Collision Metastasis of Chronic Lymphocytic Leukemia and Glioblastoma: A Case Report
Received: 27-Jan-2017 / Accepted Date: 16-Feb-2017 / Published Date: 20-Feb-2017 DOI: 10.4172/2161-0681.1000303
Abstract
Collision tumors or metastasis from an extracranial tumor to a primary brain neoplasm are rare but do occur. The majority involve metastases to meningiomas. This case presents the second documented collision of a systemic chronic lymphocytic leukemia (CLL) to a glioblastoma multiforme (GBM). Lymphomas tend to coalesce in perivascular locations, and the characteristic vascularity of glioblastomas may present a very inviting milieu for lymphoma cells.
Keywords: Collision tumor; Chronic lymphocytic leukemia; Glioblastoma
315415Introduction
Collision tumors are relatively rare entities that represent two distinct neoplasms that have invaded each other and contain different underlying histological elements. This phenomenon is relatively rare, especially in the central nervous system. We present a case of a collision tumor of a chronic lymphocytic leukemia with a glioblastoma multiforme.
Case Presentation
A 67-year-old male with a history of untreated stage 0 chronic lymphocytic leukemia (CLL)-for which he was asymptomatic and followed with 6 month CBCs-presented with left-sided incoordination, difficulties with upper extremity fine motor skills bilaterally, and difficulty walking.
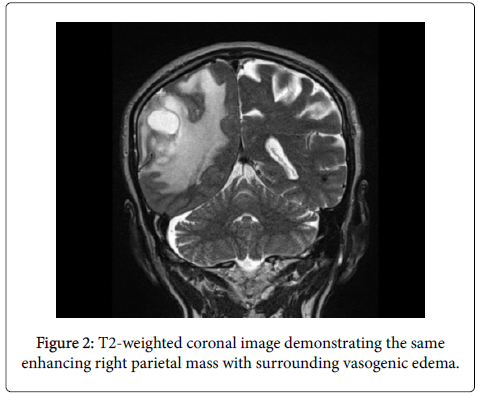
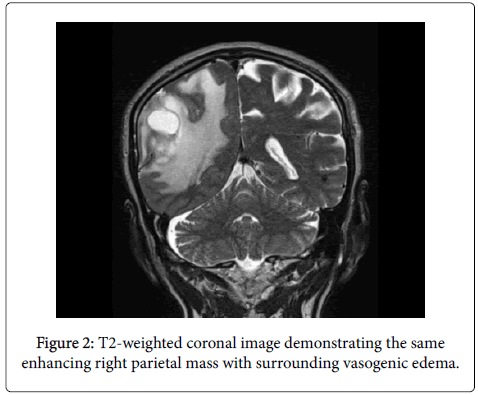
He also endorsed mild intermittent headaches and some confusion. Relevant past medical history includes hyperlipidemia, GERD, and trisomy 13. MRI revealed a large enhancing right parietal mass with surrounding vasogenic edema.
He underwent a gross total resection of the tumor and a month later, was subsequently started on fractionated external beam radiation therapy with concomitant temozolomide. He continues to do well and is being followed with close interval imaging studies.
Materials and Methods
Surgical specimens were processed in accordance with standard laboratory guidelines and were formalin-fixed and paraffin-embedded (FFPE). Routine sections were stained with hematoxylin and eosin (H&E).
Immunohistochemistry was performed using an Envision Flex Visualization, Envision+ Dual Link System, Peroxidase (Dako), ultraView Universal DAB detection kit (Ventana), or a standard streptavidin technique (Jackson) with a DAB or Nova Red (Vector) chromogen for anti-glial fibrillary acidic protein (GFAP), p53, anti- MIB 1 (Ki-67 antigen), IDH-1, CD20, CD3, CD5, CD23, and Pax-5. All controls showed appropriate reactivity (Figures 1 and 2).
Results
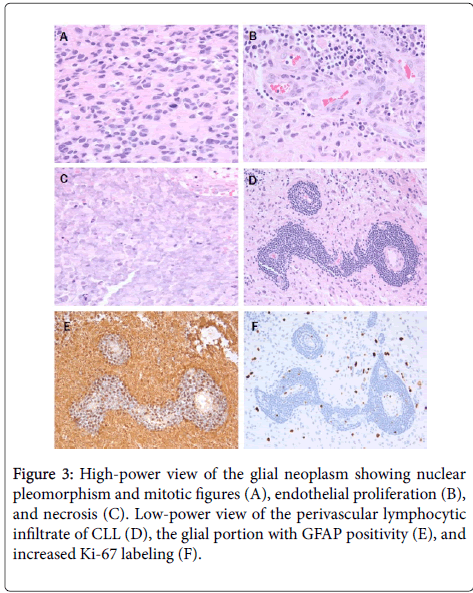
Microscopic examination shows a high-grade glial tumor with hypercellularity, nuclear atypia, frequent mitoses, endothelial proliferation, and necrosis (Figure 3).
A, B, and C photomicrographs are at 10x eyepiece and 40x objective (400x), while D, E, and F photomicrographs are at 10x eyepiece and 20x objective (200x).
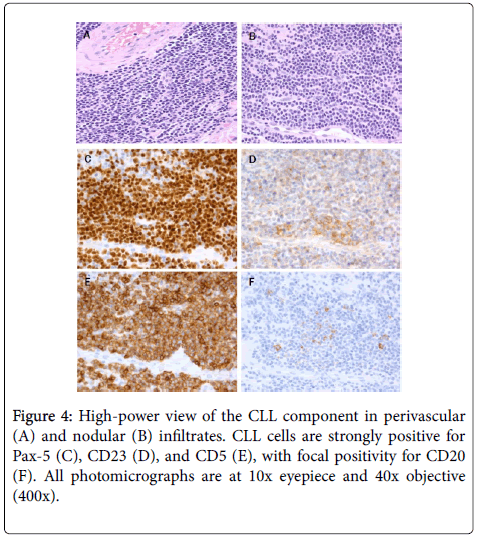
Diagnosis is glioblastoma, WHO grade IV, IDH-1 wild-type (WHO 2016). Present throughout the tumor, concentrated perivascularly (Figures 3 and 4) but also in larger nodules (Figure 4), are collections of small lymphocytes with round to slightly irregular nuclei, condensed chromatin, and scant cytoplasm.
Immunohistochemically, the glial portion was robustly positive for GFAP (Figure 3) and showed increased Ki-67 positivity (Figure 3). The lymphocytic component was strongly positive for Pax-5, CD23, and CD5 (Figure 4) with weak staining for CD20 (Figure 4) and scattered reactive CD3 positive T-cells. This phenotype is essentially the same as the systemic CLL diagnosed 6 years previously (Figure 4).
Discussion
Collision tumors, which encompass tumor-to-tumor metastases, have been described in the literature, though reports of intracranial collision tumors are rare. Meningiomas are the most common intracranial recipient tumor [1-5], with far fewer studies reporting a glioma-and more specifically, a glioblastoma-as the recipient tumor [6-8]. Many collision tumors are not identified until autopsy.
Several studies have reported a collision metastasis between multiple primary brain tumors, such as between a meningioma and a glioma [9,10]. One previous case report also demonstrated a tumor-totumor metastasis in a patient with chronic lymphoid leukemia and glioblastoma. Various hypotheses have been proposed to explain this phenomenon. Pure coincidence, genetic predisposition, and the possibility that one tumor may serve as an irritating factor that leads to the growth of the second tumor [11,12]. Among recipient intracranial tumors, meningiomas are the most common, with many studies reporting collision tumors between various primary tumors, including renal cell carcinomas, breast cancers, and lung cancers, and meningiomas [1,13-15]. The mechanism has not been fully defined, but several hypotheses include the relatively slow growth of meningiomas, which leads to a longer exposure to the donor tumor, and the highly vascular nature of meningiomas that make them susceptible to seeding from extracranial primary tumors [16-18]. Yet another potential explanation is a defective blood-brain barrier that predisposes intracerebral tumors to hematologic metastases; briefly,c once the primary tumor has vascularized, cancer cells can then invade blood vessels and migrate into distant organs, including the brain [2,6].
In our case, the patient was an elderly male with a past medical history significant for stage 0 CLL who presented with an enhancing right parietal mass. Surgical resection was completed, and formal pathololgic analysis confirmed the presence of co-exisiting CLL and glioblastoma multiforme tumor cells. We report the unique case of a metastatic hematologic tumor integrating with a gliobastoma multiforme and suggest that a dense lymphocytic infiltrate be appropriately worked-up in the presence of an obvious primary intracranial tumor.
References
- Han HS, Kim EY, Han JY, Kim YB, Hwang TS, et al. (2000) Metastatic renal cell carcinoma in a meningioma: a case report. J Korean Med Sci 15: 593-597.
- Tally PW, Laws ER Jr, Scheithauer BW (1988) Metastases of central nervous system neoplasms. Case report. J Neurosurg 68: 811-816.
- Boice JD (1981) Cancer following medical irradiation. Cancer 47: 1081-1090.
- Lee A, Wallace C, Rewcastle B, Sutherland G (1998) Metastases to meningioma. AJNR Am J Neuroradiol 19: 1120-1122.
- Joglekar VM, Davis CH, Blakeney CG (1981) Metastasis of carcinoma to meningioma and glioma. Acta Neurochir (Wien) 58: 67-74.
- Erdogan H, Aydin MV, Tasdemiroglu E (2014) Tumor-to-tumor metastasis of the central nervous system. Turk Neurosurg 24: 151-162.
- Franke FE, Altmannsberger M, Schachenmayr W (1990) Metastasis of renal carcinoma colliding with glioblastoma. Carcinoma to glioma: an event only rarely detected. Acta Neuropathol 80: 448-452.
- Posnikoff J, Stratford J (1960) Carcinoma metastasis to malignant glioma; case report. Arch Neurol 3: 559-563.
- Prayson RA, Chowdhary S, Woodhouse S, Hanson M, Nair S (2002) Collision of a syncytial meningioma and malignant astrocytoma. Ann Diagn Pathol 6: 44-48.
- Vaquero J, Coca S, MartÃnez R, Jiménez C (1990) Convexity meningioma and glioblastoma in collision. Surg Neurol 33: 139-141.
- Tokunaga T, Shigemori M, Hirohata M, Sugita Y, Miyagi J, et al. (1991) Multiple primary brain tumors of different histological types-report of two cases. Neurologia medico-chirurgica 31: 141-145.
- Lee EJ, Chang CH, Wang LC, Hung YC, Chen HH (2002) Two primary brain tumors, meningioma and glioblastoma multiforme, in opposite hemispheres of the same patient. J Clin Neurosci 9: 589-591.
- Bhargava P, McGrail KM, Manz HJ, Baidas S (1999) Lung carcinoma presenting as metastasis to intracranial meningioma: case report and review of the literature. Am J Clin Oncol 22: 199-202.
- Bucciero A, del Basso de Caro M, Vizioli L, Carraturo S, Cerillo A, et al. (1992) Metastasis of breast carcinoma to intracranial meningioma. Case report and review of the literature. J neurosur scien 36: 169-172.
- Cervoni L, Salvati M, Gagliardi D, Delfini R (1994) Metastasis of breast carcinoma to intracranial meningioma. Case report. Neurosurg Rev 17: 233-236.
- Schmitt H P (1984) Metastases of malignant neoplasms to intracranial tumours: the "tumour-in-a-tumour" phenomenon. Virchows Archiv 405: 155-160.
- Gore I, Barr R (1958) Metastasis of cancer to cancer. AMA Arch Pathol 66: 293-298.
- Bouillot S, Vignes JR, Guerin J, Dubus P, Vital A (2003) Leukemic dissemination within a glioblastoma in a patient with chronic lymphoid leukemia. Clin Neuropathol 22: 10-13.
Citation: Yan SC, Lakis NS, Stopa EG, Doberstein CE (2017) Collision Metastasis of Chronic Lymphocytic Leukemia and Glioblastoma: A Case Report. J Clin Exp Pathol 7:303. DOI: 10.4172/2161-0681.1000303
Copyright: © 2017 Yan SC, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 5104
- [From(publication date): 0-2017 - Jul 02, 2025]
- Breakdown by view type
- HTML page views: 4216
- PDF downloads: 888