Research Article Open Access
Coal Fired Power Plants: Emission Problems and Controlling Techniques
Shahzad Baig K1* and Yousaf M2
1Department of Chemical Engineering, Ryerson University, Toronto, Canada
2Department of Chemistry and Biomolecular Sciences, University of Ottawa, Ottawa, Canada
- *Corresponding Author:
- Shahzad Baig K
Department of Chemical Engineering
Ryerson University, Toronto, Canada
Tel: (647) 866-5317
E-mail: k2shahza@ryerson.ca
Received Date: June 19, 2017 Accepted Date: August 03, 2017 Published Date: August 07, 2017
Citation: Shahzad Baig K, Yousaf M (2017) Coal Fired Power Plants: Emission Problems and Controlling Techniques. J Earth Sci Clim Change 8: 404. doi: 10.4172/2157-7617.1000404
Copyright: © 2017 Shahzad Baig K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Earth Science & Climatic Change
Abstract
Coal-fired power generation; Emission control techniques; Environmental impact; Particulate matter; Sustainable
Keywords
Coal-fired power generation; Emission control techniques; Environmental impact; Particulate matter; Sustainable
Introduction
The electricity generation was 21.6 trillion kilowatt-hours (T-kWh) in 2012, expected to rise to 25.8 T-kWh in 2020 and 36.5 T-kWh in 2040 by EIA [1]. Hence, net electricity generation of our world will increase 69% by 2040. There were around 50,000 coal fired power plants working in 2007 and this total is expected to increase worldwide by PRTR [2]. All educated people in the world are worried about the environmental problems caused by coal based power plants. The burning of coal adds mainly to increase acid rain and hence increase air pollution which in turn is a cause of global warming, harm to flora and fauna and damage of property. It is the chemical composition of coal which make it difficult to remove impurities from the coal prior to its burning. The coal fired power plants using modern technologies pollute less than firstborn designs due to these new technologies that filter the flue gases in stacks; however various pollutants are still being emitted in several times higher amounts than natural gas based and other power plants. The most abundant energy source in our world is coal. Depending on the source (type) of coal the emissions from the burned coal contain pollutants such as sulfur dioxide (SO2), sulfur trioxide (SO3), nitrogen oxides (NOx), particulate matter (PM), condensable PM, mercury (Hg), trace metals and radioactive nucleoids. Environmental regulations for coal-fired power plants in the world cover a comprehensive range of very tedious requirements. New regulations were implemented from 2014 in China, USA and European Union which fixed the ‘emission limits’ very low for SO2, NOx, mercury particulate for coal-fired power generation plants. Now it is the time to evaluate emission control technologies whether the technologies can be helpful in achieving the new lower emission limits. The technologies available for emissions control and to sustain the multi-pollutant emission regulatory requirements are: Selective Catalytic Reduction (SCR), Electrostatic Precipitators (ESP), Fabric Filters (FF), Flue Gas Desulfurization (FGD), wet ESP, Dry Sorbent Injection (DSI), and Mercury Control Methods (MCM). The articles published till now on the problems of coal fired power plants were studied and found that none of the review integrated coal characteristics, pollutants by coal fire power generation plants, remedies and equipment’s used to bring the level of pollution within compliance. This article touched all these issues collectively. In addition to the use of new technologies and equipment to control the level of pollution due to coal fired power generation plants there are two other solutions as well: i) retire the coal fired plants, ii) cogenerate power by coal and natural gas.
Retirement of coal fired plants
USA has retired processively 175 coal fired power plants, with capacity of 27 gigawatts, till 2016 by Gerhardt [3]. On the other hand, Britain has built 30 gas fired power plants because gas reserves from North Sea Oil wells has become possible to use, in 1990. France only produces 4% of its electricity needs from coal fired plants and even then, she has closed down 7 coal-fired units totaling 1,758 MW of capacity in 2015 by Schwartzkopff and Littlecott [4]. Solar and wind farms (renewable energy resources) have generated 40.65 gigawatts of power in Germany in 2015. The power generated by other renewable energy resources (biomass and hydropower) was 47.9 gigawatts and the required peak power stress was 61.1 gigawatts. Between 2011 and 2015 Germany has opened 10.7 GW of new coal fired power stations. Italy’s gross energy consumption is 163 Mtoe. Fossil fuel makes up 86% of its primary energy consumption (38% crude oil, 38% gas, coal 10% (i.e., 16 Mtoe=10 GW)) by Deloitte [5]. Italy is almost totally dependent on imports of coal, which make it the 3rd largest importer of coal in Europe by Littlecott in 2015. The Environment Ministry in Japan has given the green light for the construction of new coal-fired power plants in next 12 years. Now Japan has a total of 90 coal-fired units with total capacity of 40.5 GW. A list of the proposed plants (43), which will have a total capacity of 20.5 GW by Rogers in 2016 has been issued by Japanese government. Japan seem to have got their ideas in reverse after Fukushima Daiichi reactors accident. The global incentive is to cut out fossil fuel not to add more to the melting pot. Canada used to consume 60 million tons of coal: 52 million tons of it was used for its power generation. Canadian consumption has been reduced to 42% since 2005. Canada utilized nearly 41 million tons of coal in 2014, 35 million tons of this coal was used for power generation. The decrease is due to a reason that Canada, is gradually stopping power generation by using coal-fired power plants. The use of mixed fuel in the USA has reduced consumption of coal and increase natural gas power based power generation system, resulted in decreased carbon dioxide emissions. The amount of CO2 emitted was measured in the first quarter of 2012 and it was the lowest recorded CO2 emission for any year since 1992. On the other hand, another issue was identified i.e., leakage of methane into the atmosphere by natural gas fracking and very large gas delivery system. The methane leakage needs special considerations.
Environmental Impacts
Pollution caused by coal burning
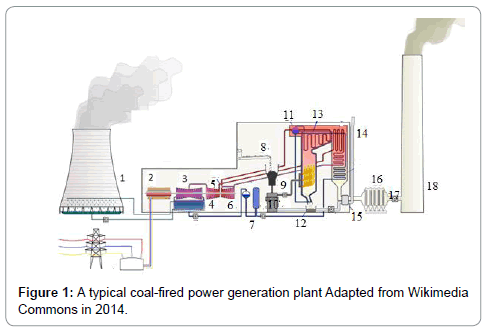
Coal is divided into three classes: anthracite, bituminous, and lignite. Empirical formulas obtained by elemental analysis are C137H97O9NS for bituminous coal and C240H90O4NS for high-grade anthracite. Anthracite coal is a hard rock with a metallic luster and it has jet black appearance. (Tables 1 and 2) gives the heating characteristics of coals A typical coal fired power generation plant is (Figure 1) and the important equipment used in the plant (Table 3) (Figure 1). Coal is used in a coal-fired power generation plant to turn water into steam and steam drives turbine generators to generate electricity. In this process, coal is first pulverized and the fineness achieved is as that of a talcum powder (200 mesh to 325 mesh). It is then stir together with hot air in a skillful way and injected in the burning chamber (firebox) of a boiler. The coal/air mixture is almost completely combusted, hence, generate maximum possible heat. Purified water is pumped through tubes of the boiler, is converted into steam by the supplied heat. The temperature of steam reaches up to 1,000 degrees Fahrenheit and pressures is raised up to 3,500 psi, and this high-pressure steam is conveyed to the turbine. The huge pressure of steam pushes the blades of turbine which move the shaft of turbine. The shaft of turbine is coupled to the shaft of a generator. The generator magnets spin inside the wire coils to produce electromagnetic field to produce electricity. After moving turbines, the steam is injected into a condenser where cooling water from a nearby source is pumped in the condenser through a network of tubes. The cooling water in the tubes transforms the steam back into water that can be recycled in the plant or returned to its source without being contaminated (not even at high temperature, ideally), and the steam is returned to the boiler and this cycle is repeated. Heat is obtained by combustion operation. The combustion involves combinations of coal with oxygen.
| Coal type | Ignition temperature |
Volatile initial release temperature |
|---|---|---|
| Lignite | 250-450 | 130-170 |
| Bituminous | 400-500 | 200-300 |
| Anthracite | 700-800 | 380-400 |
Table 1: Heating characteristics of coals.
| Pollutant | Anthracite | Lignite |
|---|---|---|
| CO2 (g/GJ) | 94,600 | 101,000 |
| SO2 (g/GJ) | 765 | 1,361 |
| NOx (g/GJ) | 292 | 183 |
| CO (g/GJ) | 89.1 | 89.1 |
| Organic compounds-Non methanic (g/GJ) | 4.92 | 7.78 |
| PM (g/GJ) | 1,203 | 3,254 |
| Total volume of flue gases (m3/GJ) | 360 | 444 |
Note: The average emission amount of flue gases from coal fired power plants reported by European Environment Agency (EEA 2008).
Table 2: Average emission of flue gases from coal burning.
| 1 | Colling tower | 7 | Deaerator | 13 | Superheater |
| 2 | Generator | 8 | Coal conveyor | 14 | Air Intake |
| 3 | Low pressure turbine | 9 | Coal hopper | 15 | Air preheater |
| 4 | Condenser | 10 | Pulverized fuel mill | 16 | Precipitator |
| 5 | Intermediate pressure turbine | 11 | Boiler drum | 17 | Induced draught fan |
| 6 | High pressure turbine | 12 | Ash hopper | 18 | Stack |
Table 3: Some important parts of coal-fired power plant in Figure 1.
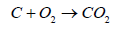 (1)
(1)
On consumption of oxygen, the following reaction (Equation 2) becomes possible.
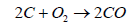 (2)
(2)
Carbon moNOxide (CO) is produced by burning of carbon in insufficient oxygen. If concentration of CO is increased over 2000 ppm it causes death because CO interferes with the dissemination of oxygen in the body. The molecules of hemoglobin in blood carries O2 to every part of body. The molecule of CO has almost the same shape as the O2 and mix with the hemoglobin with the result in inhibiting the distribution of O2. If breathed for 8 hours in CO concentration of at 30 ppm, it is estimated that 10% of the hemoglobin may be converted to carboxyhemoglobin. The carboxyhemoglobin formed due to CO affect the ability to see clearly. As it is given in the Equation 1, CO2 is the main product of coal combustion. The other products originate from the sulfur (S) and nitrogen (N) contents of coal. The nitrogen is transformed from N2 to NO2 gas and sulfur to SO2 which are the culprits of acid rain and lungs cancer.
Particulate matter
The particulate matter emitted from coal power plant (CPP) have a very grave effect on public health. The main contributor to the particulate matter is coal fly ash, and minor are sulfate and nitrate by Nel [6]. Coal fly ash is the incombustible materials that is 20% of the collected coal-ash by Grahame and Schlesinger [7]. Particulate matter cause irritation and/or obstruction in the delicate and fine airways of lungs, obstructions in airways may cause asthma, chronic bronchitis by Grahame and Schlesinger [7]. For every 4 tons of burnt coal, one ton of ash is produced. It is further estimated that one ton of ash can spread over up to 150,000 square kilometers (60,000 square miles). Fly ash can travel up to 40 km to 50 km in the down wind direction. It settles down subsequently causing land degradation, severe air and water pollution and diseases in plants and animals, including human being by Pandey [8]. Under this coverage area the harmful substances have been detected even in the milk of cows. Exposure to PM 2.5 increases the risk of death from heart disease, respiratory diseases and lung cancer by EEA [1] and WHO [9]. It has been estimated that the concentration of 238 Th and 226 Rd, 40 K in the upper 30 cm layer of soil within 20 km of coal fired power stations is increased, annually, in the range of 0.03% to 0.12% of the corresponding typical natural concentration in soil.
Carbon dioxide
Based on Equation 1 Carbon dioxide (CO2) is formed when 1 atom of C unites with 2 atoms of O. To discuss this relation in terms of weight, we know that atomic weight of C is 12, O has 16 and CO2 has 44. In the case of complete combustion 1 pound of C combines with 2.667 pounds of O to produce 3.667 pounds of CO2. Let’s go one step further, coal with a C content of 78% emits about 204.3 pounds of CO2 per million Btu of heat generated by complete burning. Hence, complete combustion of 1 ton of coal will produce about 2.86 tons of CO2 by Hong and Slatick [10]. Using more efficient and higher combustion temperature, CO2 emissions could be reduced. Another alternative is Carbon Capture and Storage (CCS) but the technology is under development and the use of CCS may increase the cost of coal based power production.
Nitrogen oxides and sulfur oxides
Modern day coal power plants pollute less than older designs due to new technologies that filter the exhaust air in smoke stacks; yet emission levels of various pollutants are greater than the emissions from natural gas power plants. Pollution from coal-fired power plants comes from the emission of gases such as CO2, NOx and SO2 into the atmosphere. These gases react with the atmospheric air to create acidic compounds such as H2SO3, HNO2, H2SO4 and which precipitate as rain, hence it is called acid rain. Burning of fossil fuels emits SO2 which in turn forms H2SO4 as given in Equations 3 to 6
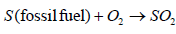 (3)
(3)
SO2 react with rain water or moisture from air and form sulfurous acid
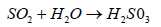 (4)
(4)
SO2 also oxidizes to form a sulphate ion
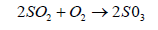 (5)
(5)
Sulphate ion joins with hydrogen ion from air and become sulphuric acid (H2SO4) and falls back on earth.
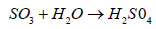 (6)
(6)
Similarly, molecular nitrogen and molecular oxygen react to give nitric oxide as given in Equations 7-9:
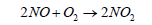 (7)
(7)
Nitric oxide (NO) then reacts quickly with excess of oxygen (O2) to give nitrogen dioxide (NO2), It is to be noted that nitrogen dioxide is responsible for brown smog
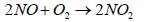 (8)
(8)
When nitrogen dioxide dissolves in water, it forms a 1:1 mixture of nitrous acid and nitric acid:
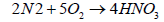 (9)
(9)
It is important to remember that the acidity of any solution is measured in pH (potential hydrogen) scale ranging from 1 to 14, with pH 7 taken as neutral. pH values higher than 7 are considered alkaline (the pH of baking soda is eight); pH values lower than 7 are considered acidic (the pH of lemon juice is two). Also remember that the pH scale is a logarithmic measure which means that every pH change of one (ΔpH=1) is a 10-fold change in acid content. Therefore, a decrease from pH 7 to pH 6 is a tenfold increase in acidity; a drop from pH 7 to pH 5 is a 100-fold increase in acidity; and a drop from pH seven to pH 4 is a 1000-fold increase. NO2 changes vegetation. NOx and SOx contribute to the growing respiratory disease.
Radioactive contamination
Coal contains trace impurities of uranium (U), thorium (Th), Radon (Rd) and other natural radioactive
https://www.omicsonline.org/scholarly/radioisotopes-for-medicine-journals-articles-ppts-list.php |
s. On burning of coal, this radioactive contamination is released to the environment. Burning of coal in large quantum raise the trace amount to a considerable amount. The radioactive emissions produced from the fly ash of a CPP are 100 times more than a nuclear power generation of same energy producing capacity by McBride et al., in 1978. For example, A 1,000 MW CPP could release 5.2 tons per year of uranium U235 and 12.8 tons thorium by Gabbard in 1993. While a nuclear power plant of 1000 MW capacity could generate about 750 Kg of high-level radioactive waste and 225 Kg Plutonium per year by NIRS in 1997 and by WNA in 2016.
Toxic contamination of water and air
A study conducted by US EPA found that the underground dumped ash (produced by coal-fired power plants) has contaminated ground water due to its with toxic contents [11]. The toxic contaminants include Arsenic and lead. Arsenic cause skin, bladder and lung cancer and lead damage nervous system [12]. Local aquatic life is also disrupted due to the coal ash life cycle because coal ash also transmits various types of toxic contaminants into the local atmosphere which travels to other sites with air. The carried over coal ash is dropped and dissolved in ponds, lakes and rivers. A research conducted by Stuttgart University estimates that the air pollution caused by coal-fired power plants was responsible for 22,300 premature deaths in the EU in 2010.
Mercury contamination
Water streams are contaminated by mercury from the emissions of coal fired power plant by Gottlieb [13]. USA-EPA has determined in a study that 25% of fish had mercury levels higher than the safety levels, even it was found in the fish of isolated countryside waterways. A research conducted by University of Stuttgart under commission from Greenpeace found that coal-fired power plants were the largest source of mercury air emissions in the EU, such as 200,000 babies are born each year in the EU with mercury levels harmful to their mental and neurological development.
Pollution by using natural gas
On the other hand, due to natural gas fracking process and increased gas distribution, methane is leaked into the atmosphere. Methane is 10 to 25 times more effective as a greenhouse gas than the carbon dioxide. Methane contents in atmosphere are around 0.00018% while CO2 content are around 0.039% (CO2 is about 200 times). Though CH4 amount is so small even then a scientific estimate says that onesixth of recent global warming is due to the methane emissions [14]. To assess emissions from gas pipelines across a major city, Philips [15] recorded CH4 leaks across the city of Boston using a cavity-ring-down mobile CH4 analyzer. They identified 3356 CH4 leaks from 785 road miles of pipelines. It was found that the leakage was 15 times more than the global background level [15]. In another study, forty separate types of equipment/instruments were found to be potential sources of methane emissions during the production and processing of natural gas and oil by hydraulic fracturing, or fracking [16]. The estimation of the amount of methane escaping during the fracking and processing of oil and gas is exceedingly difficult. It is estimated that the methane emissions range from 1.5 percent [12] to 9 percent [17] of gross natural gas production. For a 20-year interlude, one ton of CH4 has a potential to increase global warming 84 to 87 times more than that of CO2. In spite of all these, natural gas plants are appreciated more in the world and their contribution to the world’s total electricity generation was 22% in 2014.
Techniques to Reduce the Environmental Impacts
Hansen propagated through an open letter to President Barack Obama and through his book ‘Storms of My Grandchildren’ that there should be a moratorium for coal-fired power plants that do not capture their CO2 emissions and sequests CO2". The zero emission (emissionless) is achieved by carbon capture and sequester. An example of this type of plant is Elsam power station at Esbjerg, Denmark (European Communities, 2006). One recommendation is that the coal used for power plants should be clean coal. "Clean coal" is a term used by coal industry to describe a type of coal from where minerals and impurities are chemically washed of and processed (gasified, steam treated). In order to run coal-fired power plants effectively, a cost-effective method is to run the plant on a diverse type of fuel, such as conversions to biomass or municipal waste based power plants. The emission level from this type of plants is estimated to be 20% less CO2 than a coal fired unit operating at a same capacity.
Combined heat and power
Combined Heat and Power (CHP) is a process to generate electricity and process heat. Instead of discharging heat at a higher than ambient temperature, it is used to heat the buildings. This expertise is commonly practiced in some countries, for example Denmark and other Scandinavian countries and parts of Germany. Hansen [18] has shown that CHPDH is the low-cost method of reduction in carbon emissions.
Options for fossil fuel power plants
The choices other than coal-fired power plants include hydroelectric power, nuclear power, solar power, wind power, geothermal power, tidal and new renewable energy techniques. Some of the power production technologies are proven on large industrial scale (i.e., hydroelectric, nuclear, wind, and tidal power) while others are in prototype stage.
Cost by power generation source
The costs for a fossil fuel based power plant with a life of 30 years to 50 years is charming for investor due to the low initial investment i.e., around $1000 to $1300 per kilowatt electricity as compared to $2000 per kilowatt from an onshore wind farm. This cost calculation is only true when it strictly includes the cost of electricity production and does not consider the indirect costs supplementary to the pollutants generated due to fossil fuels burning (e.g., increased respiratory diseases).
Particulate matter control
Particulate matter (PM) is often classified as PM 2.5 and PM 10. PM 2.5 is particulate matter of size 2.5 μm and less. PM 10 is particulate matter 10 μm and less and it includes PM 2.5. PM 2.5 is considered to have more harmful health effects than the relatively coarser particles. Impaired visibility is one of the effects of particulate matter emissions. Particles of sizes 2.5 μm and greater than 2.5 μm are regulated while the sizes less than 0.1 μm are presently difficult to regulate. (Table 4) gives the permissible limits set by different countries for emission of PM.
| Emission limit | Country |
|---|---|
| 10 mg/Nm3 per day | EU |
| 5 mg/Nm3 per day. | China |
| 4 mg/Nm3 per day | Japan |
| 3 mg/Nm3 per day | EU New* |
Note: *new emission standards of European Union
Table 4: Limits of PM emission limit from a coal fired power plant.
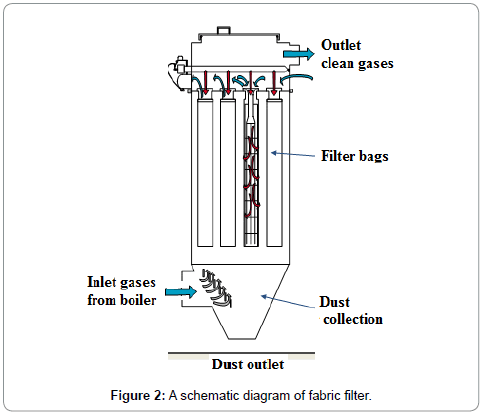
A particulate matter (PM) control device (equipment) remove the PM from the exhaust gas stream, stop the PM from re-entering the exhaust gases, and remove the collected PM. The main PM control equipment in use are Electrostatic Precipitators (ESP), Fabric Filters (FF), Mechanical Collectors (MC) and Venturi Scrubbers (VS). Each type of PM control equipment is based on a different PM collection technique. The FF contains baghouse which collects the particulate matter by using finely netted filters, electrostatic precipitators creates an electromagnetic field to catch particles, and centrifugal force is used by cyclone collectors to separate particles ESP and FF are good to meet stringent EPA requirements of high efficiency and reliability. A FF consists of a number of joint enclosures. Each enclosure contains up to over a thousand fabric bags made of small diameters and are attached with vertical supports. The flue gas passes through the fabric bags and PM from the flue gas is accumulated on the bag surface. The cake formed can contribute significantly to remove other constituents of flue gas, such as SO2 and mercury. A schematic diagram of the fabric filter is given in Figure 2. Advantages of a FF include high particle collection efficiency for a wide particle size range, high-level of reliability, resistance to flow changes because of these reasons FF is preferred on ESP. There is no universal formula for FF collector to predict particle collection efficiency. A number of variables are considered in design of baghouses: pressure drop of flue gases, filter bag drags, air-to-fabric ratio, and collection efficiency for PM. The pressure drop across a filter is predicted from a derivation of Darcy's law for the flow of fluids through porous media and it is given as Equation 10:
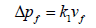 (10)
(10)
Where: Δpf=pressure drop through the clean fabric filter, in. H2O (cm H2O)
k1=resistance of fabric, in. H2O/(ft/min) (cm H2O/(cm/sec))
vf =velocity of filtration, ft/min (cm/sec)
Deposited dust on the fiber start acting as a cake, the pressure drop through the collected dust cake can be predicted by using Equation 11 [19]. The Billings and Wilder [19] formula is again a derivative from Darcy's law:
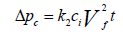 (11)
(11)
Where: Δpc=pressure drop through the dust cake, in. H2O (cm H2O)
k2=resistance of the dust cake, in. H2O/(lb/ft2-ft/min)
(cm H2O/(g/cm2-cm/sec))
ci=concentration of duct loading, lb/ft3 (g/cm3)
vf=velocity of filtration, ft/min (cm/sec)
t=time of filtration, min (sec)
The coefficient of the resistance of dust cake to filter gas, k2, is verified by conducting experiments and it is dependent upon flue gas viscosity, dust particle density and dust cake porosity. The dust cake porosity is further related to the fabric permeability. According to American Society of Testing and Materials (ASTM) standard D737-69, the permeability for the fabric is the volume of air that passes through one square foot of filter cloth having a pressure drop of no more than 0.5 inches of water. It is to be noted that the term k2 is reliant on the size of the particles in the gas stream. For the particles of size less than 2 μm, k2 is high. When k2 is high (10 in. H2O or 25 cm H2O) as a result the pressure drop will increase and frequent bags cleaning will be required.
The total pressure drop is the sum of pressure drop through the filter and the pressure drop through the dust cake and it is given in Equation 12 and Equation 13 as:
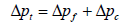 (12)
(12)
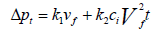 (13)
(13)
The baghouses are operated in a pressure drop range 4 to 10 in. H2O. But it is customary to operate units at less than 6 in of H2O because bag cleaning is not so frequent at this pressure drop. It is found that particles of less than 1 μm in diameter can be collected in a baghouse. Emission regulations for coal fired power plants control of PM 2.5. In order to determine collection efficiency, it is required to divide the emissions mg m3 by the incoming loading mg m3. FF is available in two types: reverse-air and pulse-jet. The pulse-jet type is preferred due to its small size and low cost over the reverse-air type. In a pulse-jet FF, the cake formed on the bags is removed by a reverse pulsate of high pressure air. This cleaning can be performed online, unlike a reverseair FF where the enclosure needs to be removed from service line.
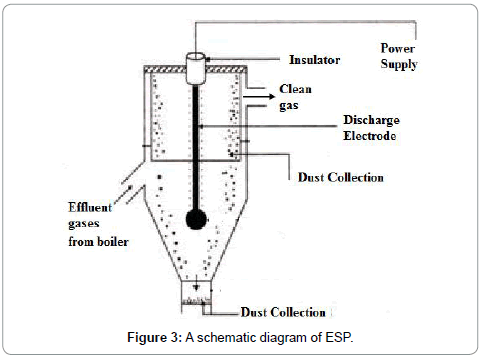
An ESP unit consist of a number of parallel and/or vertically placed plates and flue gases pass through these plates. PM from the fly ash is charged due to the electric field (Figure 3).
The 99.9% efficiency of an ESP is proven at medium and higher levels of ash containing coals with and the outlet emissions levels found were approximately 12 mg/Nm3 to 36 mg/Nm3 at 6% O2. Therefore, it is a good technology to offer for emission control. The performance and efficiency of an ESP is calculated by using Deutsch-Anderson equation. In order to enhance accuracy of the Deutsch-Anderson equation cases where all particles are not uniform in size, a parameter called the effective precipitation rate (we) can be replaced for the migration velocity in the equation. Therefore, Dr. Harry White proposed modifying the Deutsch-Anderson Equation 14 by using the term ‘We’ instead of ‘w’ (White 1990)
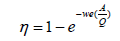 (14)
(14)
η=fractional collection efficiency
We=terminal drift velocity, m/s
A=total Collection area, m2
Q, volumetric
Migration Velocity (w) denotes to the speed of an individual charged particle at which is migrated to the collection electrode, while the Effective Precipitation Rate (We) indicates the average speed at which all particles in the entire dust mass move toward the collection electrode. The variable, We, is calculated from field experience rather than from theory; values for We are obtained from data banks build up from ESP systems in the similar industries or from the studies conducted by pilot-plant. Application of the Deutsch-Anderson equation in this manner could be particularly useful when trying to determine the amount of additional collection area needed to upgrade an existing ESP to meet more stringent regulations or to improve the performance of the unit working in a coal-fired power generation plant. An alternative way to determine the total efficiency of precipitation at ESP is calculate by measuring the dust concentration before and after ESP. The benefits of an ESP are high-level of efficiency, high-level of reliability, and reduction in the flue gas pressure loss, resistance to change in moisture and temperature, and low-level of maintenance.
Mechanical collectors (MC) are also called cyclone separators they work on gravity, inertia or centrifugal force. MC are efficient for PM greater than 20 microns. Below 20 microns the collection efficiency drops and it is almost negligible below 5 microns. Current EPA codes on PM cannot be met by using MC alone, when a fraction of PM is less than 10 microns. A typical cyclone separator (Figure 4). Drag, centrifugal and buoyant forces are used to separate the particles from flue gases. MC are available in vertical and horizontal forms. A highspeed rotation is established in the air flow in a cyclone separator. PM particles are denser than gases and they attain high inertia by following the spiral movement of the air stream, strike with the wall of cyclone and fall down to the bottom of the separator from where they can be removed.
In the field of environmental engineering, while working with the design and analysis of coal fired power plants gas streams, it is always important to analyze the particle removal from gas streams. The step by step calculation are useful to understand how the values are being used in this η calculation, however, when it comes to quick calculations, the following Equation 15 is used
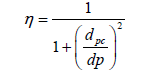 (15)
(15)
where
η=fractional particle collection efficiency
dpc=characteristic diameter of particles collected with 50% efficiency, (µm).
dp=diameter of the partciles of interests, (µm).
Cyclones have often been considered as low-efficiency particle collectors, However, efficiency depends on particle size and cyclone design. Advanced design work has greatly improved cyclone efficiency. The cyclones are good devices to achieve efficiencies of 90% for particles larger than 15 µm to 20 µm while some of the cyclone manufacturers claimed to provide 98% efficiency through their fabricated cyclones for particles larger than 5 µm.
In a venturi scrubber (VS), flue gases containing PM passes through a ‘venturi throat’ where water is injected into the gas stream. The difference in velocity and pressure make small and larger water droplets. These droplets collide with PM and stick with them. At the expanded end of the ‘venture throat’ the velocity is reduced and it allows droplets of water containing PM to combine together and make larger droplets. The large particle falls out of the gas stream due to gravity. A large cyclonic section is recommended after the venturi to improve drop out of PM-loaded water droplets. The dust removal efficiency is calculated from the following Equation 16,
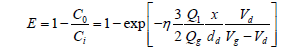 (16)
(16)
C=dust concentration (g/m3)
Cd=drag coefficient (dimensionless)
d=diameter (m)
E=efficiency (dimensionless)
g=acceleration due to gravity (m/s2)
Q=volumetric flow rate (m3/h)
v=velocity (m/s) × length (m)
g=nertial impaction parameter (dimensionless) l viscosity (N s/m2)
d=droplet
di=initial value of droplet
g=gas
i=inlet
l=liquid
o=outlet
p=particle
The efficiency of VS for very small particles is much lower. VS generate a wastewater stream which is needed to be subjected to treatment before discharge. Disposal of this wet sludge by-product is again a problem.
NOx control
The original coal burners are replaced with new Low NOx burners. The Low NOx burner apply advance fluid dynamics and flame thermodynamics techniques to reduce flame temperature, hence, less NOx.
NOx is controlled by using Selective Catalytic Reduction (SCR) systems and/or Non-Catalytic Reduction (SNCR) system. In these technical treatment systems through a series of reactions with a chemical reagent injected into the flue gas, NOx is reduced to N2 and H2O. The most commonly used chemical agents are NH3 and urea ((NH2)2CO) for SNCR. SNCR system introduce urea into temperature range of 760°C to 1100°C (1400°F to 2012 °F). Within this range, urea may react with available oxygen to form NOx and in this way the NOx removed ranges from 15% to 35%.
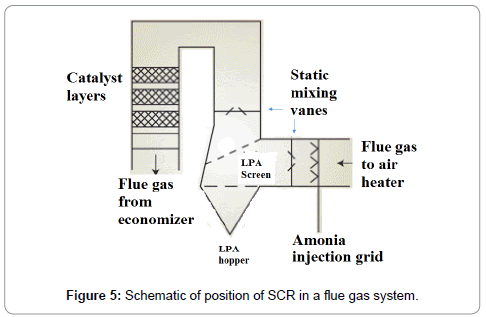
SCR decomposed NOx into ammonia (NH3), carbon dioxide (CO2) and water (H2O) which further react with flue gases and convert flue gas to N2 and H2O. SCR is the most effective method of reducing NOx emissions from 70% to 90%. The SCR reactions take place in a temperature range of 230°C to 450°C (446°F to 842°F) and optimum reaction is achieved in the range of 360°C to 450°C (680°F and 842°F). The minimum effective temperature varies due to fuel type, flue gas composition and catalyst (Figure 5). The worldwide installations of SCR on coal-fired power generation plant is given in Tables 5 and 6.
| Emission limits | Country |
|---|---|
| 180 mg/Nm3 | EU |
| 30 to 50 mg/Nm3 | China |
| 40 mg/Nm3 | EU New* |
Note: *new emission standards of European Union
Table 5: NOx emission limits set.
| Country/Region | Capacity, MWe |
|---|---|
| Austria | 1,200 |
| Germany | 33,000 |
| Japan | 7,700 |
| Netherlands | 1,000 |
| Scandinavia | 1,100 |
| United States | 2,000 |
| Total | 46,000 |
Note: The worldwide installations of SCR on coal-fired power generation plant
Table 6: SCR installations in world.
SO2 control
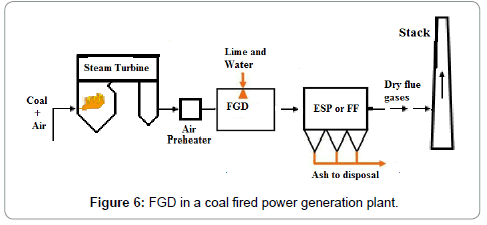
The emissions of SO2 can be controlled by three approaches: 1) blending of fuel, 2) switching fuel, with a fuel having lower sulfur contents, or 3) removing the SO2 from the flue gases. SOx emission limits set by various countries are given in Table 7. A variety of technologies are available to remove SO2. Among these technologies the prominent are: wet flue gas desulphurization (FGD), dry flue gas desulphurization. The dry FDG use a spray dryer absorber (SDA) or circulating dry scrubber (CDS), or dry sorbent injection (DSI). Conventionally used wet FGD systems include a wet limestone process which forced oxidized S to remove as SO2 and gypsum is obtained as a byproduct. SO2 removal efficiency achieved by Limestone process is 98%. The net reaction is given in Equation 17:
| Emission limit | Country |
|---|---|
| 20 to 60 mg/Nm3 | EU |
| 5 to 15 mg/Nm3 | USA (some plants) |
| 20 mg/Nm3 | EU New* |
Note: *new emission standards of European Union
Table 7: SOx emission limits.
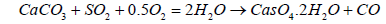 (17)
(17)
Wet FGD systems are designed for various types of chemicals including magnesium-enriched lime, seawater, and soda ash (sodium carbonate, Na2CO3). Some limestone-based systems use an organic acid to enhance SO2 removal. Wet FGDs was successfully used for coals such as lignite, anthracite, bituminous, and sub-bituminous types. Figure 6 shows the locations of the flue gas desulfurization (FGD) option in plant. It may be of interest that in China, the installed capacity of FGD systems is increasing from 379 GWe at end 2008 to 723 GWe in 2020 which represents 75% of all the new FGD to be installed worldwide each year [20]. A Spray Dry Flue Gas Desulfurization Systems (SDA) is an example of dry FGD system. In SDA, lime slurry is atomized and applied over the exhaust gases to absorb the SO2 and other gases. The subsequent dry material with absorbed gases is collected in a downstream PM control equipment, such as a FF or ESP. A small quantity of the dry material can be recycled to minimize the usage of lime. The SDA cools the flue gas from 340 K to 350 K before the flue gas passes through the FF. Extremely low PM emissions are possible, including Pm2.5. Approximately 96% of SO2 can be removed with the use of this technology which make it suitable to for compliance of new emission limits. Advantages of dry FGD as compare to wet FGD include: 1) Low construction cost, 2) Simple unit operations, 3) Less water consumption, 4) lLss power consumption, 5) Use of alkalinity to control the fly ash for SO2 absorption as well, and 6) Dry solid byproduct (easy to manage).
Circulating Dry Srubber (CDS) is also a type of dry FGD system. A fluidized bed of hydrated lime is used for controlling SO2 in the flue gas. A mixture of products formed such as calcium sulfite (CaSO3) or calcium sulfate (CaSO4), unreacted lime (CaO), and fly ash is collected to a downstream PM collector. Most of the waste is mixed with fresh calcium hydroxide to regenerate and reuse. Water spray on the fluidized bed in addition to enhance SO2 absorption for minimum lime used. It has been observed that the adsorbent (CaSO3, CaSO4, or CaO) used at close to the saturation humidification level give 10% more SO2 removal than the less saturated level [21]. CDS can provide 98% of SO2 removal depending on the application conditions.
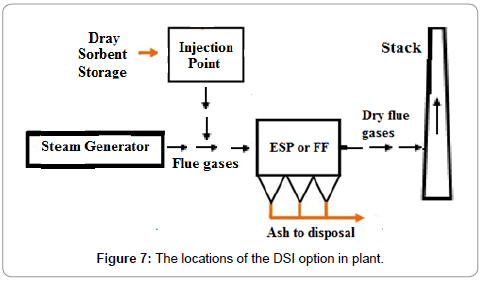
Dry Sorbent Injection (DSI) is a minimal cost solution. The DSI involves the injection of trisodium hydrogen dicarbonate dihydrate also sodium sesquicarbonate dihydrate (Na2CO3.NaHCO3.2H2O), sodium bicarbonate (NaHCO3) or lime (CaO) into the ductwork after the boiler. SOx present in flue gases bonds with the dry sorbent, and the product is dropped out in a PM control device as given in Figure 7. The SO2 adsorbed depends on the type of adsorbent and the capacity of absorbent as given in Table 8. The adsorbents were evaluated at a Ca/S stoichiometric ratio of 2.0 while burning 1.6% sulfur coal. The particle size of adsorbent effect SO2 removal, for example, SO2 removed was 48% with particle size less than 10 μm and it decreased to 39% for particle size of less than 44 μm. There is no requirement of a separate adsorbent chamber, capital costs are less. DSI are commonly applied on small plants where the initial cost is an issue and / or when lower SO2 removal efficiency (50% to 70%) is required. Dry sorbent injection (DSI) is a pollution control technology that may play a role in the removal of Mercury as well [22].
| Adsorbent | SO2 removed |
|---|---|
| Linestone | 30% |
| Dolomite Lime | 58% |
| Calcitic Lime | 62% |
| Ligno Lime | 68% |
Table 8: SO2 removed by some adsorbent.
Mercury control
Mercury (Hg) combines with water and forms water soluble compounds, one of them is methyl-mercury. The water-soluble compounds of Hg accumulate in fish and the consumption of such a fish is a risk to humans. Annual limits set for Hg emission control are given in Table 9. Approximately 95% mercury is captured by using powdered activated carbon (PAC). It is suitable to achieve new set of emission limits by using PAC. All possible species (compounds) of mercury such as the elemental, oxidized and particulate are collected in PM control devices (ESP or fabric filter). A modification of PAC, such as halogenation of PAC improves the ability to capture mercury. PAC is a porous material, hence, contaminants, such as mercury, gets adsorbed in the porous area of the PAC particles. PAC with high chlorine contents is considered to be effective for 90% mercury control (Hg-control) in flue gases at temperatures less than 440 K for bituminous coal. PAC with low chlorine contents can remove up to 60% of Hg. Brominated PAC is the most effective for Hg- control because of the more reactivity of bromine (Br) over chlorine (Cl). It is expected that total mercury removals would increase from 10% to 50% to 50% to 90% with brominated PAC injection. A problem for PAC injection is its poisoning by SO3. The consumption of PAC increases as the concentration of SO3 increases above 5 ppm and when concentration of SO3 increases more than 15 ppm, the PAC become less effective for mercury removal. It is the most difficult to remove Hg from the flue gases if it is coming out in flue gases in elemental form. To mitigate poisoning of SO3, a DSI should be installed to remove SO3 before the point of injection of PAC. The larger the DSI unit, the greater the conversion of elemental Hg to oxidized Hg with efficient Hg-removal. The oxidized type of Hg can also be effectively removed by Wet FGD systems.
| Emission limit | Country |
|---|---|
| 1.0 µg/Nm3 | EU |
| 0.3 µg/Nm3 | China |
| <0.5 µg/Nm3 | EU New* |
Note: *new emission standards of European Union
Table 9: Annual limits for Hg emissions.
Discussion and Conclusion
Particulate matter can be removed and the use of FF is preferred on ESP. NOx can be released at the emission control limits by using decomposition technique SCR along with the temperature control. SOx emissions can be controlled by CDS and DSI chemical reaction techniques. Mercury emission can be restrained by use of DSI and FDG techniques. Control of fly ash will control radioactive emission to atmosphere. New air emission limits set for coal fired power plants are stringent but practicable. It is recommended that a coal power plant working at these emission limits should work otherwise it should retire or plan to go for cogeneration with natural gas. To make world a safer place some people are expecting more stringent emission control limits which means there should be search for more efficient new control technologies.
References
- Eia (2016) International energy outlook independent statics and analysis. US Energy Information. Report No.: DOE/EIA-0484.
- PRTR (2016) Europäisches Emissionsregister.
- Gerhardt T (2012) By the numbers: Coal plant retirements over the next five years. A record number of coal power plants retire". E-Magazine.
- Schwartzkopff J, Littlecott C (2015) G7 coal phase out: France. A review from oxfam international. E3G. 47 Great guildford street, London SE1 0ES.
- Deloitte C (2015) European energy market reforms. Country profile, Italy deloitte conseil, Zurich, Switzerland. 42168.
- Nel A (2005) Air pollution-related illness: Effects of particles. Science 308: 804-806.
- Grahame T, Schlesinger R (2007) Health effects of airborne particulate matter: Do we know enough to consider regulating specific particle types or sources? Inhalation toxicology 19: 457–481.
- Pandey SK (2014) Coal fly ash: Some aspects of characterization and environmental impacts. JECET 3: 921-937.
- WHO (2013) Outdoor air pollution a leading environmental cause of cancer deaths. Lyon/Geneva, Switzerland.
- Hong BD, Slatick ER (1994) Carbon dioxide emission factors for coal. Energy information administration, quarterly coal report, January-April 1994, DOE/EIA-0121(94/Q1) Washington, USA. pp. 1-8
- McElrath WA (2009) Office of investigations special report: Response to EPA administrator’s request for investigation into allegations of a cover-up in the risk assessment for the coal ash rulemaking. report no. 10-N-0019. US-EPA.
- Harrison MR, Shires TM, Wessels JK, Cowgill RM (1996) National USA Environmental protection agency (EPA). 2007. Human and ecological risk assessment of coal combustion
- Gottlieb B, Gilbert SG, Evans LG (2010) Coal ash: The toxic threat to our health and environment. A report from physicians for social responsibility and earthjustice. Earth justice. 1625 Massachusetts Ave. NW, Suite 702 Washington, DC 20036. Physicians for social responsibilities 1875 Connecticut avenue, NW, Suite 1012 Washington.
- Shindell D, Kuylenstierna JC, Vignati E, Van-Dingenen R, Amann M, et al. (2012) Simultaneously mitigating near-term climate change and improving human health and food security. Science 335: 183-189.
- Phillips NG, Ackley R, Crossen ER, Down A, Hutyra LR, et al. (2013) Mapping urban pipeline leaks: Methane leaks across Boston. Environmental Pollution 173: 1-4.
- Drouin M (2014) Texting, textese and literacy abilities: A naturalistic study 37: 250-267
- Shauk Z (2013) A methane problem in Utah. Houston Chronicle, USA.
- Hansen J (2009) Storms of my grandchildren. London: Bloomsbury Publishing. p. 242. ISBN 1-4088-0745-9.
- Billings CE, Wilder J (1970) Fabric filter systems study, handbook of fabric filter technology. Springfield, VA: HRD Press.
- Minchener A (2010) Developments in China’s coal-fired power sector. IEA clean coal centre. Report CCCé163. ISBN 92: 483-492.
- Jiang XM (2000) Flue gas desulfurization technologies for coal-fired power plants. Presentation at coal-tech 2000 International conference held on Nov 13-14.
- "Sources climate change" US EPA (2011). Retrieved august 26, 2012.
Relevant Topics
- Atmosphere
- Atmospheric Chemistry
- Atmospheric inversions
- Biosphere
- Chemical Oceanography
- Climate Modeling
- Crystallography
- Disaster Science
- Earth Science
- Ecology
- Environmental Degradation
- Gemology
- Geochemistry
- Geochronology
- Geomicrobiology
- Geomorphology
- Geosciences
- Geostatistics
- Glaciology
- Microplastic Pollution
- Mineralogy
- Soil Erosion and Land Degradation
Recommended Journals
Article Tools
Article Usage
- Total views: 47691
- [From(publication date):
July-2017 - Apr 03, 2025] - Breakdown by view type
- HTML page views : 44889
- PDF downloads : 2802