Mini Review Open Access
Coexistence of Primary Biliary Cirrhosis and Immune Thrombocytopenic Purpura
Toru Shizuma*Department of Physiology, School of Medicine, Tokai University, Japan
- *Corresponding Author:
- Toru Shizuma
Department of Physiology
School of Medicine, Tokai University
143, Shimokasuya, Isehara, Kanagawa, Japan
Tel : +81-0463-93-1121
Fax: +81-0463-93-6684
E-mail: shizuma@is.icc.u-tokai.ac.jp
Received date: March 13, 2015, Accepted March 30, 2015, Published date: April 7, 2015
Citation: Shizuma T (2015) Coexistence of Primary Biliary Cirrhosis and Immune Thrombocytopenic Purpura . J Gastrointest Dig Syst 5:273. doi:10.4172/2161-069X.1000273
Copyright: © 2015 Shizuma T. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Gastrointestinal & Digestive System
Abstract
Although autoimmune diseases are often concomitant, the coexistence of primary biliary cirrhosis (PBC) and immune (idiopathic) thrombocytopenic purpura (ITP) is rare. This is a review of the English-language literature regarding concomitant cases of PBC and ITP. Among 17 concomitant cases reported, including four diagnosed with Evans syndrome, which includes ITP symptoms, PBC was diagnosed first in five cases, ITP was diagnosed first in two cases, and both were almost simultaneously diagnosed in remaining 10 cases. Standard pharmacotherapy was ursodeoxycholic acid (UDCA) for PBC and corticosteroids for ITP/Evans syndrome. Among these 17 cases, one fatality was observed although there appears to be no trend for worse outcome compared with either PBC or ITP alone.
Abstract
Although autoimmune diseases are often concomitant, the coexistence of primary biliary cirrhosis (PBC) and immune (idiopathic) thrombocytopenic purpura (ITP) is rare. This is a review of the English-language literature regarding concomitant cases of PBC and ITP. Among 17 concomitant cases reported, including four diagnosed with Evans syndrome, which includes ITP symptoms, PBC was diagnosed first in five cases, ITP was diagnosed first in two cases, and both were almost simultaneously diagnosed in remaining 10 cases. Standard pharmacotherapy was ursodeoxycholic acid (UDCA) for PBC and corticosteroids for ITP/Evans syndrome. Among these 17 cases, one fatality was observed although there appears to be no trend for worse outcome compared with either PBC or ITP alone.
Keywords
Primary biliary cirrhosis; Immune thrombocytopenic purpura; Idiopathic thrombocytopenic purpura; Thrombocytopenia; Evans syndrome
Introduction
Primary biliary cirrhosis (PBC) is an autoimmune liver disease of unknown etiology characterized by chronic progressive cholestasis that occurs prominently in middle-aged women [1-4]. Immune (or idiopathic) thrombocytopenic purpura (ITP), including Evans syndrome, is characterized by low platelet count and presence of autoantibodies to platelet surface antigens [5-7]. These ITP symptoms may be accompanied by the simultaneous presence or sequential occurrence of autoimmune hemolytic anemia (AIHA), in which case the diagnosis is Evans syndrome (i.e., a combination of AIHA and ITP) [5-7].
Distinct autoimmune diseases often coexist, but the coexistence of PBC and ITP is uncommon. Moreover, it is unclear whether the cases of concomitant PBC and ITP occur by chance or have a common immunological or genetic basis, although a common immunogenetic basis has long been suspected.
There have been sporadic case reports in the literature of concomitant PBC and ITP (including Evans syndrome), but few systematic literature reviews. We conducted a literature search for all English language reports on concomitant PBC and ITP, including cases of Evans syndrome, and summarized the clinical findings. We also speculated on possible unique and shared pathogenic mechanisms.
Methods
The literature search was performed using PubMed. We excluded reports of cases in which ITP developed a week after the administration of influenza vaccine in patients with PBC [8] because influenza virus infection or administration of influenza vaccine with or without adjuvant can, in rare instances, induce ITP or AIHA [9,10].
Primary biliary cirrhosis
Primary biliary cirrhosis is characterized by chronic progressive cholestasis due to destruction of the small intrahepatic bile ducts [1-3], particularly the interlobular bile ducts. It affects middle-aged women more frequently than men [4]. The clinical features and course of PBC vary substantially among individuals, from asymptomatic condition to cirrhosis or liver failure [11]. Jaundice and pruritus from cholestasis are typical symptoms in patients with PBC; up to 60% may have no clinical symptoms.
Useful laboratory characteristics for PBC diagnosis are elevated serum biliary enzymes, such as alkaline phosphatase and presence of antimitochondrial antibodies (AMA). The presence of AMA is particularly useful for the serological diagnosis of PBC as 90%–95% of patients are AMA positive [2]. Florid bile duct lesions, such as chronic nonsuppurative destructive cholangitis and epithelioid granuloma formation, are common histopathological findings in PBC [11].
Administration of ursodeoxycholic acid (UDCA) is the standard pharmacotherapy for PBC; however, up to 40% of patients with PBC do not exhibit a complete response to UDCA. Patients with end-stage liver failure require an organ transplant [12].
Silveria et al. [13] reported that among 67 patients with PBC, 32 (48%) had at least one extrahepatic autoimmune disease. Similarly, Floreani et al. [14] reported that among 361 patients with PBC followed for 8.0 ± 6.9 years, 221 (61.2%) had atleast one extrahepatic autoimmune disease. The most commonly reported comorbidities in PBC are Sjögren’s syndrome (SjS), a disease characterized by autoimmune destruction of exocrine glands, and Hashimoto’s thyroiditis (chronic thyroiditis) [12]. Other complications include rheumatoid arthritis, scleroderma, systemic lupus rythematosus (SLE), type I diabetes mellitus, and pernicious anemia [12].
Immune (or idiopathic) thrombocytopenic purpura
ITP is characterized by systemic hemorrhagic diathesis due to excessive thrombocyte destruction. ITP can be classified according to its clinical course into an acute (newly diagnosed) type, more commonly observed in infants, and a chronic type, more commonly observed in adults [9,15]. Furthermore, ITP can be classified as primary or secondary ITP according to the underlying cause. Primary ITP is defined as an acquired autoimmune disorder characterized by isolated thrombocytopenia in the absence of other causes or disorders that may be associated with thrombocytopenia [16,17]. In contrast, secondary ITP is defined as thrombocytopenia associated either with related disorders (e.g., hematological malignancies, collagen diseases, and infectious diseases) or induced by administration of vaccines or drugs [16,17].
No single test can establish ITP diagnosis. Rather, ITP is a diagnosis of exclusion according to medical history, physical findings, serum tests (complete blood count or anti-platelet antibody levels), and peripheral blood smears [16]. Primary ITP diagnosis requires the presence of isolated thrombocytopenia (<100,000 cells/μL) only [16]. Moreover, the presence of serum platelet-associated immunoglobulin G (PAIgG) is not specific for ITP.
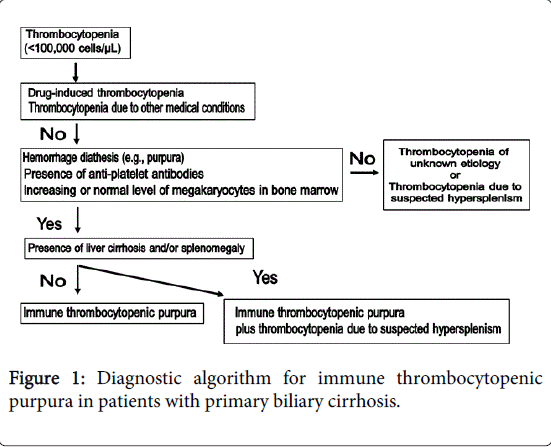
Although the mechanisms underlying ITP have yet to be completely elucidated, immune dysregulation and the production of autoantibodies appear to play major roles in ITP [18]. Because thrombocytopenia is commonly associated with liver cirrhosis and chronic hepatitis, distinguishing between concomitant ITP and thrombocytopenia due to PBC is critical in cases of PBC with thrombocytopenia. A diagnostic algorithm for ITP complicated by PBC is shown in Figure 1.
Evans syndrome
Evans syndrome is diagnosed by the simultaneous presence or sequential occurrence of AIHA and ITP in the absence of an underlying etiology [5,6]. This syndrome is characterized by hemolytic anemia, thrombocytopenia, and the production of antibodies, complement, or both that attack red blood cells (RBCs) and platelets [6,7]. Evans syndrome is occasionally associated with SLE, thyroid diseases, and scleroderma [6].
Suspected mechanisms for the coexistence of PBC and ITP
Immunogenetic factors are strongly suspected in the development of simultaneous PBC and ITP. The relationship between HLA alleles and susceptibility has been investigated separately in patients with either PBC or ITP alone, and alleles DQB1*0601 and DRB1*0803 have been linked to both diseases [19]. However, HLA polymorphisms were not investigated in most reported cases of concomitant PBC and ITP.
Although most PBC cases are not cirrhotic, liver cirrhosis often involves hypersplenism and thrombocytopenia. Thrombocytopenia in PBC may be due to splenic pooling of blood cells or thrombopoietin deficiency, but could also arise due to immunomediated platelet destruction. This destruction of platelets and RBCs may be associated with the development of ITP and Evans syndrome in PBC patients [6,18].
Bassendin et al. [20] reported elevation of serum PAIgG in approximately 40% (25/62) of PBC patients, and in all nine PBC patients with thrombocytopenia. Moreover, Panzer et al. [21] reported that autoantibodies, eluted from a patient with PBC and ITP, reacted with the platelet glycoprotein complex II b/IIIa and bound to an epitope of a rat mitochondrial protein. They also reported that glycoprotein complex II b/IIIa and the mitochondrial protein showed partial amino acid sequence homology, suggesting the possibility of a common antibody binding site [19,21].
Demographic and clinical characteristics of concomitant PBC and ITP
The main characteristics of the 17 reported cases of concomitant PBC and ITP/Evans syndrome are summarized in Table 1 [6,19,21-35]. Among the 17 cases, 12 (71%) were female and five (29%) were male. PBC was diagnosed first in five cases, ITP in two cases, and both diseases were diagnosed almost simultaneous in the remaining 10 cases.
| Case (year) | Sex | Age at diagnosisof PBC (years) | Age at diagnosis of ITP (years) | PBC prior to ITP | Scheuer’s classification | Complications | Remarks | References |
| 1(1987) | F | 60 | 60 | Sim | 1? | Familial PBC | [22] | |
| 2(1987) | F | 43 | 43 | Sim | Insulin receptor autoantibodies(+) | [23] | ||
| 3(1989) | F | 59 | 59 | Sim | [24] | |||
| 4(1990) | F | 63 | 67 | + | [21] | |||
| 5(1996) | M | 59 | 59 | Sim | 2 | [25] | ||
| 6(1997) | F | 35 | 41 | + | Liver transplantation | [26] | ||
| 7(1998) | F | <60 | 62 | + | Liver transplantation | [27] | ||
| 8(2001) | M | 37 | 37 | Sim | 1? | [28] | ||
| 9(2004) | F | 64 | 34 | - | AIH, Hashimoto’s thyroiditis | [29] | ||
| 10(2007) | F | 81 | 81 | Sim | AIHA(Evans syndrome) | [30] | ||
| 11(2007) | M | 44 | 59 | + | AIHA(Evans syndrome), Hypothyroidism | Liver transplantation | [31] | |
| 12(2008) | F | 67 | 74 | + | 3 | Sepsis → death | [19] | |
| 13(2009) | F | 58 | 58 | Sim | 3? | AIH, Scleroedema | [32] | |
| 14(2009) | M | 55 | 55 | Sim | AIHA(Evans syndrome), Sjögren’s syndrome | [33] | ||
| 15(2011) | F | 40 | 40 | Sim | 2? | AIH, Polymyositis | [34] | |
| 16(2012) | M | 45 | 42 | 2? | IgG4-related cholangitis | [35] | ||
| 17(2013) | F | 53 | 53 | Sim | 2? | AIHA(Evans syndrome) | [6] | |
| PBC: primary biliary cirrhosis; ITP: immune thrombocytopenic purpura; F: female; M: male; Sim: simultaneous;AIH: autoimmune hepatitis; AIHA: autoimmune hemolytic anemia | ||||||||
Table 1: Characteristics of 17 patients with comorbid primary biliary cirrhosis and immune thrombocytopenic purpura.
Age at diagnosis ranged from 37 to 83 years, but the patients were predominantly middle-aged. The interval between diagnosis of the primary and concomitant diseases ranged from 0–30 years. Among the 17 cases, one had familial PBC (her sister also developed PBC) [22].
The most prevalent complicating disease, aside from AIHA (in Evans syndrome), was autoimmune hepatitis (AIH) (AIH-PBC overlap) in 3 of the 17 cases [29,32,34]. In patients who underwent liver biopsy, the pathological findings of PBC varied from stage I to III according to the Scheuer classification.
Standard pharmacotherapy for concomitant cases was administration of UCDA for PBC. Although liver transplantation was performed in three concomitant cases, ITP developed after liver transplantation in each case [26,27,31]. The therapies for ITP were immunosuppressive treatments, such as the administration of corticosteroids, azathioprine, or intravenous immunoglobulin, or splenectomy in concomitant cases.
One case was fatal due to sepsis [31]. However, there was no obvious tendency for poor prognosis in concomitant cases, as compared to either PBC or ITP alone.
Conclusion
Seventeen cases of concomitant PBC and ITP were retrieved from PubMed. Among them, PBC was diagnosed first in five cases, ITP was diagnosed first in two cases, and remaining cases were diagnosed almost simultaneously. Four cases were of Evans syndrome (ITP plus AIHA) and three cases were AIH-PBC overlap. At present, it remains uncertain whether these concomitant diseases occur by chance or arise together due to a common etiology. However, a shared etiology is possible given common HLA polymorphisms and epitopes among candidate pathogenic targets.
References
- Harada K, Nakanuma Y (2014) Prevalence and risk factors of hepatocellular carcinoma in Japanese patients with primary biliary cirrhosis.Hepatol Res 44: 133-140.
- Shizuma T, Kuroda H (2011) A case of primary biliary cirrhosis which developed eight years after diagnosis of systemic lupus erythematosus.Intern Med 50: 321-324.
- Matsumoto T, Kobayashi S, Shimizu H, Nakajima M, Watanabe S, et al. (2000) The liver in collagen diseases: pathologic study of 160 cases with particular reference to hepatic arteritis, primary biliary cirrhosis, autoimmune hepatitis and nodular regenerative hyperplasia of the liver.Liver 20: 366-373.
- Selmi C, Bowlus CL, Gershwin ME, Coppel RL (2011) Primary biliary cirrhosis.Lancet 377: 1600-1609.
- Shlamovitz GZ, Johar S (2013) A case of Evans' syndrome following influenza vaccine.J Emerg Med 44: e149-151.
- Korkmaz H, Bugdaci MS, Temel T, Dagli M, Karabagli P (2013) Autoimmune hepatitis-primary biliary cirrhosis overlap syndrome concomitant with immune hemolytic anemia and immune thrombocytopenic purpura (Evans syndrome). Clin Res Hepatol Gastroenterol 37: e45-e50.
- Chen H, Jia XL, Gao HM, Qian SY (2011) Comorbid presentation of severe novel influenza A (H1N1) and Evans syndrome: a case report.Chin Med J (Engl) 124: 1743-1746.
- Mamori S, Amano K, Kijima H, Takagi I, Tajiri H (2008) Thrombocytopenic purpura after the administration of an influenza vaccine in a patients with autoimmune liver disease. Digestion 77: 159-160.
- Rand ML, Wright JF (1998) Virus-associated idiopathic thrombocytopenic purpura.Transfus Sci 19: 253-259.
- Shizuma T (2013) A patient with alcoholic liver cirrhosis who developed autoimmune hemolytic anemia following infection with influenza type A. JSM Biotechnol Bioeng 2: 1019.
- Bowlus CL, Gershwin ME2 (2014) The diagnosis of primary biliary cirrhosis.Autoimmun Rev 13: 441-444.
- Floreani A, Franceschet I, Perini L, Cazzagon N, Gershwin ME, et al. (2014) New therapies for primary biliary cirrhosis. Clin Rev Allergy Immunol.
- Silveira MG, Mendes FD, Diehl NN, Enders FT, Lindor KD (2009) Thyroid dysfunction in primary biliary cirrhosis, primary sclerosing cholangitis and non-alcoholic fatty liver disease.Liver Int 29: 1094-1100.
- Floreani A, Franceschet I, Cazzagon N, Spinazza A, Buja A, et al. (2014) Extrahepatic autoimmune conditions associated with primary biliary cirrhosis. Clin Rev Allergy Immunol.
- Casoli P, Tumiati B (1989) [Acute idiopathic thrombocytopenic purpura after anti-influenza vaccination].Medicina (Firenze) 9: 417-418.
- Lo E, Deane S (2014) Diagnosis and classification of immune-mediated thrombocytopenia.Autoimmun Rev 13: 577-583.
- McKenzie CG, Guo L, Freedman J, Semple JW (2013) Cellular immune dysfunction in immune thrombocytopenia (ITP).Br J Haematol 163: 10-23.
- Chong BH, Ho SJ (2005) Autoimmune thrombocytopenia.J Thromb Haemost 3: 1763-1772.
- Toshikuni N, Yamato R, Kobashi H, Nishino K, Inada N, et al. (2008) Association of primary biliary cirrhosis with idiopathic thrombocytopenic purpura.World J Gastroenterol 14: 2451-2453.
- Bassendine MF, Collins JD, Stephenson J, Saunders P, James OF (1985) Platelet associated immunoglobulins in primary biliary cirrhosis: a cause of thrombocytopenia?Gut 26: 1074-1079.
- Panzer S, Penner E, Nelson PJ, Prochazka E, Benda H, et al. (1990)Identification of the platelet glycoprotein IIb/IIIa complex as a target antigen in primary biliary cirrhosis-associated autoimmune thrombocytopenia. Evidence that platelet-reactive autoantibodies can alsobind to the mitochondrial antigen M2. J Autoimmun 3: 473-483.
- Chalmers EA, Chan Lam D, Holden RJ, Fitzimons EJ (1987) Familial primary biliary cirrhosis and autoimmune thrombocytopenia.Scott Med J 32: 152.
- Selinger S, Tsai J, Pulini M, Saperstein A, Taylor S (1987) Autoimmune thrombocytopenia and primary biliary cirrhosis with hypoglycemia and insulin receptor autoantibodies. A case report.Ann Intern Med 107: 686-688.
- Wallerstedt S, Westin J, Hansson G (1989) Primary biliary cirrhosis presenting as idiopathic thrombocytopenic purpura with deterioration after splenectomy.J Intern Med 225: 279-283.
- Mizukami Y, Ohhira M, Matsumoto A, Murazumi Y, Murazumi K, et al. (1996) Primary biliary cirrhosis associated with idiopathic thrombocytopenic purpura.J Gastroenterol 31: 284-288.
- Yoshida EM, Mandl LA, Erb SR, Buckley AB, Scudamore CH, et al. (1997) Idiopathic thrombocytopenic purpura in a liver transplant recipient with previous primary biliary cirrhosis.J Clin Gastroenterol 24: 274-275.
- Fickert P, Trauner M, Sill H, Hinterleitner TA, Stauber RE (1998) Successful steroid treatment of idiopathic thrombocytopenic purpura after orthotopic liver transplantation for primary biliary cirrhosis. Am J Gastroenterol 93: 1985-1986.
- Takahashi T, Saitoh T, Imai K (2001) Idiopathic thrombocytopenic purpura complicated with asymptomatic primary biliary cirrhosis.J Gastroenterol 36: 214-215.
- Arakawa Y, Amaki S, Miyakawa H, Sakai T, Gotou I, et al. (2004) PBC-AIH overlap syndrome with concomitant ITP and Hashimoto's disease with positivity for anti-centromere antibody.J Gastroenterol 39: 490-495.
- Azad A, Berera V, Jayarajan J, Lim K (2007) Evans syndrome and primary biliary cirrhosis.Int J Lab Hematol 29: 145-148.
- Retana AK, Kaplan MM, Erban JK (2007) Autoimmune hemolytic anemia in patients with liver transplants for primary biliary cirrhosis: Three case reports and a review of the literature.Am J Gastroenterol 102: 197-200.
- Toyoda M, Yokomori H, Kaneko F, Yoshida H, Hoshi K, et al. (2009) Primary biliary cirrhosis-autoimmune hepatitis overlap syndrome concomitant with systemic sclerosis, immune thrombocytopenic purpura. Intern Med 48: 2019-2023.
- Tian Y, Wang C, Liu JX, Wang HH (2009) Primary Biliary Cirrhosis-Related Autoimmune Hemolytic Anemia: Three Case Reports and Review of the Literature.Case Rep Gastroenterol 3: 240-247.
- Kurihara Y, Shishido T, Oku K, Takamatsu M, Ishiguro H, et al. (2011) Polymyositis associated with autoimmune hepatitis, primary biliary cirrhosis, and autoimmune thrombocytopenic purpura.Mod Rheumatol 21: 325-329.
- Naitoh I, Nakazawa T, Hayashi K, Miyabe K, Shimizu S, et al. (2012) A case of IgG4-related sclerosing cholangitis overlapped with primary biliary cirrhosis.Intern Med 51: 1695-1699.
Relevant Topics
- Constipation
- Digestive Enzymes
- Endoscopy
- Epigastric Pain
- Gall Bladder
- Gastric Cancer
- Gastrointestinal Bleeding
- Gastrointestinal Hormones
- Gastrointestinal Infections
- Gastrointestinal Inflammation
- Gastrointestinal Pathology
- Gastrointestinal Pharmacology
- Gastrointestinal Radiology
- Gastrointestinal Surgery
- Gastrointestinal Tuberculosis
- GIST Sarcoma
- Intestinal Blockage
- Pancreas
- Salivary Glands
- Stomach Bloating
- Stomach Cramps
- Stomach Disorders
- Stomach Ulcer
Recommended Journals
Article Tools
Article Usage
- Total views: 16905
- [From(publication date):
April-2015 - Apr 04, 2025] - Breakdown by view type
- HTML page views : 12268
- PDF downloads : 4637