Cognitive Profile of Glaucoma Patients
Received: 04-Jul-2022 / Manuscript No. JADP-22-69212 / Editor assigned: 07-Jul-2022 / PreQC No. JADP-22-69212 (PQ) / Reviewed: 21-Jul-2022 / QC No. JADP-22-69212 / Revised: 28-Jul-2022 / Manuscript No. JADP-22-69212 (R) / Published Date: 04-Aug-2022 DOI: 10.4172/2161-0460.1000546
Abstract
Aim: The study was to evaluate neuropsychological functions in patients with glaucoma.
Materials and methods: A complete examination of 90 patients was performed, which were divided into 3 groups of 30 people each: Alzheimer's disease group, vascular open-angle glaucoma. All patients underwent clinical, neurological and neuropsychological examination.
Results: The data on the similarity of the structure of cognitive deficit in patients with Alzheimer's disease and glaucoma were obtained. More than half (50%) of patients with open-angle glaucoma were newly diagnosed with moderate cognitive impairment.
Conclusion: The results indicate the need for a comprehensive neurological and neuropsychological examination of patients with glaucoma for the early diagnosis of cognitive disorders, the appointment of timely therapy and an improvement in the prognosis of the disease.
Keywords
Alzheimer's disease; Glaucoma; Neuropsychological research; Cognitive impairment
Introduction
Primary open-angle glaucoma and Alzheimer's Disease (AD) are the leading causes of disability and addiction among working-age patients worldwide. Recently, in Russia, the leading cause of primary disability for ophthalmic diseases is glaucoma. Glaucoma occupies one of the first places among the causes of amaurosis, becoming an important medical and social problem, since every 3rd person suffering from blindness is of working age. Over 20 years, the number of glaucoma patients in Russia has increased by 40%, glaucoma accounted for more than 15% of the causes of blindness [1]. With an increase in the life expectancy of the population, the prevalence of glaucoma is expected to increase from 64.3 million in 2013 to 111.8 million by 2040. According to statistical analysis, from 2020 to 2040, the number of patients suffering from glaucoma will increase by 1.5 times [2].
The prevalence of dementia is similar to the prevalence of glaucoma, the global incidence in 2010 was estimated at 35.6 million people, it is expected to increase the number of patients to 75 million people by 2030, and by 2050 the number of patients will be 130 million people. It is expected to increase the number of patients with BA by 1.5 times from 2030 to 2050. BA occupies a leading place in the structure of dementia, accounting for 56.6% of cases. The second most important cause of severe cognitive decline is vascular dementia, which accounts for 14.6% of cases [3].
The comorbidity of BA and glaucoma is very high. The overall prevalence of glaucoma in the population is estimated at about 3%, while the prevalence among patients with AD is about 25.9% [4,5]. However, the diagnosis of ophthalmological diseases, in particular glaucoma, causes great difficulties in patients suffering from AD.
Firstly, the diagnostic criterion for the diagnosis of glaucoma is static perimetry, this is a non-invasive method of assessing visual fields, and the patient reports the appearance of an object during the study. This diagnostic method requires understanding and performing a new task, focusing attention for about 20 minutes, which is difficult for patients with AD [6].
Secondly, patients with AD are characterized by visual agnosia, in particular simultaneous agnosia, or as it is also called Balint syndrome. The patient perceives only the object that is in close attention, that is, the ability to simultaneously perceive several objects or to cover the situation depicted in the plot picture is lost. Therefore, the results of statistical perimetry will not be reliable.
The increase in the prevalence of AD and glaucoma with age, the common embryological origin of the brain and eye, common pathogenetic mechanisms (protein metabolism disorders, oxidative stress, and tissue hypoxia) suggest a fairly close relationship between these diseases [7]. It is assumed that glaucoma occupies a middle position between ophthalmological and neurological pathology [8]. Back in 1990, S. Fedorov postulated that the majority of elderly patients (up to 45%) who are diagnosed with ophthalmological pathology requiring treatment by an ophthalmologist already have various neurological diseases, some of which are observed by a neurologist [9]. Quite often (56.8%) ophthalmologists pay their attention to the combined damage of the nervous system and the eye. To date, scientists around the world consider glaucoma as a progressive degeneration of the optic nerve, which is accompanied by visual disturbances secondary to the loss of retinal ganglion cells [10]. Gupta et al., for the first time discovered neurodegenerative changes on autopsy material of the brain in patients with glaucoma, in the form of amyloid deposition in the chiasm, cranial bodies, and visual radiation and even in the occipital lobes, which is pathognomonic for neurodegenerative diseases [11]. In 2012, a group of scientists led by Erichev, examining the autopsy material of the brain of patients with open-angle glaucoma, found damage to most of the axons in the optic nerve, as well as a decrease in the lateral cranial bodies of the thalamus. Microscopic examination of the material showed a decrease in the thickness of the cell layer in the visual cortex of the brain, as well as the accumulation of the pigment lipofuscin, which is one of the markers of atrophy [12]. According to the results of these studies, it is possible to assume a diffuse spread of the neurodegenerative process in patients with glaucoma.
Drug therapy for AD and glaucoma are another common feature of these diseases. The first line of drug therapy for AD are anticholinesterase drugs, which include rivastigmine, donepizil, galantamine, when prescribing these drugs, ophthalmologists revealed a decrease in intraocular pressure [13]. In ophthalmological practice in glaucoma, nootropic drugs are used to protect retinal ganglion cells, in particular choline alfoscerate, which has a complex neurotrophic and antioxidant effect [14].
A similar embryological origin, related microvascular anatomy and histological structure between the microvascular network of the brain and the retina have long been noted. Recent studies have proven the role of vascular factors in the development and progression of glaucoma, and vascular dysfunction has been identified as one of the important causes of glaucoma. According to the literature, it was found that long-term arterial hypertension can lead to a decrease in the relative area of retinal vessels, due to diffuse narrowing of arterioles, as well as remodeling of arterial vessels with a subsequent decrease in the internal lumen. Long before the appearance of patients' complaints, changes in the vascular system appear. In middle-aged patients with signs of retinal vascular changes (i.e., with signs of retinopathy), a marked decrease in cognitive functions has been proven [15-17].
The aim of our study is to compare the structure of neuropsychological changes in patients with Glaucoma, AD and DM.
Materials and Methods
The study was conducted at the N. I. Pirogov State Clinical Hospital No. 1 of the Moscow Department of Health and the Federal State Budgetary Institution "NMIC GB named after Helmholtz" Ministry of Health of Russia. The study included 90 people who were on inpatient treatment.
According to diagnostic criteria, patients were divided into three groups of 30 people each:
• The first group consisted of patients with an already established diagnosis of open-angle glaucoma, of which 70% were women (21) and 30% men (9), the average age of patients was 69 years (median 70 [62; 76]). Upon admission to the glaucoma department, no pathology was detected during a routine examination by a neurologist.
• The second group included patients with an established diagnosis of AD according to the NINDS-ADRDA criteria, of which 70% were women (21) and 30% were men (9), the average age of patients was 64 years (median 65 [61; 67]).
• The third group also included 30 patients diagnosed with diabetes according to the NINDS-AIREN criteria, of which 70% were women (21) and 30% men (9), most patients had post–stroke cognitive impairment (76.6% of cases), age-64.5 years (median 66 [60; 69]).
For the study, patients aged 40 to 90 years with diagnosed openangle glaucoma, BA and DM, without organic pathology, concomitant somatic pathology in the decompensating stage, mental disorders, gross ophthalmological pathology, at the time of the study, visual acuity in patients above 0.6, without increased intraocular pressure, who are able to understand and sign informed consent were selected.
According to statistics, women suffer from AD more often than men, as well as with glaucoma, women are 3 times more likely to suffer from this disease, which our study showed in three groups, the number of women prevails over men [18]. However, the predominance of women in the DM group is explained by the development of more severe vascular distress in men, which did not allow them to be included in our study.
The neurological status of patients was assessed [19]. The neuropsychological state of the patients was assessed using the following generally accepted neuropsychological scales and tests:
• Mini-mental State Examination-MMSE (Brief Mental Status Scale) [20].
• The Montreal Cognitive Assessment (МоСА) [21].
• Frontal Assessment Battery (FAB) [22].
• Clock drawing and copying test
• Fluency test (literal and categorical associations)
The present study corresponded to the ethical standards of the local Ethics Committee of the N.I. Pirogov RNIMU. All the subjects signed an informed consent.
Statistical analysis was performed in the IBM SPSS Statistics v25 program (IBM Corp., USA). Statistically significant differences were determined at 0.95 error-free judgment probability level or p<0.05.
Results
When assessing the state of cognitive functions based on the MoCA scale, the average score in patients with glaucoma was 19.5 (12;22), in patients with AD was 20(18;24), patients with SoD-24(22;26) (p<0.001). According to the MMSE scale, the average score in patients with glaucoma is 14(10;18), with BA-22(19;23), with SoD-26(24;28) at the same time (p<0.001). When evaluating the clock drawing test in the glaucoma group, the average score was 4 (3;5), in the BA group-6 (5;6), in the SoD group-8(4;9) (p<0.001).
During the dial copying test, the average score in the glaucoma group corresponded to 6(4;6), in the BA group 7(6;8), in the SoD group-9(7;10) (p<0.001). When assessing semantic and phonetic verbal fluency, the average score in the glaucoma group was 9,5 (7;10)/8(6;9), in the BA group 11(11;12)/9,5(9;10) accordingly, in the SoD group 13(11;14)/11(9;13) at the same time (p<0.001). Pairwise differences were revealed between the groups of glaucoma and SoD, as well as the group of BA and SoD on the scales of MoCA, MMSE, and clock copying test, semantic and phonetic verbal fluency.
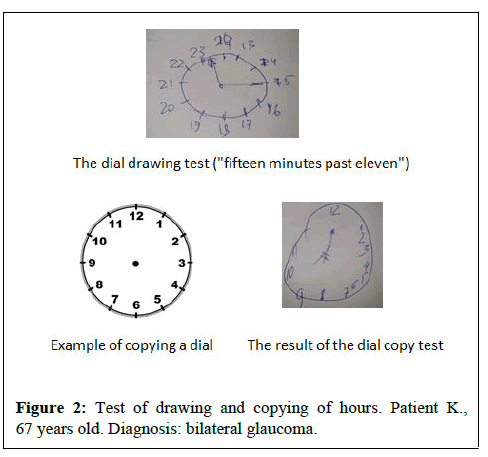
It should be said that in patients with glaucoma, when assessing the neuropsychological status, disorders of visual-spatial functions were primarily detected, which are associated with a violation of the parietal cortex. In some patients, difficulties were revealed in drawing a clock, a cube and a pentagon, but the greatest difficulties were observed in patients with self-drawing a dial (8 (4; 9) points) (Figures 1 and 2). Most of the patients in the auditory-speech memory test (5 words from the MoCA scale) showed a violation of delayed reproduction, reproduction did not improve when giving the patient hints by categories and a variety of choices, this indicates a violation of semantic encoding of memory, that is, primary memory impairment (according to the hippocampal type).
In patients from the BA group in the auditory-speech memory test, violations of delayed reproduction were observed, categorical prompts did not improve the performance of the task, as well as false recognitions of previously presented material were noted. This indicates amnestic disorders of the hippocampal type. Verbal fluency tests revealed violations of semantic and phonetic speech associations, respectively (9.5(7;10) points)/(8(6;9) points). When conducting tests for visual-spatial functions, the cortical component was interested, patients were not able to draw the dial themselves or copy the clock, however, the greatest difficulties were revealed when performing the dial drawing test (4 (3; 5) points) than copying the dial (6(4;6) points).
During neuropsychological testing, dysregulatory cognitive deficit was observed in patients with DM in the form of impaired attention, slowness of mental activity, impaired planning (building an algorithm of actions), decreased cognitive flexibility, and activity control. Memory disorders were also detected, when providing hints of categories and a variety of choices, playback improved, which indicates the difficulty of extracting information. However, memory impairment was not as pronounced as in AD and glaucoma. These patients were characterized by speech disorders, difficulties in naming objects, the nominative function of speech suffered, the repetition of complex logical and grammatical constructions, errors were noted in the fluency test, mostly associated with a decrease in the number of phonemic verbal fluency (9.5 (9;10) points) with relative preservation of semantic verbal fluency (11 (11;12) points), which is pathognomonic for subcortical-frontal dysfunction. Also, one of the characteristic signs of frontal lobe dysfunction during neuropsychological testing is the presence of errors in the clock drawing test, when they are copied safely.
Discusssion
Summarizing the data obtained by us, it should be noted that during neuropsychological testing in patients from the group of AD and glaucoma, a decrease in memory of the hippocampal type, visualspatial function, and impaired verbal fluency was revealed, which indicates the similarity of cognitive deficits of these groups. This cognitive deficit is associated with dysfunction in the temporal limbic system and parietal cortex. Patients from the DM group had impaired executive functions as a result of damage to the frontal-subcortical divisions.
A detailed analysis of neuropsychological changes on the MoCA scale revealed moderate cognitive impairment in more than half (63.3%) of patients, only 36.7% of patients did not have cognitive deficits. According to the Short Mental Status Assessment Scale (MMSE), the norm was observed in 36.7% of people, the UKN– 36.7% (11 people), and in 26.7% of cases a mild degree of dementia was observed. According to the Frontal Dysfunction Test Scale (FAB), dementia was detected in 4 people (13.3%), moderate frontal dysfunction in 14 people (46.7%) and the norm in 12 people (40%). Thus, more than half of patients with open-angle glaucoma had cognitive changes. It should be particularly noted that previously, during a routine examination by a neurologist in a polyclinic, no neurological pathology was detected.
Conclusion
The changes revealed in the course of this study indicate a diffuse spread of the neurodegenerative process in patients with glaucoma, similar to that in AD. Patients with open-angle glaucoma for early detection of cognitive disorders need to conduct a comprehensive neurological and neuropsychological examination to prescribe timely treatment and improve the prognosis of the disease. Until now, the question remains, what is primarily affected by glaucoma-neurons in the visual cortex or retina?
References
- Kuroyedov AV, Nagornova ZM, Tibieva ZU, Krinitsyna EA, Sergeeva VM (2018) Additive and combination therapy for glaucoma: Principles and practice. Russ Ophthalmol J 11: 71-81.
- Flaxman SR, Bourne RR, Resnikoff S, Ackland P, Braithwaite T (2017). Global causes of blindness and distance vision impairment 1990-2020: A systematic review and meta-analysis. Lancet Glob Health 5: e1221-34.
- Koberskaya NN. Alzheimer's disease (2019). Neurol Neuropsychiatrist Psychosom 11: 52.
- Prum BE, Herndon LW, Moroi SE, Mansberger SL, Stein JD, et al., (2016) Primary angle closure preferred practice pattern guidelines. Ophthalmology 123: 1-40.
- Bayer AU, Ferrari F, Erb C (2002) High occurrence rate of glaucoma among patients with Alzheimer’s disease. Eur Neurol 47: 165-168.
- Erichev VP, Panyushkina LA, Ronzina IA (2016) Comparative analysis and diagnostic value of static perimetry and electrophysiological studies in glaucoma and Alzheimer’s disease. J Glaucoma 15: 11-18.
- Tamura H, Kawakami H, Kanamoto T, Kato T, Yokoyama T, et al., (2006) High frequency of open-angle glaucoma in Japanese patients with Alzheimer's disease. J Neurosci 246: 79-83.
- Yucel YH, Zhang Q, Gupta N, Kaufman PL, Weinreb RN (2000) Loss of neurons in magnocellular and parvocellular layers of the lateral geniculate nucleus in glaucoma. Arch Ophthalmol 118: 378-384.
- Nesterov AP (2000) Primary open-angle glaucoma: Pathogenesis and principles of treatment. Clin Ophthalmol 1: 4-5.
- Weinreb RN, Khaw PT (2004) Primary open-angle glaucoma. The lancet 363: 1711-1720.
- Gupta N, Ang LC, De Tilly LN, Bidaisee L, Yucel YH (2006) Human glaucoma and neural degeneration in intracranial optic nerve, lateral geniculate nucleus, and visual cortex. Br J Ophthalmol 90: 674-678.
- Erichev VP, Tumanov VP, Panyushkina LA (2012) Glaucoma and neurodegenerative diseases. Journal of NII GB RAMS. 62-68.
- Kirby E, Bandelow S, Hogervorst E (2010) Visual impairment in Alzheimer's disease: A critical review. J Alzheimers Dis 21: 15-34.
- Yucel YH, Zhang Q, Gupta N, Kaufman PL, Weinreb RN (2000) Loss of neurons in magnocellular and parvocellular layers of the lateral geniculate nucleus in glaucoma. Arch Ophthalmol 118: 378-384.
- Schmidl D, Garhofer G, Schmetterer L (2011) The complex interaction between ocular perfusion pressure and ocular blood flow-relevance for glaucoma. Exp Eye Res 93: 141-55.
- Kubarko A I, Bur EA, Kubarko YuА, Avdey LL (2017) The state of retinal vessels, light sensitivity of the visual system and their relationship with structural changes in the brain in patients with arterial hypertension. Emer cardiol cardiovas risks 1: 89-98.
- Wong TY, Klein R, Sharrett AR, Couper DJ, Klein BE, et al., (2002) Cerebral white matter lesions, retinopathy, and incident clinical stroke. Jama 288: 67-74.
- Laws KR, Irvine K, Gale TM (2018) Sex differences in Alzheimer's disease. Curr Opin Psychiatry 31(2):133-139.
- Gusev E I, Grechko VE, Burd GS. Nervous diseases. Medicine 1988: 639
- Folstein MF, Folstein SE, McHugh PR (1975) “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12: 189-198.
- Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, et al., (2005) The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53: 695-9.
- Dubois B, Slachevsky A, Litvan I, Pillon BF (2000) The FAB: A frontal assessment battery at bedside. Neurology 55: 1621-1626.
Citation: Bogolepova AN, Makhnovich EV, Kovalenko EA, Osinovskaya NA, Jyravleva AN (2022) Cognitive Profile of Glaucoma Patients. J Alzheimers Dis Parkinsonism. 12: 546. DOI: 10.4172/2161-0460.1000546
Copyright: © 2022 Bogolepova AN, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2215
- [From(publication date): 0-2022 - Apr 02, 2025]
- Breakdown by view type
- HTML page views: 1844
- PDF downloads: 371