Colchicine and Covid-19: Could there still be Hope for this Old Low-Cost Drug?: Multicenter Observational Study in Hospitalized Patients
Received: 11-Aug-2022 / Manuscript No. jcidp-22- 71673 / Editor assigned: 16-Aug-2022 / PreQC No. jcidp-22- 71673 (PQ) / Reviewed: 30-Aug-2022 / QC No. jcidp-22- 71673 / Revised: 05-Sep-2022 / Manuscript No. jcidp-22- 71673 (R) / Published Date: 12-Sep-2022
Abstract
Background: Usefulness of colchicine in hospitalized COVID-19 patients with pneumonia has been compared with standard of care (SOC) in several studies around the world with many different results. Taken on account the variability of patient’s characteristics and their SOC according to the country, we carried out the following analysis in order to contribute with more data to the knowledge of this treatment.
Objective: To evaluate whether treatment with colchicine reduced the rate of death in COVID-19 hospitalized patients.Length of stay, differences in response by sex and age, response to colchicine according to inflammatory markers, comorbidity and concomitant drugs prescribed to treat these patients were also analyzed.
Methods: Real-world, controlled, retrospective study carried out in two tertiary hospitals in Spain. Outcomes werecompared in patients who received colchicine (colchicine group-CG) with those inpatients who did not (non-colchicine group-NCG). Controls were matched to the CG 1:1 by age (±2 years), sex and severity of the disease.
Results: 222 patients were evaluated (111 treated with colchicine), median age 79 years (66-87). There were 19(17.1%) deaths in the CG and in 32(29.4%) in the NCG (OR: 0.497;95%CI:0.261–0.946;p=0.031). A longer hospital stays in CG with respect to NCG (13[7-20] vs. 10 [6-15], respectively; p=0.019) was observed. Proportion of deaths were significantly higher in the NCG than in CG in patients≥ 70years (p=0.012). A greater benefit of colchicine treatment was detected for men even though our data did not reach significant differences. There were differences in deaths between the CG and NCG in patients with CRP high levels (p=0.046). 88.7% of patients had comorbidities, most frequently systemic hypertension, diabetes, COPD and cardiovascular disease, with no differences in deaths between both groups. Almost all patients received antimicrobials (91.9%) concomitantly, mainly azithromycin and ceftriaxone.We found differences in death rate between two groups in patients using antibiotics (38% in CG vs. 62% in NCG) (p=0.023).
Conclusion: Our findings support that colchicine may reduce mortality in COVID-19 hospitalized patients with pneumonia. These results indicate that, despite the available data, more RCTs are still needed in order to identify which patients hospitalized for COVID-19 pneumonia may benefit from this safe and inexpensive drug.
Introduction
To date a large number of hospitalized patients with COVID-19 around the world have received off-label treatments. Most of them have been prescribed with limited clinical evidence supporting their efficacy and safety against this new disease [1]. Among drugs currently used to treat these patients, some of them very expensive, only dexamethasone proved to reduce mortality [2].
In this scenario, colchicine has been postulated as a potential effective drug for these patients. This is a tricyclic alkaloid (C22H25NO) extracted from Colchicum autumnale that has historically been used for the gout treatment. It was first recommended for the treatment of gout by Alexander of Tralles in the 6th century BC. Colchicine is a powerful anti-inflammatory drug that acts by inhibiting the polymerization of tubulin with an effect on the inflammasome and pro-inflammatory mediators [3]. Consequently, it was thought that it could be useful in the control of the inflammatory complications of COVID-19.
Numerous studies that have analyzed the usefulness of this drug in hospitalized patients with different results, in many cases discrepant [4,5,6]. Chiu, et al. in a meta-analysis (16,248 patients) observed that, excluding the Recovery trial [7], which reported no difference in mortality, colchicine seems to be associated with a lower risk of mortality among ICU patients and non-ICU patients, in observational studies and in randomized controlled trials.
Given that colchicine is generally a well-tolerated drug, it has a good safety profile as shown in a meta-analysis on the adverse effects of colchicine [8] and bearing in mind that it is a very low-cost medicine, there is still a potential for further investigation in the use of colchicine for COVID-19 inpatients.
In this context, taking into account that most studies compare the effect of colchicine with standard of care, and that this has varied between different countries, we have believed it of interest to contribute with our data to the knowledge of the impact of this drug on a particular region where these patients have received similar standard of care. Thus, we carried out the following study in Madrid (Spain) where the same care settings were implemented over time since the start of the pandemic. We consider this to be important due to the variability in the data available both in the characteristics of hospitalized COVID-19 pneumonia patients and their standard of care in the different regions.
The primary efficacy endpoint was a composite of death or discharge. As secondary endpoints we considered: the length of stay (LOS), differences in response to colchicine according to the total dose, gender and age, response to colchicine according to inflammatory markers, comorbidities, and other drugs prescribed to treat coronavirus.
Methodology
We conducted a real-world, controlled, retrospective cohort study in two of the hospitals with the largest number of patients admitted with COVID-19 pneumonia in Madrid, the epicenter of the pandemic in Spain. Physicians prescribed therapies according to the guidelines in our region regarding the pharmacological management of COVID-19 inpatients. Physicians prescribed treatments using a computerized physician order entry (CPOE) programme where they also recorded the diagnosis and severity. Treatments of all admitted adults who tested positive were reviewed. The Institutional Review Board at both centres that participated in this study approved the protocol.
Study Population
We included all individuals, 18 years or older, who were hospitalised in both hospitals (a total of 1,586 beds) with a diagnosis of COVID-19 pneumonia from March to June 2020, who received colchicine (colchicine therapy group [CG]). Patients not hospitalised or discharged from the emergency department after a stay of less than 24 hours were not included. Also, we excluded COVID-19 patients admitted to critical care units.
Control group comprised patients who met inclusion criteria and did not received colchicine (non-colchicine therapy group [NCG]). Controls were matched to the CG 1:1 by age (± 2 years), sex, comorbidity and severity of the disease. To select controls, we chose the consecutively next admitted patient after one treated with colchicine. This allowed us to select control subjects at a close time and place to cases, that is, under similar circumstances in terms of patient care settings. Severity of the disease was evaluated according to the Spanish Official Document on the management of COVID-19. It considered mild pneumonia as oxygen saturation higher than 90%, with no signs of severity and a CURB-65 pneumonia severity score lower than 2; and severe pneumonia as organ failure, oxygen saturation lower than 90% or respiratory rate higher than 30 [9].
To diagnosis COVID-19, test result for COVID-19 virus from analysis of nasopharyngeal or oropharyngeal swab samples were obtained during hospitalization. These tests were carried out by hospital health personnel and results were registered by physicians in the Electronic Medical Record (EMR).
From the EMR, we obtained data from all prescription events of hospitalised patients with COVID-19 in both groups. The data obtained included patients’ demographic details, comorbidities, systemic inflammatory response to COVID-19 (ferritin, fibrinogen, D-dimer and C-reactive protein [CRP]) and medication administration data. Inflammatory biomarkers were collected the day that the colchicine treatment was initiated in the CG, and in the NCG we registered them the same day used to its CG matched patient.
Statistical Analysis
A database was designed to reflect the case report content form, in which a data entry matrix with possible ranges or values was established, along with the various consistency rules between variables. The quality of information received through exploratory analysis was aimed at detecting discrepancies in the values, out-of-range or missing values. An exploratory analysis also provided information on the distribution of the main variables to be studied and provided guidance on possible transformations.
Quantitative variables were expressed as medians [interquartile range] and categorical data as frequencies (proportion). For the non-parametric analysis, the Mann-Whitney U test was used. For the associations between qualitative variables, the chi-squared test (or Fisher´s test when necessary) was used. The hospital LOS was dichotomized intro ≤ 10 and > 10 days, given it corresponded to the median of the included population. For this purpose, statistical significance was set at a p value ≤ 0,05. The SPSS (IBM SPSS Statistic version 19.0.) programme was used to analyze the data.
Results
Characteristics of the Cohort
The pharmacotherapy of 222 (111 treated with colchicine) hospitalised patients with COVID-19 was analyzed. The median age of participants was 79 years [66 – 88] (81 years [66-87] in CG vs. 79 years [66-88] in NCG; p=0.978). In total, 52.3% were men (54.1% in CG vs. 50.5% in NCG, p=0.591). Patient´s characteristics are shown in [Table 1].
| Characteristic | CG | NCG | p |
|---|---|---|---|
| N=111 | N=111 | ||
| Age, years | 81 [66 – 87] | 79 [66 – 88] | 0.978 |
| ≥70 years | 81 (73) | 80 (72.1) | 0.88 |
| Males | 60/111 (54.1) | 56/111 (50.5) | 0.591 |
| Comorbidity | 98 (88.3) | 99 (89.2) | 0.832 |
Table 1: Patient´s characteristics
Data expressed as median [interquartile range] or number (percentage). Comparisons between groups by Mann-Whitney U test and chi-squared test
Abbreviations: CG=Colchicine therapy group; NCG=Non-colchicine therapy group
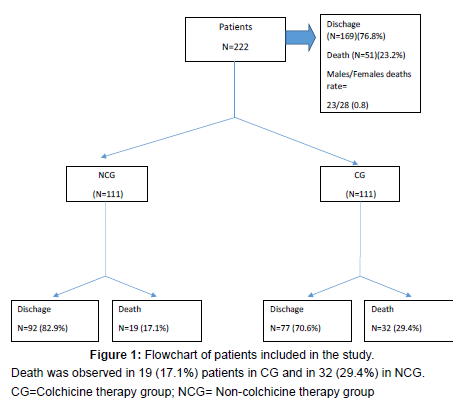
Primary endpoint of death occurred in 19 (17.1%) patients in the CG as compared with 32 (29.4%) in the NCG (OR: 0.497; 95%CI: 0.261–0.946; p=0.031). [Figure 1] shows a flowchart of the study and rate of deaths and discharges in both groups.
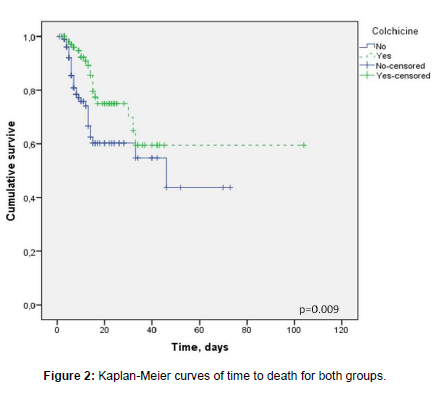
Differences in the proportion of deaths in both groups were supported by a higher hospital LOS in CG with respect to NCG (13 [7-20] vs. 10 [6-15], respectively; p= 0.019). When hospital LOS was categorized as > 10 or ≤10 days (10 days was the median length of hospitalization of these patients), the use of colchicine was associated with a longer hospital LOS when comparing with the control group (OR 1.856; 95% CI: 1.089 – 3.162; p=0.022) [Table 2]. Likewise, time to death was longer in the CG than in the NCG (p=0.009) [Figure 2].
| Analysis | CG | NCG | P value |
|---|---|---|---|
| (N=111) | (N=111) | ||
| >10 days | 66 (57.4%) | 49 (42.6%) | 0.022 |
Table 2: Association between colchicine use and the length of stay in hospital categorised as > or ≤ 10 days
Data expressed as number (percentage). Comparisons between groups by chi-squared test
Abbreviations: CG=Colchicine therapy group; NCG=Non-colchicine therapy group
Median total dose of colchicine administered during hospitalization in the CG was 7.5 mg [3.5-12]. In the CG, no differences in death rates were found when categorizing total colchicine doses by ≤ 7.5 mg or > 7.5 mg [Table 3].
| Total dose of colchicine | N | Death | Death rate | p |
|---|---|---|---|---|
| ≤ 7.5 mg | 64 | 8 | 12.5% | 0.132 |
| > 7.5 mg | 47 | 11 | 23.4% | |
Table 3: Death rate in the colchicine group according to categorized total dose of colchicine received during hospitalization
Data expressed as number (percentage). Comparisons between groups by chi-squared test
Abbreviations: CG=Colchicine therapy group; NCG=Non-colchicine therapy group
Data were disaggregated by age (< or ≥ 70 years), and by sex. Proportion of deaths were higher in the NCG than in CG in patients ≥ 70 years (p=0.012). With respect to sex, distribution of deaths showed that in males nine deaths occurred in the CG whereas 14 in the NCG; and in females 10 deaths were identified in the CG and 18 in the NCG without significant differences founded [Table 4].
| Characteristic | CG death | NCG death | p |
|---|---|---|---|
| Age ≥ 70 years | 17/81 (21%) | 31/79 (39.2%) | 0.012 |
| Age < 70 years | 2/30 (6.7%) | 1/30 (3.3%) | 1 |
| Males | 9/60 (15%) | 14/56 (25%) | 0.177 |
| Females | 10/51 (19.6%) | 18/53 (34%) | 0.099 |
Table 4: Differences in the primary endpoint according to age and sex
Data expressed as number (percentage). Comparisons between groups by chi-squared test
Abbreviations: CG=Colchicine therapy group; NCG=Non-colchicine therapy group
Regarding systemic inflammatory response against COVID-19, patients were categorized according to the upper-limits of normal levels of the systemic inflammatory biomarkers. We observed a significant higher proportion of deaths in the CG compared with the NCG according to their CRP levels [Table 5].
| Inflammatory marker | Death | p | |
|---|---|---|---|
| CG | NCG | ||
| Ferritin ≥ 322 ng/dL | 2/6 (33.3%) | 4/6 (66.7%) | 0.349 |
| Fibrinogen ≥450 mg/dL | 15/40 (37.5%) | 25/40 (62,5%) | 0.057 |
| D-dimer ≥ 500 ng/mL | 16/35 (45.7%) | 19/35 (54.3%) | 0.167 |
| CRP ≥ 5 mg/mL | 19/50 (38%) | 31/50 (62%) | 0.046 |
Table 5: Differences in the death rate in colchicine and non-colchicine group according to systemic inflammatory response
Data expressed as number (percentage). Comparisons between groups by chi-squared test
Abbreviations: CG=Colchicine therapy group; CRP=C-reactive protein; NCG=Non-colchicine therapy group
In our study we also evaluated differences in response to colchicine treatment according to the comorbidity of the patients. In total, 88.7% of patients had comorbidities, most frequently systemic hypertension, diabetes, COPD and cardiovascular disease, with no differences between both groups.
Finally, in terms of pharmacotherapy against COVID-19, we observed that the most frequent treatment used was a combination of hydroxichloroquine and ceftriaxone. Almost all patients received antimicrobials (91.9%) concomitantly, most frequently azithromycin and ceftriaxone, both in patients treated and not treated with colchicine. When comparing both groups, the greatest difference in death rate between two groups was detected for patients using antibiotics (p= 0.023) [Table 6].
| Death | |||
|---|---|---|---|
| Concomitant treatments | CG | NCG | p |
| Hidroxicloroquine | 16/39 (41%) | 23/39 (59%) | 0.076 |
| Lopinavir/ritonavir | 0 | 4/4 (100%) | 0.236 |
| Corticosteroids | 12/28 (42.9%) | 16/28 (57.1%) | 0.096 |
| Tocilizumab | 3/6 (50%) | 3/6 (50%) | 0.669 |
| Remdesivir | 0 | 0 | NC |
| Antibiotic | 19/50 (38%) | 31/50 (62%)) | 0.023 |
| Azitromicine | 9/19 (47.4%) | 10/19 (52.6%) | 0.517 |
| Ceftriaxone | 16/44 (36.4%) | 28/44 (63.6%) | 0.022 |
| Levofloxacine | 4/12 (33.3%) | 8/12 (66.7%) | 0.232 |
Table 6: Differences in deaths of colchicine and control group according to their concomitant treatments for COVID-19
Data expressed as number (percentage). Comparisons between groups by chi-squared test
Abbreviations: CG=Colchicine therapy group; NCG=Non-colchicine therapy group
Discussion
The present study suggests a significant clinical benefit from colchicine in patients hospitalized with COVID-19. Our results show that the risk of death was significantly lower among admitted patients who received colchicine (OR: 0.497; 95CI: 0.261 –0,946; p=0.031). Different results, in many cases discrepant have been published on this topic. Many other authors have observed mixed results on mortality in hospitalized patients who received colchicine to treat this disease [10- 17].
It is known that COVID-19 is linked to the hyper inflammatory response of the body characterized by pathological cytokine levels [18]. As it has been already described, SARS-CoV-2 activate the NLRP3 inflammasome, which is inhibited by colchicine leading to decreased levels of interleukins (IL-1β, IL-18 and IL-6) and CRP [19,20]. This could explain the potential efficacy of these anti-inflammatory drugs for COVID-19 patients.
In terms of LOS, we found a longer hospitalization period in patients treated with colchicine compared with the control group. However, other studies analysing this outcome detected a shortened period of hospitalization for patients treated with colchicine [5,13,15,21].
The potential role of gender was also evaluated since susceptibility to the SARS-Cov-2 infection seems to affect more to men [22, 23]. Our data showed that apparently women were more beneficiated by colchicine treatment than men even though there was not statistical significance. It is known that men have higher plasma concentrations of IL-18 and IL-8. By contrast, female patients had more robust T cell activation than male patients during SARS-CoV-2 [19]. which could explain these differences. Moreover, epidemiological findings reported higher vulnerability in males than females probably due to a higher expression of angiotensin-converting enzyme-2 (ACE 2; receptors for coronavirus) and immunological differences driven by differences in hormones between them [20] Some authors, in agreement with us, have proved a worse clinical evolution in men, with higher rates of mortality [11, 24].Moreover, in our study a greater benefit of colchicine treatment in patients older than 70 years was detected.
Since inflammatory markers have been used to predict the risk of progression to severe disease [25], we also compared groups according to these parameters before initiating colchicine treatment. With the exception of ferritin, our results revealed a higher proportion of patients with inflammatory markers above the normal range in the control group. Statistical significance was reached only for CPR in terms of better response to colchicine.
Deftereos et al. [26] observed that COVID-19 patients decreased their levels of D-dimer once treated with colchicine. This effect has anti-inflammatory properties on the endothelium [27,28], which may be relevant in patients with COVID-19, elevated D-dimer levels have been reported in patients with worse prognosis [29,30]. Higher ferritin levels have been associated with poor prognosis of COVID-19 patients as well [31].
As in other observational cohorts [11,13,15] our results reveal that patients with coronavirus had numerous comorbidities (88.7%). In agreement, the most common of which were systemic hypertension [32-37]. Nevertheless, we did not find remarkable differences between patients treated and not treated with colchicine in terms of comorbidity.
The most common concomitant treatment for admitted COVID-19 patients was hydroxychloroquine and corticosteroids. When comparing deaths in both groups according to COVID-19 treatments we found significant differences in favor of the colchicine group in death rate for patients treated concomitantly with hydroxychloroquine and antibiotics, mainly ceftriaxone (p<0.05). Currently, the evidence available for the management of patients affected by COVID-19 is limited. Among the drugs currently recommended by the Spanish Ministry of Health for the treatment of hospitalized patients with COVID-19 are remdesivir and dexamethasone (the only one with which a reduction in mortality has been confirmed) [38]. Scarci et al. [11] in line with us observed that among the colchicine group, there was no difference between patients who were treated or not with dexamethasone or with hydroxychloroquine.
The assessment of colchicine prescription in a setting previously scarcely described such as COVID-19 hospitalized patients in our region renders clinical relevance and strength to this study. However, it is limited by weaknesses inherent in an observational retrospective study. Other limitations may be that there could have been patients who met inclusion criteria but were not assessed because of missing data for some variables and potential for inaccuracies in the electronic health records such as lack of documentation of coexisting illness for some patients.
In conclusion, our findings support that colchicine may reduce mortality in COVID-19 hospitalized patients with pneumonia. These results indicate that, despite the available data, more RCTs are still needed to prove the usefulness of this safe and inexpensive drug.
References
- Kalil AC (2020) Treating COVID-19-Off-Label Drug Use, Compassionate Use, and Randomized Clinical Trials During Pandemics. JAMA. 323: 1897-1898.
- Llover MN, Jiménez MC (2021) Estado actual de los tratamientos para la COVID-19. FMC. 1: 40-56.
- Villamañán E, Larrubia Y, Ruano M (2012) Colchicine: what's up, doc? Med Clin. 139: 295-299.
- Chiu L, Lo CH, Shen M, Chiu N, Aggarwal R, et al. (2021) Colchicine use in patients with COVID-19: A systematic review and meta-analysis. PLoS One. 16: e0261358.
- Toro-Huamanchumo CJ, Benites-Meza JK, Mamani-García CS, Bustamante-Paytan D, Gracia-Ramos AE, et al. (2022) Efficacy of Colchicine in the Treatment of COVID-19 Patients: A Systematic Review and Meta-Analysis. J Clin Med. 11: 2615.
- Zein AFMZ, Raffaello WM (2022) Effect of colchicine on mortality in patients with COVID-19 - A systematic review and meta-analysis. Diabetes Metab Syndr. 16: 102395.
- RECOVERY Collaborative Group (2021) Colchicine in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet Respir Med. 9: 1419-1426.
- Sandhu T, Tieng A, Chilimuri S, Franchin G (2020) A Case Control Study to Evaluate the Impact of Colchicine on Patients Admitted to the Hospital with Moderate to Severe COVID-19 Infection. Can J Infect Dis Med Microbiol. 2020: 8865954.
- Clinical management of COVID-19 hospital care.
- Lopes MI, Bonjorno LP, Giannini MC, Amaral NB, Menezes PI, et al. (2021) Beneficial effects of colchicine for moderate to severe COVID-19: a randomised, double-blinded, placebo-controlled clinical trial. RMD Open. 7: e001455.
- Scarsi M, Piantoni S, Colombo E, Airó P, Richini D, et al. (2020) Association between treatment with colchicine and improved survival in a single-centre cohort of adult hospitalised patients with COVID-19 pneumonia and acute respiratory distress syndrome. Ann Rheum Dis. 79: 1286-1289.
- Manenti L, Maggiore U, Fiaccadori E, Meschi T, Antoni AD, et al. (2021) Reduced mortality in COVID-19 patients treated with colchicine: Results from a retrospective, observational study. PLoS One. 16: e0248276.
- Chiu L, Lo CH, Shen M, Chiu N, Aggarwal R, et al. (2021) Colchicine use in patients with COVID-19: A systematic review and meta-analysis. PLoS One. 16: e0261358.
- Toro-Huamanchumo CJ, Benites-Meza JK, Mamani-García CS, Bustamante-Paytan D, Gracia-Ramos AE, et al. (2022) Efficacy of Colchicine in the Treatment of COVID-19 Patients: A Systematic Review and Meta-Analysis. J Clin Med. 11: 2615.
- Zein AFMZ, Raffaello WM (2022) Effect of colchicine on mortality in patients with COVID-19 - A systematic review and meta-analysis. Diabetes Metab Syndr. 16: 102395.
- Lan SH, Hsu CK, Lai CC, Chang SP, Lu LC, et al. (2022) Effect of colchicine on the outcomes of patients with COVID-19: a systematic review and meta-analysis of randomised controlled trials. Ann Med. 54: 1956-1965.
- Kow CS, Lee LH, Ramachandram DS, Hasan SS, Ming LC, et al. (2022) The effect of colchicine on mortality outcome and duration of hospital stay in patients with COVID-19: A meta-analysis of randomized trials. Immun Inflamm Dis. 10: 255-264.
- Fara A, Mitrev Z, Rosalia RA, Assas BM (2020) Cytokine storm and COVID-19: a chronicle of pro-inflammatory cytokines. Open Biol. 10: 200160.
- Tardif JC, Kouz S, Waters DD, Bertrand OF, Diaz R, et al. (2019) Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. N Engl J Med 381: 2497-2505.
- Martínez GJ, Robertson S, Barraclough J, Xia Q, Mallat Z, et al. (2015) Colchicine acutely suppresses local cardiac production of inflammatory cytokines in patients with an acute coronary syndrome. J Am Heart Assoc. 4: e002128.
- Kow CS, Lee LH, Ramachandram DS, Hasan SS, Ming LC, et al. (2022) The effect of colchicine on mortality outcome and duration of hospital stay in patients with COVID-19: A meta-analysis of randomized trials. Immun Inflamm Dis. 10: 255-264.
- Bwire GM (2020) Coronavirus: Why men are more vulnerable to covid-19 than women? SN Compr Clin Med. 4: 1-3.
- Takahashi T, Ellingson MK, Wong P, Israelow B, Lucas C, et al. (2020) Sex differences in immune responses that underlie COVID-19 disease outcomes. Nature. 588: 315-320.
- Mi J, Zhong W, Huang C, Zhang W, Tan L, et al. (2020) Gender, age and comorbidities as the main prognostic factors in patients with COVID-19 pneumonia. Am J Transl Res. 12: 6537-6548.
- Chilimuri S, Sun H, Alemam A, Mantri N, Shehi E, et al. (2020) Predictors of mortality in adults admitted with covid-19: Retrospective cohort study from New York City. West J Emerg Med. 21: 779-784.
- Deftereos SG, Giannopoulos G, Vrachatis DA, Siasos GD, Giotaki SG, et al. (2020) Effect of colchicine vs. standard care on cardiac and inflammatory biomarkers and clinical outcomes in patients hospitalized with coronavirus disease 2019: The GRECCO-19 randomized clinical trial. JAMA Netw Open. 3: e2013136.
- Angelidis C, Kotsialou Z, Kossyvakis C, Vrettou AR, Zacharoulis A, et al. (2018) Colchicine pharmacokinetics and mechanism of action. Curr Pharm Des. 24: 659-663.
- Leung YY, Yao Hui LL, Kraus VB (2015) Colchicine-update on mechanisms of action and therapeutic uses. Semin Arthritis Rheum. 45: 341-350.
- Zhou F, Yu T, Du R, Fan G, Liu Y, et al. (2020) Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 395: 1054-1062.
- Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, et al. (2020) COVID-19 and thrombotic or thromboembolic disease: Implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J Am Coll Cardiol. 75: 2950-2973.
- Cheng L, Li H, Li L, Liu C, Yan S, et al. (2020) Ferritin in the coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis. J Clin Lab Anal. 34: e23618.
- Goyal P, Choi JJ, Pinheiro LC, Schenck EJ, Chen R, et al. (2020) Clinical Characteristics of Covid-19 in New York City. N Engl J Med. 382: 2372-2374.
- Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, et al. (2020) Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with covid-19 in the New York City area. JAMA. 323: 2052-2059.
- Zhou F, Yu T, Du R, Fan G, Liu Y, et al. (2020) Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 395: 1054-1062.
- Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, et al.(2020) Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 382: 1708-1720.
- Huang C, Wang Y, Li X, Ren L, Zhao J, et al.(2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 395: 497–506.
- Borobia AM, Carcas AJ, Arnalich F, Álvarez-Sala R, Monserrat-Villatoro J, et al. (2020) A cohort of patients with COVID-19 in a major teaching hospital in Europe. J Clin Med. 9: 1733.
- Llover MN, Jiménez MC (2021) Estado actual de los tratamientos para la COVID-19. FMC. 28: 40-56.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Villamanan E, Sobrino C, Carpio C, Mateos C, Larrubia Y, Zamarron E, et al. (2022) Colchicine and Covid-19: Could there still be Hope for this Old Low- Cost Drug?: Multicenter Observational Study in Hospitalized Patients. J Clin Infect Dis Pract, 7: 158.
Copyright: © 2022 Villamanan E, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Usage
- Total views: 3445
- [From(publication date): 0-2022 - Dec 08, 2025]
- Breakdown by view type
- HTML page views: 2937
- PDF downloads: 508