Clinicopathological Correlation in Patients with Oral Lichen Planus
Received: 03-Jun-2023 / Manuscript No. jdpm-23-102435 / Editor assigned: 05-Jun-2023 / PreQC No. jdpm-23-102435 (PQ) / Reviewed: 23-Jun-2023 / QC No. jdpm-23-102435 / Revised: 23-Jun-2023 / Manuscript No. jdpm-23-102435 (R) / Published Date: 30-Jun-2023 DOI: 10.4172/jdpm.1000154
Abstract
Background: Oral Lichen Planus is a chronic immunological mucosal disorder. Histopathological evaluation of oral biopsy is necessary because of varied clinical manifestations that might mimic other disease and its premalignant potential. However, a lack of universally accepted diagnostic criteria leads to a very low clinic-pathological correlation.
Methodology: The current prospective hospital based observational study was carried from 2016 to 2022 at Department of Dermatology, College of Medical Sciences, Bharatpur. All patients with oral lesions suspicious of Oral Lichen Planus were included in our study and routine histopathological evaluation was carried as per standard protocols. Clinical and histopathological diagnosis were made as per World Health Organization criteria.
Results: The average age of patients in our study was 41.14 years and majority of them were females (68.8%).Areca nut and tobacco chewing habbit was present in 24.4% and 16.7% patients respectively. Majority of patients were asymptomatic while 41.1% patients complained of burning sensation upon intake of food, especially in erosive form of disease. Involvememt of buccal mucosa (55.6%) followed by tongue (7.8%) and labial mucosa (7.8%) were observed. Reticular Oral Lichen Planus (53.3%) was the most common morphological type followed by erosive OLP (23.3%). Clinicopathological correlation was achieved in 65.6% of cases. Band like stromal infiltrate (100%) and basal layer vacuolization (100%) were the most consistent histopathological findings.
Conclusion: There is low clinicopathological correlation in cases of Oral Lichen Planus using World Health Organization criteria and a universally accepted diagnostic criteria is the need of hour.
Keywords
Oral Lichen Planus; Clinicopathological correlation; Histopathology
Introduction
Oral lichen planus (OLP) is a T-cell–mediated chronic inflammatory disorder affecting the oral mucosa with global prevalence ranging from 0.2 to 2% [1,2]. It usually affects middle aged individuals and has a gender predilection with a female to male ratio of 2:1 [3]. Mucosal involvement in cutaneous Lichen Planus (LP) is reported to be 60% but oral lesion can be the only manifestation of disease [4].
Although the cause remains largely unknown, genetic and environmental factors are reported to be associated with OLP.4 Environmental factors such as drugs, dental materials, infectious agents, psychological stress, autoimmunity, personal habits (smoking, and chewing betel nuts), and systemic diseases (Diabetes Mellitus, Hypertension, and Thyroid dysfunction) are reported to be associated with OLP [3]. The auto-reactive cytotoxic CD8 + T cells trigger apoptosis of the basal cells of the oral epithelium that expresses altered self-antigens on their surface via tumor necrosis factor (TNF)-α, Fas- FasL-mediated or granzyme B [3].
Oral Lichen Planus has distinctive clinical features within the oral cavity and characteristic morphological types reported include atrophic, bullous, erosive, papular, plaque like and reticular. Most common type is reticular which commonly affects buccal mucosa bilaterally and the lesions are asymptomatic [6].
OLP is classified as potentially malignant disorder with low and questionable risk for malignant transformation. However, histopathological confirmation is required because of premalignant potential, especially in, non-reticular types, unilateral presentation or lesions present at the cancer-risk oral sites (lower lip, lateral border of tongue, floor of the mouth). Currently, World Health Organization has given histopathological criteria for OLP and is accepted in clinical practice [7]. Present prospective hospital based study is carried out to study epidemiological characteristics and clinico-histopathological correlation in Oral Lichen Planus patients.
Materials and Methods
An observational prospective hospital based study was conducted from 2016 to 2022 at the department of Dermatology, College of Medical Sciences and Teaching Hospital, Bharatpur, Nepal. A total of 90 patients with clinical diagnosis of OLP were enrolled in the study. Patients previously diagnosed as OLP under treatment and those not giving consent for participation were excluded from the study. Detailed history and clinical examination utilizing WHO criteria was performed [8]. Informed consent was obtained from all patients and serological tests (HIV, VDRL, Hepatitis B and Hepatitis C) were performed before subjecting them to oral biopsy. After standard processing and staining with H&E (Haematoxylin and Eosin) stain, histopathological diagnosis was established utilizing WHO criteria. Data were recorded and photographs were taken when necessary. Statistical analysis of the data was done by using standard statistical protocol with the help of Statistical Package for the Social Sciences (SPSS) software, version 26.
Results
The mean age of the patients included in study was 41.14 +13.3 years. The maximum number (n=28, 31.1%) of cases were between 31- 40 years of age. The female to male ratio was 2.21:1. Arecanut chewing (n=22, 24.4%) was the most common among our patient followed by tobacco chewing (n=15, 16.7 %), smoking (n=11, 12.9%), and alcohol consumption (n=8, 8.9%) but none of our patients had history of contact allergen. Type 2 Diabetes mellitus (n=4, 4.4%) and Hypertension (n=5, 5.6 %) were the most common systemic diseases associated with OLP followed by Hypothyroidism (n=3, 3.3%). Serological tests for Hepatitis C was negative in all patients.
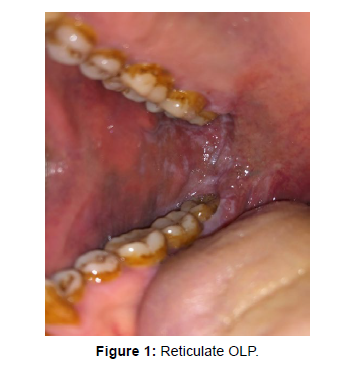
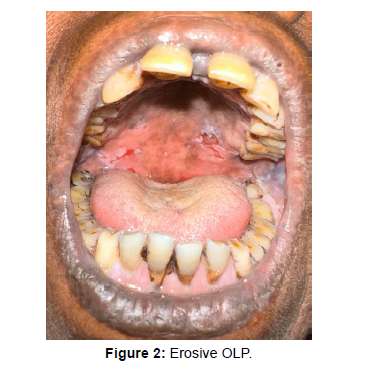
The mean duration of the disease was 13.32+9.03 months. Majority of patients were asymptomatic (n=53, 58.9%) whereas 41.1% patients complained of burning sensation upon intake of food. Burning sensation was most prevalent in erosive form of OLP (n= 17, 18.8%). Buccal mucosa (n=50, 55.6%) followed by tongue (n=7, 8.6%) and labial mucosa (n=7, 8.6%) were most common sites of involvement in our patients. Multiple sites were involved in 23 (25.6%) patients. Reticular OLP was the most common subtype (Figure 1) observed (n=48, 53.3%), followed by erosive (Figure 2) OLP (n=21, 23.3%). Plaque and atrophic form of OLP accounted about 6.7 % and 4.4% in our patients.
Involvement of skin was seen in 15 (16.6%) patients. 13 patients had classical papular Lichen planus, whereas Lichen Planus Pigmentosus and Lichen Planus Hypertrophicus were seen in one patient each. Nail Changes were seen in seven patients (7.7%). Longitudinal ridging and pterygium formation were seen in five and two patients respectively. Genital involvement was seen in 3 patients.
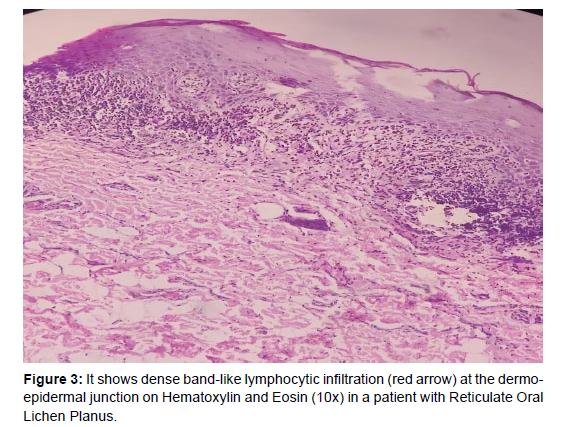
Clinicopathological correlation for individual subtype of OLP is summarized in Table 1 and histopathological findings observed in Confirmed cases of OLP (n=59) are summarized in Table 2. Among 90 patients with clinical lesions suggestive of OLP, 59 patients had histopathological features consistent Table 2with clinical diagnosis. Features were non-specific in 15 (16.7%) patients, while 6 (6.7%) patients had leukoplakia, 5 (5.6%) patients had benign keratosis, 2 patients had Discoid Lupus Erythematosus, and 1 patient had Pemphigus Vulgaris. Dysplasia and frank SCC were noted in two patients. Overall a clinicopathological correlation was observed in 65.6% (59 out of 90). Highest correlation was obtained in reticular OLP (81.25%) (Figures 3 and 4).
| Clinical types of OLP | Histopathological Diagnosis |
Clinico-pathological Correlation |
|---|---|---|
| Reticular OLP (n=48, 53.3%) |
OLP (39) Non-specific (6) Benign keratosis (2) Leukoplakia (1) |
81.25% |
| Erosive OLP (n=21, 23.3%) |
OLP (10) Non-specific (7) DLE (2) Benign keratosis (1) Dysplasia/SCC (1) |
47.61% |
| Plaque OLP (n=6, 6.7%) |
OLP (2) Non-specific (1) Leukoplakia (2) Dysplasia/SCC (1) |
33.3% |
| Atrophic OLP (n=4, 4.4%) |
OLP (2) Leukoplakia (1) Benign keratosis (1) |
50 % |
| Multiple subtypes (n=11, 12.2%) |
OLP (6) Leukoplakia (2) Non-specific (1) Pemphigus Vulgaris (1) Benign Keratoses (1) |
54.5 % |
Table 1: It shows the clinical types of Oral lichen planus, its histopathological diagnosis and the Clinicopathological correlation.
| Histopathological Findings | Present (%) |
|---|---|
| Parakeratosis | 91.5% |
| Hypergranulosis | 74.5% |
| Irregular Acanthosis with saw toothed rete edges | 89.8% |
| Basal layer Vacuolation | 100% |
| Stromal Infiltrate | 100% |
| Civatte body | 35.6% |
| Melanin incontinence | 44% |
| Atypical cells | 0 % |
Table 2: It shows histopathological findings in confirmed cases of OLP.
Discussion
Oral lichen planus is a chronic inflammatory disease and has a reported prevalence of 0.2 to 2%.2 The mean age of patients included in our study was 41.14+13.3 years which is similar to the study carried out by Irani et al.8 However, Alvarej et al reported a higher median age of onset (57 years) [9]. A female preponderance (female to male ratio 2.21:1) was observed in our study and is in concordance with a study reported [10]. However, Munde et al reported a male preponderance in their study [11].
Use of tobacco, gutkha and betel nut in South Asian countries have been highlighted as possible etiologic factors in development of OLP [10,12,13]. In our study, areca nut and tobacco chewing was observed in 41.1% patients. A study conducted by Mankapure et al. reported tobacco, gutkha and betel nut chewing habit in 36.1% of the patients, which is in accordance with our study [14]. Although, studies have reported smoking as causative agent in development of OLP Studies have refuted the role of alcohol abuse and smoking as possible etiological factors in development of OLP [15 ]. Only a small number of patients gave a history of smoking (12.2%) and alcohol abuse (8.9%) in our study [16].
Although, correlation of OLP with various systemic diseases has been reported, our study along with studies conducted by Xue JL, et al. [17] and Shen ZY, et al. [10]. point to a minimal role of systemic disease in causation of OLP as 14.5% of our patients only had systemic illness such as hypertension (5.6%), Diabetes Mellitus (4.4%), and hypothyroidism (2.8%). Serology for HCV was negative in all cases included in our study however, a positive association of HCV infection with development of OLP due to genetic susceptibility i.e HLA-DR6 and geographical variation in the prevalence of HCV infection has been reported [18-20].
Burning sensation upon intake of food was reported in 41.1% of our patients and majority of symptomatic patients had erosive form of OLP (18.8%) which is in concordance with Xue et al. In our study, buccal mucosa was the most frequent site of involvement (55.6%), followed by tongue and labial mucosa, 7.8% each which is in accordance with various studies reported [10,11]. Reticular form of OLP (52.9%) was the most common morphological type observed, followed by erosive type (21.4%). This is in accordance with studies conducted in past [10,11]. Bullous and papular forms of OLP were not observed in our study as these are rare morphological types [21 ].
Only a minority of OLP patients develop cutaneous lesions [4]. This is similar to the observation made in our study where only 16.6% patients had skin involvement. Nail changes, including pterygium and longitudinal ridging, were rare (7.7%).This was in concordance with study conducted by Eisen D, et al. [22]. Genital mucosa involvement was seen in 3.3% patients in our study. This is considerably lower when compared to study by Eisen D, et al. [22] Involvement of other mucosal surfaces like esophageal, laryngeal, and conjunctival mucosa were not observed in our study and this finding is consistent with rarity of involvement of these mucosal sites [6].
Oral Lichen Planus is reported to show a low clinic-pathological correlation ranging from 52.2% to 58% [7,23,24]. In a study by Van der Meij EH, et al. 42% of their reported cases in which all clinicians agreed about clinical diagnosis being diagnostic of OLP, there appeared no consensus on histopathological diagnosis and a clinico-pathological correlation could be achieved in 58% of the cases [7]. Similarly, in our study, clinico-pathological correlation was observed in 65.6% of our cases. This highlights the need for biopsy in all suspected cases of OLP. However, a study conducted by Rad et al. found higher clinicopathological correlation when using modified WHO criteria (93.87%) versus WHO criteria (68%) [25].
Band like stromal infiltrate (100%) and basal layer vacuolization (100%) were the most consistent histopathological features in confirmed cases of OLP in our study (figure 3 and 4). Parakeratosis (91.5%) and irregular acanthosis with saw toothed rete ridges (89.8%) were other frequent features. Hypergranulosis, melanin incontinence and civatte body were noted in 74.5%, 31.1%, and 23.3% respectively. These findings were similar to a study conducted by Gonzalez et al. who also observed band like stromal infiltrate (100%) and basal layer liquefaction (100%) in all their cases [26]. Cellular atypia was not noted in confirmed cases of OLP in our study however the reported malignant transformation varies from 0.7% to 2.8% in several studies [27-29]. World Health Organization classifies OLP as a pre malignant disorder [30]. This highlights the need for histopathological evaluation and subsequent follow up of all patients.
Conclusion
Cases of Oral Lichen Planus possess a diagnostic dilemma with a low clinico-pathological correlation observed in 65.6%, in our study. Hence, our study highlights the need for routine histopathological evaluation of OLP cases for accurate diagnosis and management.
Limitation
The current study was limited by its cross sectional design and lack of follow up of patients at a later stage. Acknowledgement We would like to thank Prof. Dr. T. Sheshagiri Rao, Head of the Department, Department of Pathology, College of Medical Sciences and Teaching Hospital, Bharatpur for his support and guidance.
References
- Thornhill MH, Sankar V, Xu XJ, Barrett AW, High AS, et al. (2006) The role of histopathological characteristics in distinguishing amalgam-associated oral lichenoid reactions and oral lichen planus. J Oral Pathol Med 35: 233-40.
- Hiremath SK, Kale AD, Charantimath S (2011) Oral lichenoid lesions: clinico-pathological mimicry and its diagnostic implications. Indian J Dent Res 22:827-34.
- Gupta S, Jawanda M (2015) Oral Lichen Planus: An Update on Etiology, Pathogenesis, Clinical Presentation, Diagnosis and Management. Indian J Dermatol 60:222-9.
- Cheng YSL, Gould A, Kurago Z, Fantasia J, Muller S (2016) Diagnosis of oral lichen planus: a position paper of the American Academy of Oral and Maxillofacial Pathology. Oral Surg Oral Med Oral Pathol Oral Radiol 122:332-54.
- Chitturi RT, Devy AS, Nirmal RM, Sunil PM (2014) Oral Lichen Planus: A Review of Etiopathogenesis, Clinical, Histological and Treatment Aspects. J Interdiscipl Med Dent Sci 2:1-5.
- Gorouhi F, Davari P, Fazel N (2014) Cutaneous and mucosal lichen planus: a comprehensive review of clinical subtypes, risk factors, diagnosis, and prognosis. Sci World J 1-22.
- Van der Meij EH, Van der Waal I (2003) Lack of clinicopathologic correlation in the diagnosis of oral lichen planus based on the presently available diagnostic criteria and suggestions for modifications. J Oral Pathol Med 32:507-12.
- Irani S, Esfahani AM, Ghorbani A (2016) Dysplastic change rate in cases of oral lichen planus: A retrospective study of 112 cases in an Iranian population. J Oral Maxillofac Pathol 20:395-399.
- Boñar-Alvarez P, Pérez Sayáns M, Garcia-Garcia A, Chamorro-Petronacci C, Gándara-Vila P, et al. (2019) Correlation between clinical and pathological features of oral lichen planus: A retrospective observational study. Medicine (Baltimore) 98:e14614.
- Shen ZY, Liu W, Zhu LK, Feng JQ, Tang GY, et al. (2012) A retrospective clinicopathological study on oral lichen planus and malignant transformation: analysis of 518 cases. Med Oral Patol Oral Cir Bucal 17:943-7.
- Munde AD, Karle RR, Wankhede PK, Shaikh SS, Kulkurni M (2013) Demographic and clinical profile of oral lichen planus: A retrospective study. Contemp Clin Dent 4:181-5.
- Trivedy CR, Craig G, Warnakulasuriya S (2002) The oral health consequences of chewing areca nut. Addict Biol 7:115-25.
- Reichart PA, Warnakulasuriya S (2012) Oral lichenoid contact lesions induced by areca nut and betel quid chewing: a mini review. J Investig Clin Dent 3:163-6.
- Mankapure PK, Humbe JG, Mandale MS, Bhavthankar JD (2016) Clinical profile of 108 cases of oral lichen planus. J Oral Sci 58:43-7.
- Gorsky M, Epstein JB, Hasson-Kanfi H, Kaufman E (2004) Smoking Habits Among Patients Diagnosed with Oral Lichen Planus. Tob Induc Dis 2:9.
- Carbone M, Arduino PG, Carrozzo M, Gandolfo S, Argiolas MR, et al. (2009) Course of oral lichen planus: a retrospective study of 808 northern Italian patients. Oral Dis 15:235-43.
- Xue JL, Fan MW, Wang SZ, Chen XM, Li Y, et al. (2005) A clinical study of 674 patients with oral lichen planus in China. J Oral Pathol Med 34:467-72.
- Lodi G, Olsen I, Piattelli A, D'Amico E, Artese L, et al. (1997) Antibodies to epithelial components in oral lichen planus (OLP) associated with hepatitis C virus (HCV) infection. J Oral Pathol Med 26:36-9.
- Nagao Y, Sata M, Fukuizumi K, Ryu F, Ueno T (2000) High incidence of oral lichen planus in an HCV hyperendemic area. Gastroenterology 119:882-3.
- Carrozzo M, Francia Di Celle P, Gandolfo S (2001) Increased frequency of HLA-DR6 allele in Italian patients with hepatitis C virus-associated oral lichen planus. Br J Dermatol 144(4):803-8.
- Canto AM, Müller H, Freitas RR, Santos PS (2010) Oral lichen planus (OLP): clinical and complementary diagnosis. An Bras Dermatol 85:669-75.
- Eisen D (1999) The evaluation of cutaneous, genital, scalp, nail, esophageal, and ocular involvement in patients with oral lichen planus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 88:431-6.
- Mravak-Stipetić M, Lončar-Brzak B, Bakale-Hodak I, Sabol I, Seiwerth S, et al. (2014) Clinicopathologic correlation of oral lichen planus and oral lichenoid lesions: a preliminary study. Sci World J 1-6.
- Hiremath S, Kale AD, Hallikerimath S (2015) Clinico-pathological study to evaluate oral lichen planus for the establishment of clinical and histopathological diagnostic criteria. Turk J Pathol 31:24-9.
- Rad M, Hashemipoor MA, Mojtahedi A, Zarei MR, Chamani G, et al. (2009) Correlation between clinical and histopathologic diagnoses of oral lichen planus based on modified WHO diagnostic criteria. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 107:796-800.
- Fernández-González F, Vázquez-Álvarez R, Reboiras-López D, Gándara-Vila P, García-García A, et al. (2011) Histopathological findings in oral lichen planus and their correlation with the clinical manifestations. Med Oral Patol Oral Cir Bucal 16:641-6.
- Tsushima, F, Sakurai J, Uesugi A, Oikawa Y, Ohsako T, et al. (2021) Malignant transformation of oral lichen planus: a retrospective study of 565 Japanese patients . BMC Oral Health 21:1-9.
- Giuliani M, Troiano G, Cordaro M (2019) Rate of malignant transformation of oral lichen planus: A systematic review. Oral Dis 25:693-709.
- Guan G, Mei L, Polonowita A, Hussaini H, Seo B, et al. (2020) Malignant transformation in oral lichen planus and lichenoid lesions: a 14-year longitudinal retrospective cohort study of 829 patients in New Zealand. Oral Surg Oral Med Oral Pathol Oral Radiol 130:411-418.
- Rosa EA, Brietzke AP, de Almeida Prado Franceschi LE, Hurtado Puerto AM, Falcão DP, et al. (2018) Oral lichen planus and malignant transformation: The role of p16, Ki-67, Bub-3 and SOX4 in assessing precancerous potential. Exp Ther Med 15:4157-66.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Schpolar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Schpolar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Mathur M, Jha A, Thakur N, Das G, Shah S, et al. (2023) ClinicopathologicalCorrelation in Patients with Oral Lichen Planus. J Dent Pathol Med 7: 154. DOI: 10.4172/jdpm.1000154
Copyright: © 2023 Mathur M, et al. This is an open-access article distributed underthe terms of the Creative Commons Attribution License, which permits unrestricteduse, distribution, and reproduction in any medium, provided the original author andsource are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2446
- [From(publication date): 0-2023 - Jul 08, 2025]
- Breakdown by view type
- HTML page views: 2161
- PDF downloads: 285