Research Article Open Access
Clinico-Pathological Differences of Oral Squamous Cell Carcinoma among Younger and Older Patients
1Dental Implant Research Center, Department of Oral and Maxillofacial Pathology, School of Dentistry, Isfahan University of Medical Sciences, Isfahan, Iran
2Dental Materials Research Center, Department of Oral and Maxillofacial Pathology, School of Dentistry, Isfahan University of Medical Sciences, Isfahan, Iran
- *Corresponding Author:
- Saeedeh Khalesi
Dental Materials Research Center, Department of Oral and Maxillofacial Pathology
School of Dentistry, Isfahan University of Medical Sciences, Isfahan, Iran
Tel: 989131079487
E-mail: S_khalesi@dnt.mui.ac.ir
Received date: June 02, 2017; Accepted date: July 14, 2017; Published date: July 17, 2017
Citation: Razavi SM, Khalesi S (2017) Clinico-Pathological Differences of Oral Squamous Cell Carcinoma among Younger and Older Patients. J Clin Exp Pathol 7:316. doi: 10.4172/2161-0681.1000316
Copyright: © 2017 Razavi SM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Clinical & Experimental Pathology
Abstract
Introduction: Over 95% of the oral cancers are squamous cell carcinoma (OSCC). The incidence of OSCC in earlier than 40 years old has been reported from 0.4 to 3.9% of all patients. Recent studies have indicated the increasing number of young and very elderly adults. The purpose of this study is evaluating the clinicopathological features of OSCC among younger and older patients.
Materials and Methods: In this retrospective study, files of 80 OSCC patients were retrieved from Oral Pathology Department. Demographic data including gender, age, clinical feature and location of lesions were collected. Archival formalin fixed paraffin embedded tissue blocks were used to prepare hematoxylin and eosin stained for grading OSCC based on Broder’s, Anneroth et al. and Bryne et al. classification among younger and older patients. The variables data were analyzed using Chi square, Mann Whitney and Fisher exact tests. The significant level was set at P<0.05.
Results: Comparison of clinical criteria between young and old patients did not appear statistically significant). Furthermore, we did not found a statistically significant between Broder’s, Anneroth et al. and Bryne et al. grading systems on two groups (P>0.05).
Conclusion: Within the limitations of the present study, we show that there are not any specific histpathological parameters of OSCC in young and old patients. The incidence of OSCC in young patients was low compared to old patients. Although, further research need to access genetic, hereditary, diet and demographic factors with more patients.
Keywords
Squamous cell carcinoma; Pathology; Oral cancer
Introduction
Head and neck cancers (HNCs) is the fifth most common malignant neoplasm in the worldwide and over 95% of the oral cancers are squamous cell carcinoma (SCC) [1,2]. Squamous cell carcinoma of head and neck is typically diagnosed in fifth to seventh decade of life [3]. The incidence of oral squamous cell carcinoma (OSCC) in earlier than 40 years old has been reported from 0.4 to 3.9% of all patients [4]. However, recent studies have indicated the increasing number of young and very elderly adults [5]. The incidence of oral cancer in younger patients is reported 6% of all oral neoplasms in United Kingdom and 4.3-5.5% in the Sri Lankan [6,7]. Long term exposures to some classic risk factors such as alcohol consumption, smoking and betel nut chewing have been strongly associated with occurrence of OSCCs in all patients [5,8]. But, according to recent studies additional conditions such as chronic inflammation, genetic alterations, and viral infection may be other predisposes factors to young and very elderly adult patients [5]. Furthermore, some studies showed the more biological behavior and clinical course of OSCC in young patients [2,9,10]. But in other studies observed no difference between clinical course and prognosis of OSCC in two groups of age [4,11,12]. Even, Udeabor and et al. showed that young patients have a better prognosis than old patients [8]. Various histopathological grading systems of OSCC such as Broder’s, Anneroth et al. and Bryne et al. grading systems have been discussed in literature that these systems provide valuable diagnostic and predictive information of OSCC [13-15]. The purpose of this study is evaluating the clinicopathological features of OSCC among younger and older patients. Furthermore, this study attempts to evaluate aggression of OSCC histopathological features with Broder’s, Anneroth et al. and Bryne et al. grading systems in patients above forty and below forty years of age.
Materials and Methods
This retrospective study is conducted on the records of the patients in the archive of the Oral and Maxillfacial Pathology Department of Isfahan Dental Faculty. In this study, eighty samples of oral squamous cell carcinoma were included. Demographic data including gender, age, clinical feature and location of lesions were collected. The samples were divided into two groups, as young (40 years old) and old (>40 years old). The sections from all archived paraffin-embedded tissue specimens from 80 cases of OSCC were stained with hematoxylin and eosin (H & E). A section containing the full thickness of the tumors were used for histopathological gradings. Broder’s, Anneroth et al. and Bryne et al. classification grading systems (Tables 1 and 2) were used to detect the histopathological parameters [13-15]. SPSS version 22.0 Statistical Software was used for analysis. The variables data were analyzed using Chi square, Mann Whitney and Fisher exact tests. The significant level was set at P<0.05.
| Broder’s grading system | Differentiated (%) | Undifferentiated (%) | ||
|---|---|---|---|---|
| Grade I | 100-75 | 0-25 | ||
| Grade II | 75-50 | 25-50 | ||
| Grade III | 50-25 | 50-75 | ||
| Grade IV | 25-0 | 75-100 | ||
| Anneroth et al. grading system | Score1 | Score2 | Score3 | Score4 |
| Degree of keratinization | Highly keratinized | Moderately keratinized | Minimal keratinized | No keratinized |
| Nuclear aberrations | Few | Moderate | Abundant | Abundant + anaplastic nuclei |
| Number of mitoses | Few (0-2 cells) | Moderate (3-4 cells) | Numerous (5-6 cells) | Extremely numerous |
| (more than 6 cells) | ||||
| Pattern of invasion | Large islands | Small islands | Thin strands | Individual tumor cells |
| + pushing border | (<5 cells thickness) | |||
| Host immune response | Dense | Moderate | Light | None |
| Bryne et al. grading system | Score1 | Score2 | Score3 | Score4 |
| Degree of keratinization | Highly keratinized | Moderately keratinized | Minimal keratinized | No keratinized |
| Nuclear aberrations | Few | Moderate | Abundant | Abundant + anaplastic nuclei |
| Pattern of invasion | Large islands | Small islands | Thin strands | Individual tumor cells |
| + pushing border | (<5 cells thickness) | |||
| Host immune response | Dense | Moderate | Light | None |
Table 1: Three grading systems for squamous cell carcinomas.
| Score | Grade | |
|---|---|---|
| Anneroth et al. | 05-Oct | I (Well differentiated) |
| Nov-15 | II (moderately differentiated) | |
| 16-20 | III (poorly differentiated) | |
| Bryne et al. | 04-Aug | I (Well differentiated) |
| 09-Dec | II (moderately differentiated) | |
| 13-16 | III (poorly differentiated) |
Table 2: Scoring system of Anneroth et al.’s and Bryne et al. grading systems.
Results
The frequency of OSCCs based on location, clinical feature and duration between two age groups showed in Tables 3 and 4. Comparison of these clinical criteria between young and old patients did not appear statistically significant (P>0.05). Furthermore, we did not found a statistically significant between Broder’s, Anneroth et al. and Bryne et al. grading systems on two groups (P>0.05). Distribution of histopathological criteria according to three grading classification is shown in Table 4, which comparison between the young and older groups of patients did not demonstrate statistically significant (P>0.05) (Figure 1).
| Young (n=11) | Old (n=69) | Total (n=80) | P-value | |
|---|---|---|---|---|
| Number (%) | Number (%) | Number (%) | ||
| Sex | ||||
| Male | 10(90.9) | 28(40.6) | 38(47.5) | 0.002 |
| Female | 1(9.1) | 41(59.4) | 42(52.5) | |
| Tumor location | ||||
| Alveolar mucosa | 5(45.5) | 26(37.7) | 31(38.8) | 0.416 |
| Buccal mucosa | 2(18.2) | 14(20.3) | 16(20.0) | |
| Tongue | 2(18.2) | 19(27.5) | 21(26.3) | |
| Floor of the mouth | 0(0.0) | 4(5.8) | 4(5.0) | |
| Palate | 2(18.2) | 2(2.9) | 4(5.0) | |
| Lip | 0(0.0) | 4(5.8) | 4(5.0) | |
| Tumor duration | ||||
| <6 month | 6(54.5) | 48(69.6) | 54(67.5) | 0.59 |
| 6-12 month | 4(36.4) | 5(7.2) | 9(11.3) | |
| 1-2 years | 0(0.0) | 9(13.0) | 9(11.3) | |
| >2 years | 1(9.1) | 7(10.1) | 8(10.0) | |
| Clinical feature | ||||
| Exophytic lesion | 4(36.4) | 30(43.5) | 34(42.5) | 0.896 |
| Ulcer | 6(54.5) | 27(39.1) | 33(41.3) | |
| White plaque | 0(0.0) | 4(5.8) | 4(5.0) | |
| Red & white plaque | 1(9.1) | 8(11.6) | 9(11.3) |
Table 3: Characteristics of two groups.
| Histopathological parameter | Young (n=11) Number (%) | Old (n=69) Number (%) | Total (n=80) Number (%) | P-value |
|---|---|---|---|---|
| Degree of keratinization | ||||
| High | 5(45.5) | 30(43.5) | 35(43.8) | 0.839 |
| Moderate | 4(36.4) | 25(36.2) | 29(36.3) | |
| Light | 2(18.2) | 11(15.9) | 13(16.3) | |
| None | 0(0.0) | 3(4.3) | 3(3.8) | |
| Nuclear aberrations | ||||
| Few | 3(27.3) | 16(23.2) | 19(23.8) | 0.916 |
| Moderate | 3(27.3) | 21(30.4) | 24(30.0) | |
| Abundant | 4(36.4) | 20(29.0) | 24(30.0) | |
| Abundant+anaplastic | 1(9.1) | 12(17.4) | 13(16.3) | |
| Number of mitoses | ||||
| Few (0-2) | 7(63.6) | 29(42.0) | 36(45.0) | 0.195 |
| Moderate (3-4) | 2(18.2) | 20(29.0) | 22(27.5) | |
| Numberous (5-6) | 2(18.2) | 15(21.7) | 17(21.3) | |
| Ex numerous (>6) | 0(0.0) | 5(7.2) | 5(6.3) | |
| Pattern of invasion | ||||
| Large islands | 8(72.7) | 49(71) | 57(71.3) | 0.789 |
| Small islands+pushing border | 1(9.1) | 12(17.4) | 13(16.3) | |
| Thin strands | 2(18.2) | 7(10.1) | 9(11.3) | |
| Indiviual tumor cells | 0(0.0) | 1(1.41) | 1(1.3) | |
| Host immune response | ||||
| Dense | 2(18.2) | 15(21.7) | 17(21.3) | 0.942 |
| Moderate | 4(36.4) | 26(37.7) | 30(37.5) | |
| Light | 5(45.5) | 17(26.4) | 22(27.5) | |
| None | 0(0.0) | 11(15.9) | 11(13.8) | |
| Broder’s grade | ||||
| I | 5(45.5) | 32(46.4) | 37(46.3) | 0.946 |
| II | 4(36.4) | 23(33.3) | 27(33.8) | |
| III | 2(18.2) | 11(15.9) | 13(16.3) | |
| IV | 0(0.0) | 3(4.3) | 3(3.8) | |
| Anneroth et al. grading | ||||
| I | 8(72.7) | 36(52.2) | 44(55.0) | 0.193 |
| II | 3(27.3) | 31(44.9) | 34(42.5) | |
| III | 0(0.0) | 2(2.9) | 2(2.5) | |
| Bryne’s garding | ||||
| I | 9(81.8) | 38(55.1) | 47(58.8) | 0.094 |
| II | 2(18.2) | 30(43.5) | 32(40.0) | |
| III | 0(0.0) | 1(1.4) | 1(1.3) | |
Table 4: Distribution of histopathological parameters in two groups.
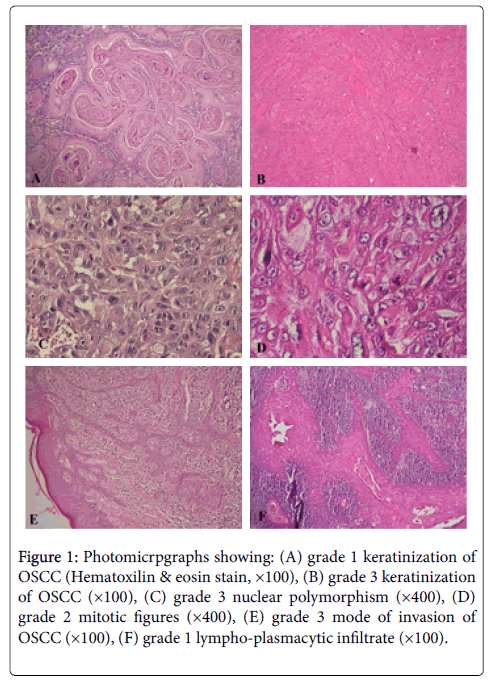
Figure 1: Photomicrpgraphs showing: (A) grade 1 keratinization of OSCC (Hematoxilin & eosin stain, ×100), (B) grade 3 keratinization of OSCC (×100), (C) grade 3 nuclear polymorphism (×400), (D) grade 2 mitotic figures (×400), (E) grade 3 mode of invasion of OSCC (×100), (F) grade 1 lympho-plasmacytic infiltrate (×100).
Discussion
In this study, we retrospective reviewed the clinical and histopathological parameters of oral squamous cell carcinoma and compared in young and old patients. The cutoff age of 40 years patients with OSCC was indicated because other studies have found significant relations between these groups [2,5]. We used three histopatholgical grading systems. Broder’s grading of OSCC suggested the quantitative grading of cancer in 1920 and has been used for many years [13]. In 1987, Anneroth et al.’s grading system suggested in order to obtain a more precise morphological evaluation of OSCC [14]. Recently, Bryne and et al. introduced a multifactorial grading system only for the deep invasive margins of the SCC [15].
In the present study, the comparison of OSCC between young and old groups according to three grading systems showed no statistically significant difference (P>0.05). This result was resembles to Sirwardena and Sosaki studies [4,16]. In contrast to our study, Rahman’s study showed statistically significant difference between younger and older patients. However, two groups of Rahman’s study have not statistically significant difference based on Broder’s criteria, but the young patients have greater aggression of OSCC because differences in nuclear polymorphism, mitosis index and depth of invasion were higher grades among young patients when compared to old patients [2]. In this present study, all individual histopathological parameters was not different statistically significant between two groups (P>0.05), but we found higher nuclear aberrations in the old patients that was contradictory with the results of Siriwardena’s study [16]. Furthermore, the results showed the lower host immune response in the younger patients. Some studies suggested that the OSCC in young patients have more aggressive behavior [17]. Considering the important role of immune response in control to tumor cell proliferation, lower immune response in younger patient’s maybe reason for more aggressive behavior in this age group.
In other hands, two groups of the present studies were more or less equally distributed within each grade in three grading systems. These results were resemble to Falaki, Sun and Iype’s studies that in they have been suggested well differentiated SCC (WDSCC) as the nost common type followed by moderate differentiated SCC (MDSCC) and poorly differentiated SCC (PDSCC) [10,18,19]. But in Rahman and Siriwardena’s studies, the most of young patients have MDSCC [2,16]. In contrast to these studies, Sasaki and et al. reported a tendency for OSCC in younger patients to be WDSCC in contrast to tumors in old group which have more often MDSCC [4].
In many studies, OSCC is predominantly a disease of men in all age groups [1,10,20], But few studies showed a female predilection in young patients of OSCC [21,22]. In contrast to these studies, we found a higher incidence of OSCC in males from the younger group, with a ratio of 10 (males): 1 (females), resemble to Sun et al. study [18].
The most common site of OSCC was the tongue according to other studies in UK, Sri Lanka and Iran [4,7,10], But in some other Asian countries such as Thailand, Taiwan and India, buccal mucosa was the most common location that was related to betel quid/tabacco chewing habits [10]. However, in the present study the most location of OSCC in young and old was alveolar mucosa (38.8%) and followed by tongue (26.3%) and buccal mucosa (20%). We can suggest that the risk factors of OSCC in young and old patients were not different. In some studies, oral tongue was most commonly location in young and alveolar process in older age [1,5,18]. However, different results in many studies have not yet to be explored.
The most common clinical feature of OSCC patients was exophytic lesion resemble to Falaki’s et al. study and in contrast to other studies that ulceroproliferative lesions were more common [10]. Although in the present study, ulcer lesions was most common clinical presentation in young patients.
Conclusion
Within the limitations of the present study, we showed that there are not any specific histpathological parameters of OSCCs in young and old patients. The incidence of OSCCs in young patients was low compared to old patients. Although, further research need to access genetic, hereditary, diet and demographic factors with more patients.
Acknowledgment
This study was supported by Isfahan University of Medical Sciences Research Grant # 295121. This research also was supported by Dental Materials Research Center of Isfahan University of Medical Sciences.
Conflict of Interest
The authors have no conflict of interest.
References
- Naz S, Salah K, Khurshid A, Hashmi AA, Faridi N (2015) Head and neck squamous cell carcinoma - comparative evaluation of pathological parameters in young and old patients. Asian Pac J Cancer Prev 16: 4061-4063.
- Ur Rahaman SM, Ahmed Mujib B (2014) Histopathological correlation of oral squamous cell carcinoma among younger and older patients. J Oral Maxillofac Pathol 18: 183-188.
- Fan Y, Zheng L, Mao MH, Huang MW, Liu SM, et al. (2014) Survival analysis of oral squamous cell carcinoma in a subgroup of young patients. Asian Pac J Cancer Prev 15: 8887-8891.
- Sasaki T, Moles DR, Imai Y, Speight PM (2005) Clinico-pathological features of squamous cell carcinoma of the oral cavity in patients <40 years of age. J Oral Pathol Med 34: 129-133.
- Troeltzsch M, Knösel T, Eichinger C, Probst F, Troeltzsch M, et al. Clinicopathologic features of oral squamous cell carcinoma: do they vary in different age groups? J Oral Maxillofac Surg 72: 1291-1300.
- Anonymous (2006) Opportunistic oral cancer screening. British Dental Association (BDA news) 6: 6-10.
- Siriwardena BS, Tilakaratne A, Amaratunga EA, Tilakaratne WM (2006) Demographic, aetiological and survival differences of oral squamous cell carcinoma in the young and the old in Sri Lanka. Oral Oncol 42: 831-836.
- Udeabor SE, Rana M, Wegener G, Gellrich NC, Eckardt AM (2012) Squamous cell carcinoma of the oral cavity and the oropharynx in patients less than 40 years of age: a 20-year analysis. Head Neck Oncol 4: 28.
- Sarkaria JN, Harari PM (1994) Oral tongue cancer in young adults less than 40 years of age: rationale for aggressive therapy. Head Neck 16: 107-111.
- Falaki F, Dalirsani Z, Pakfetrat A, Falaki A, Saghravanian N, et al. (2011) Clinical and histopathological analysis of oral squamous cell carcinoma of young patients in Mashhad, Iran: a retrospective study and review of literature. Med Oral Patol Oral Cir Bucal 16: e473-e477.
- Pytynia KB, Grant JR, Etzel CJ, Roberts D, Wei Q, et al. (2004) Matched analysis of survival in patients with squamous cell carcinoma of the head and neck diagnosed before and after 40 years of age. Arch Otolaryngol Head Neck Surg 130: 869-873.
- Ho HC, Lee MS, Hsiao SH, Hwang JH, Hung SK, et al. (2008) Squamous cell carcinoma of the oral cavity in young patients: a matched-pair analysis. Eur Arch Otorhinolaryngol 1: S57-S61.
- Broders AC (1920) Squamous epithelium of the lip: a study of five hundred and thirty seven cases. JAMA 74: 656-664.
- Anneroth G, Batsakis J, Luna M (1987) Review of the literature and a recommended system of malignancy grading in oral squamous cell carcinomas. Scand J Dent Res 95: 229-249.
- Bryne M, Koppang HS, Lilleng R, Kjaerheim A (1992) Malignancy grading of the deep invasive margins of oral squamous cell carcinomas has high prognostic value. J Pathol 166: 375-381.
- Siriwardena BS, Tilakaratne A, Amaratunga EA, Udagama MN, Ogawa I, et al. (2007) Analysis of histopathological and immunohistochemical differences of oral squamous cell carcinoma in young and old patients in Sri Lanka. J Oral Pathol Med 36: 357-362.
- Byers RM (1975) Squamous cell carcinoma of the oral tongue in patients less than thirty years of age. Am J Sur 130: 475-478.
- Sun Q, Fang Q, Guo S (2015) A comparison of oral squamous cell carcinoma between young and old patients in a single medical center in China. Int J Clin Exp Med 8: 12418-12423.
- Iype EM, Pandey M, Mathew A, Thomas G, Sebastian P, et al. (2001) Oral cancer among patients under the age of 35 years. J Postgrad Med 47: 171-176.
- Mallet Y, Avalos N, Le Ridant AM, Gangloff P, Moriniere S, et al. Head and neck cancer in young people: a series of 52 SCCs of the oral tongue in patients aged 35 years or less. Acta Otolaryngol 129: 1503-1508.
- Müller S, Pan Y, Li R, Chi AC (2008) Changing trends in oral squamous cell carcinoma with particular reference to young patients: 1971-2006. The Emory University experience. Head Neck Pathol 2: 60-66.
- Salem A (2010) Dismissing links between HPV and aggressive tongue cancer in young patients. Ann Oncol 21: 13-17.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 3789
- [From(publication date):
August-2017 - Nov 24, 2024] - Breakdown by view type
- HTML page views : 3045
- PDF downloads : 744