.17 | 65 | 12.8 | 2.8 - 59.0 | < 0.001 | 0.79 | <0.001 |
| Long / Short Axis | 1.6 | 80 | 64 | 2.21 | 0.31 | 67 | 7 | 2.1 - 23.0 | < 0.001 | 0.75 | <0.001 |
| EF | 0.8 | 80 | 64 | 2.21 | 0.31 | 67 | 7 | 2.1 - 23.0 | < 0.001 | 0.75 | <0.001 |
| Volume | 788 | 70 | 66 | 2.07 | 0.45 | 67 | 4.6 | 1.6 - 13.3 | 0.003 | 0.66 | 0.026 |
| Combination Variables: | |||||||||||
| SUVmax + Shape | - | 45 | 94 | 7.2 | 0.59 | 84 | 12.3 | 3.5 - 43.4 | < 0.001 | - | - |
| SUVmax + Long/Short ratio | - | 65 | 89 | 5.78 | 0.39 | 84 | 14.7 | 4.6 - 46.3 | < 0.001 | - | - |
| SUVmax + EF | - | 65 | 89 | 5.78 | 0.39 | 84 | 14.7 | 4.6 - 46.3 | < 0.001 | - | - |
| SUVmax + Volume | - | 55 | 88 | 4.4 | 0.51 | 81 | 8.6 | 2.8 - 25.8 | < 0.001 | - | - |
| SUVmax + Short Axis | - | 75 | 83 | 4.29 | 0.3 | 81 | 14.1 | 4.4 - 45.3 | < 0.001 | - | - |
| SA + Shape | - | 40 | 89 | 3.56 | 0.68 | 79 | 5.3 | 1.7 - 16.3 | 0.002 | - | - |
| Multivariate: | |||||||||||
| SUVmax | - | - | - | - | - | 1.5 | 1.0-2.1 | 0.038 | |||
| Short Axis | - | - | - | - | - | 1.4 | 1.0-1.9 | 0.047 | |||
Table 3: Detection of malignant lymph node by individual criteria.
A node was 1.9 times more likely to be malignant using SUV max (cut off 3.9) together with either EF or long axis/short axis ratio (relative LR+1.9; 95% CI 1.1–3.3; p=0.031) versus using SUV max alone (relative LR+1.9; 95% CI 1.1–3.3; p=0.031), increasing the specificity for malignancy from 73% (SUV max>3.9 alone) to 89% (SUV max with either EF or long/short axis). The combination of SUV max with reader defined shape improved specificity from 73% to 94%, but was not significantly better than SUV max alone (relative LR+2.3; 95% CI 0.96–5.68; p=0.063). SUV max with short axis (>6.5mm) did not produce a significant rLR+ improvements (rLR+1.39; 95% CI 0.97–1.99; p=0.076). Short axis combined with shape did not improve the results compared to shape alone (rLR+1.4; 95% CI 0.9–2.2; p=0.162).
Across all possible cutoffs, the best performing quantitative PET/CT and CECT variables by ROC analysis (AUC) were SUVmean (0.83), SUVmax (0.83), and short axis measurement (0.79). The AUC for SUVmax was not statistically superior to short axis measurement (p=0.54; 95% CI -0.082–0.157). In addition, the AUC for the combination of SUVmax with short axis (0.84) was not significantly greater than SUVmax alone (p=0.693; 95% CI -0.055–0.082).
Discussion
In this study, we demonstrated that the use of cross-modality combinations of lymph node imaging characteristics, particularly SUVmax combined with round shape (subjective roundness or long/short axis ratio or EF), significantly improved diagnostic accuracy for predicting malignancy in FDG-avid lymph nodes, with no imaging findings strongly suggestive of extracapsular spread or necrosis. Other groups have reported synergistic gains in diagnostic accuracy when the overall assessments for malignancy on PET/CT and CT were combined [8,9]. These studies, however, are limited by the use of combination of multiple parameters to define malignancy on each imaging modality, with any of several individual positive variables such as shape, size, necrosis or clustering, being sufficient for definition of metastasis.
In the present paper, we reinforced that while certain parameters in isolation (e.g. ill-defined margins and focal hypoattenuation) are highly specific for the diagnosis of metastasis, other parameters in isolation, such as axial size, are not sufficient. We further report that in those lymph nodes without highly specific findings for malignant involvement, cross modality combinations of criteria, specifically SUVmax >3.9 associated with a measure of node "roundness" (long/short axis ratio >1.6 cm, or EF <0.8), are highly specific for nodal metastatic disease. Although the combination of these imaging characteristics demonstrated relatively high specificity (89%), the sensitivity was lower (65%). We believe that analysis of these parameters using both CECT and PET/CT, after further validation, may serve to enhance reader accuracy and confidence in identification of metastasis in difficult to evaluate FDG-avid cervical lymph nodes.
Evaluation of FDG-avid lymph nodes in the entire cohort
Imaging findings suggestive of necrosis and extracapsular spread are considered specific characteristics for cervical nodal metastasis in HNSCC with reported specificity of 94-100% [5,19,20] and 98% [5], respectively. Nonetheless, no consensus exists on their relative value in FDG-avid nodes when metabolic parameters, such as SUVmax, are concurrently available. In the present study, we report high specificity for ill-defined margin and focal hypoattenuation as predictors of nodal metastasis of 97% and 94%, respectively.
In clinical practice, size cutoffs remain one of the most commonly used criteria for identification of abnormal lymph nodes; however, debate remains over the optimal use of short axis measurement as compared to long axis. Within nodes with increased FDG uptake, we report significantly greater accuracy for metastasis using short axis as compared to long axis as well as at optimal cut offs of 8.5mm and 14.5 mm, respectively. This is in line with increasing data that suggests that short axis may be a more reliable marker for malignancy than long axis [19,22]. These findings, however, are not reflected in current TNM guidelines for head and neck which recommend long axis (“greatest dimension”) as the only recommended size based criteria for nodal staging [3]. With further study and validation, these findings may support revision of current staging paradigms to reflect increased diagnostic reliability of short axis versus long axis.
In our analysis, we examined the role of several less well studied metrics such as MTV and three dimensional nodal volume. In our analysis, MTV performed statistically inferior to SUVmax, and volume, and though not statistically significant, trended towards inferiority compared to short axis (p=0.081). Thus presently we cannot recommend these as reliable metrics in isolation for determination of metastasis.
Evaluation of lymph nodes without focal hypoattenuation or ill-defined margin
While in the full cohort of lymph nodes, shape was not among the most accurate characteristics, in our subset of nodes without findings on imaging suggestive of extracapsular spread or necrosis, round shape was one of the most specific variables for lymph node metastasis of any criteria tested. Shape has traditionally been considered for node discrimination only in borderline nodes by size criteria [7], and the stand alone diagnostic value of shape has been questioned with reports of marginal incremental accuracy and specificity [7,19]. In this study, we illustrated that in absence of imaging findings suggestive of extracapsular spread or necrosis, shape should perhaps be considered as a stronger indicator of malignancy than short or long axis measurements based on the higher specificity at optimally defined cutoffs. Our data support continued evaluation of shape as criteria for determining malignancy, with initial preference over more widely accepted and utilized size criteria, to be used specifically in difficult to define FDG-avid nodes.
The sequential combination of SUVmax (>3.9) with a measurement of "roundness", EF (<0.8) or long axis/short axis ratio (<1.6), were the only combination variables that resulted in statistically significant improvement in the likelihood that a node truly harbors metastasis. SUVmax with reader defined round shape was the most specific combination variable for metastasis (94%); however, the combination was not significantly better than SUVmax alone (p=0.063). This data may suggest that some measure of roundness (reader defined node shape or long/short axis ratio or EF) in combination with SUVmax may be highly specific for metastasis in lymph nodes without ill-defined margin or focal hypoattenuation. These combined criteria for defining malignancy has not yet been addressed in the literature. We believe these findings warrant further investigation of roundness in combination with SUVmax for validation and description of appropriate cutoffs for use in clinical practice.
The use of size and SUVmax together has been considered as a means of improving diagnostic accuracy using cutoff based criteria. In the present study, across all cutoffs (ROC analysis), we found no statistically significant improvement using SUVmax with short axis versus SUVmax alone; however, at optimal cutoffs of SUVmax>3.9 and short axis>6.5mm, the improvement over SUVmax alone trended towards significance (p=0.076). Matsubara et al. [21] suggests using a measure of length to discriminate malignancy in nodes with increased FDG uptake. They report that long axis is significantly greater in PET/CT identified FDG-avid true positive nodes as compared to false positive nodes. Similar to our study; however, Matsubara et al. calculated no significant improvement for either long axis or short axis over performance of SUVmax alone, though their results trend towards significance. Though inconclusive, and providing little in terms of concrete recommendations, together, these findings suggest there may be a role for using SUVmax with a measure of axial length to better identify borderline FDG-avid lymph nodes.
Limitations
As with any retrospective analysis, inherent diagnostic and treatment biases may confound results. Nonetheless, the histopathological nature of the majority of the abnormal lymph nodes (137/174) was determined by either fine needle aspiration (FNA), core biopsy or excisional biopsy. Our data include lymph nodes with potentially micrometastasis or lymph nodes with metastatic involvement that do not appear hypermetabolic on PET/CT. However the purpose of this study was to identify parameters useful in clinical practice to improve assessment of FDG-avid lymph nodes in HNSCC patients.
Although only part of the PET/CT examinations were acquired with intravenous contrast, which may give advantage to the PET/CT reader over studies without contract, all of the patients also underwent thin slice CECT of the neck which was reviewed separately by blinded expert reader. Moreover the analysis of the PET/CT derived parameters was not affected by the presence or absence of contrast. Therefore we do not think that including these two imaging PET/CT protocols had substantial impact on the results.
The computer-assisted generation of select parameters may be incongruous with manually acquired measurements and difficult to replicate in the closely approximated anatomy of the neck. However, validation of our statistically valid results based on SUVmax and size measurements, can be tested without the need for the automated software used for this study.
Conclusions
In summary, the combinatorial analysis of anatomical and functional imaging parameters illustrates the incremental gains in specificity that are achievable when cross-modality combinations of imaging features are used to discriminate malignancy in indeterminate cervical lymph nodes. Our findings highlight the value of highly specific CECT criteria, such as ill-defined margin and focal hypoattenuation, as an initial step in the evaluation of any FDG-avid lymph node. In borderline lymph nodes without these highly specific findings, the sequential combination of functional and anatomic criteria, such as SUVmax plus an indicator of node roundness on axial CECT slices may also be highly specific for metastatic nodal involvement. Together, these findings address the incremental value, and optimal use, of PET/CT and CECT criteria, used alone or in combination, for the evaluation of cervical lymph nodes for malignancy in HNSCC.
References
- National Comprehensive Cancer Network. Clinical Guidelines in Oncology: Head and Neck Cancers. Version 1.2012.
- Leibel SA, Scott CB, Mohiuddin M, Marcial VA, Coia LR, et al. (1991) The effect of local-regional control on distant metastatic dissemination in carcinoma of the head and neck: results of an analysis from the RTOG head and neck database. Int J RadiatOncolBiolPhys 3: 549-556.
- Edge SB (2010) American Joint Committee on Cancer. AJCC cancer staging manual. (7thedn), Springer, New York.
- Hoang JK, Vanka J, Ludwig BJ, Glastonbury CM (2013) Evaluation of cervical lymph nodes in head and neck cancer with CT and MRI: tips, traps and a systematic approach. AJR 200: 17-25.
- Rodrigues RS, Bozza FA, Christian PE, Hoffman JM, Butterfield RI, et al. (2009) Comparison of whole-body PET/CT, dedicated high-resolution head and neck PET/CT, and contrast-enhanced CT in preoperative staging of clinically M0 squamous cell carcinoma of the head and neck. J Nucl Med 8: 1205-1213.
- Nakamura T and Sumi M (2007) Nodal imaging in the neck: recent advances in US, CT and MR imaging of metastatic nodes. EurRadiol 5: 1235-1241.
- Saindane AM (2013) Pitfalls in the staging of cervical lymph node metastasis. Neuroimaging Clin N Am 1: 147-166.
- Lee SH, Huh SH, Jin SM, Rho YS, Yoon DY, et al. (2012) Diagnostic value of only 18Ffluorodeocyglucosepositron emission tomography/computed tomography-positive lymph nodes in head and neck squamous cell carcinoma. Otolaryngol Head Neck Surg 4: 692-698.
- Yoon DY, Hwang HS, Chang SK, Rho YS, Ahn HY, et al. (2009) CT, MR, US,18F-FDG PET/CT, and their combined use for the assessment of cervical lymph node metastases in squamous cell carcinoma of the head and neck. EurRadiol 3: 634-642.
- Sadick M, Schoenberg SO, Hoermann K, Sadick H (2012) Current oncologic concepts and emerging techniques for imaging of head and neck squamous cell cancer. GMS Curr Top Otorhinolaryngol Head Neck Surg 11: 8-9.
- Roh JL, Park JP, Kim JS, Lee JH, Cho KJ, et al. (2014) 18F Fluorodeoxyglucose PET/CT in head and neck squamous cell carcinoma with negative neck palpation findings: A prospective study. Radiology 1: 153-161.
- Kuhn FP, Hullner M, Mader CE, Kastrinidis N, Huber GF, et al. (2014) Contrastenhanced PET/MR imaging versus contrast-enhanced PET/CT in head and neck cancer: how much MR information is needed?. J Nucl Med 55: 551-558.
- Queiroz MA, Hullner M, Kuhn F, Huber G, Meerwein C, et al. (2014) PET/MRI and PET/CT in follow-up of head and neck cancer patients. Eur J Nucl Med Mol Imaging 41: 1066-1075.
- Al-Ibraheem A, Buck A, Krause BJ, Scheidhauer K, Schwaiger M (2009) Clinical Applications of FDG PET and PET/CT in Head and Neck Cancer. J Oncol 2009: 208725-208729.
- Schwartz LH, Colville JA, Ginsberg MS, Wang L, Mazumdar M, et al. (2006) Measuring tumor response and shape change on CT: esophageal cancer as a paradigm. Ann Oncol 6: 1018-1023.
- Erdi YE, Mawlawi O, Larson SM, Imbriaco M, Yeung H, et al. (1997) Segmentation of lung lesion volume by adaptive positron emission tomography image thresholding. Cancer 12: 2505-2509.
- DeLong ER, DeLong DM, Clarke-Pearson DL. (1988) Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 3: 837-845.
- Macaskill P, Walter SD, Irwig L, Franco EL (2002) Assessing the gain in diagnostic performance when combining two diagnostic tests. Stat Med 17: 2527-2546.
- van den Brekel MW, Stel HV, Castelijns JA, Nauta JJ, van der Waal I, et al. (1990) Cervical lymph node metastasis: assessment of radiologic criteria. Radiology 2: 379-384.
- Kaji AV, Mohuchy T, Swartz JD (1997) Imaging of cervical lymphadenopathy. Semin Ultrasound CT MR 3: 220-249.
- Matsubara R, Kawano S, Chikui T, Kiyosue T, Goto Y, et al. (2012) Clinical significance of combined assessment of the maximum standardized uptake value of F-18 FDG PET with nodal size in the diagnosis of cervical lymph node metastasis of oral squamous cell carcinoma. AcadRadiol 6: 708-717.
- Sumi M, Ohki M, Nakamura T (2001) Comparison of sonography and CT for differentiating benign from malignant cervical lymph nodes in patients with squamous cell carcinoma of the head and neck. Am J Roentgenol 4: 1019-1024.
Citation: Marshall RA, Som PM, Uliel L, Genden E, Buckstein M, et al. (2015) Combined Evaluation of FDG-PET/CT and CT Imaging Characteristics of Cervical Lymph Nodes to Increase the Interpretation Accuracy for Nodal Metastatic Involvement in Head and Neck Cancer. Otolaryngol (Sunnyvale) 5:213. DOI: 10.4172/2161-119X.1000213
Copyright: © 2015 Marshall RA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 15025
- [From(publication date): 11-2015 - Nov 25, 2024]
- Breakdown by view type
- HTML page views: 10607
- PDF downloads: 4418