Clinicopathologic Findings of Intestinal Perineuromas of the Colon-an Institutional Experience of 78 Cases
Received: 20-Jun-2018 / Accepted Date: 29-Jun-2018 / Published Date: 06-Jul-2018 DOI: 10.4172/2161-069X.1000571
Keywords: Perineuromas; Benign fibroblastic polyp; Colonic polyps; Mesenchymal colon polyps; Mixed epithelial-stromal polyps
Introduction
An increase in screening colonoscopies over the past decade has led to the recognition of several benign mesenchymal or mixed epithelialstromal polyps, one of which is intestinal perineuroma (IP) (formerly known as benign fibroblastic polyp of the colon) [1-8]. Histologically these lesions are mucosal proliferations composed of bland spindle cells and have been reported to have an increased tendency to occur in the rectosigmoid colon. Both hyperplastic polyps (HPs) and sessile serrated adenomas/polyps (SSA/Ps) can exist concurrently with IPs [2-10].
Immunohistochemical stains for Glut-1, claudin-1, and epithelial membrane antigen (EMA) are useful in distinguishing these lesions from other spindle-cell proliferations. Compared to their epithelial counterparts, IPs remain an under-recognized and an underreported entity that have only recently and been described. We present to our knowledge, the largest series of IPs and report their available clinicopathologic and immunohistochemical features along with their coexisting epithelial proliferations [1-10].
Methods
With institutional review board approval, seventy-eight cases of intestinal perineuriomas were collected over an eight-year period from 2009 to 2017. These cases were identified through a query of our surgical pathology laboratory information system for the key words “benign fibroblastic polyp” or “intestinal perineurioma” and “biopsy”. Cases were also identified during routine daily sign out or at our institutional gastrointestinal pathology consensus conference. Hematoxylin and eosin (H&E) stained slides and existing immunochemical markers (n=14 cases) performed as part of the original diagnostic workup were blindly re-reviewed and confirmed by a gastrointestinal pathologist (ARH) who was provided the slides but not the diagnosis.
A real-time re-review was performed at a multiheaded microscope and the diagnosis was then ultimately compared to that in the laboratory information system by two of the study participants (SB, NP) at the time of re-review. The available immunohistochemical stains were also re-reviewed blinded to the reported results. No additional immunohistochemical stains were performed at time of rereview. The goal was to collect clinicopathologic data including age, gender, site, and associated epithelial polyp(s).
Results
Clinicopathologic features
A total of 79 IPs were identified in 78 cases, with one case containing two IPs at two separate sites (ascending and transverse colon). The median age of the patient cohort was 61 years (range 30-85) with a slight female predominance (35 males and 43 females).
IPs were found most commonly in the distal colon (n=40, 51%), followed by proximal colon (n=22, 27%), transverse colon (n=9, 11%), and rectum (n=8, 10%). Most IPs were associated with a HP (n=48, 61%), followed by a SSP/A (n=26, 33%). Only 6% (n=5) did not demonstrate an overlying HP or SSP/A, instead showing normal overlying intestinal epithelium.
The distribution of the associated polyp by location is as follows: proximal colon (18% HP, 77% SSP/A, 1% no overlying epithelial polyp), transverse colon (78% HP, 22% SSP/A), distal colon (73% HP, 18% SSP/A, 10% no overlying epithelial polyp), and rectum (100% HP). The age, sex, distribution, and associated polyps with IPs are outlined in Table 1.
| Site | Total | Male | Female | Age (years) (Average, Range) |
|---|---|---|---|---|
| Proximal (Ascending, Right, Cecum, Hepatic Flexure) | 22 | 12 | 10 | 61( 37-78) |
| HP | 4 | 4 | 0 | 58 (53-61) |
| SSP/A | 17 | 8 | 9 | 62 (37-78) |
| - | 1 | 0 | 1 | 60 |
| Transverse | 9 | 4 | 5 | 68(47-85) |
| HP | 7 | 3 | 4 | 71 (50-85) |
| SSP/A | 2 | 1 | 1 | 58 (47-69) |
| - | 0 | 0 | 0 | - |
| Distal (Descending, Left, Sigmoid) | 40 | 18 | 22 | 60(30-79) |
| HP | 29 | 11 | 18 | 62 (50-76) |
| SSP/A | 7 | 4 | 3 | 60 (50-76) |
| - | 4 | 3 | 1 | 61 (55-64) |
| Rectum | 8 | 2 | 6 | 60(51-65) |
| HP | 8 | 2 | 6 | 60(51-65) |
| SSP/A | 0 | 0 | 0 | N/A |
| - | 0 | 0 | 0 | N/A |
Table 1: Distribution of intestinal perineuromas by age, sex, and location, HP=Hyperplastic polyp; SSP/A=Sessile serrated polyp/adenoma; (-) no associated epithelial polyp.
Light microscopy
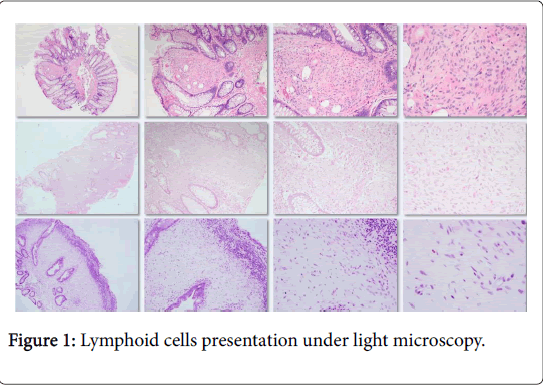
All IPs were limited to the lamina propria and were welldemarcated without infiltration (Figure 1). Histologically, they consisted of a spindle cell proliferation with uniform, spindled-shaped cells with scant eosinophilic cytoplasm and bland nuclear features. Mitotic figures were rare to absent. The spindle cell expansion of the lamina propria displaces the normal constituents of the lamina propria including the colonic crypts and lymphoid cells.
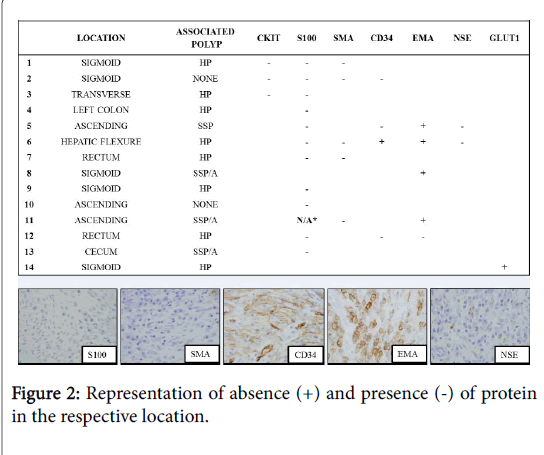
The displacement of the lymphoid cells may impart an impression of a superficial lymphoid infiltrate or aggregate (Figure 1). Adenomatous dysplasia was not identified in any of the epithelial components. Fourteen cases (18%) had immunohistochemical markers utilized at time of initial diagnosis. Stains used either in combination or alone included CKIT (CD117), S-100 protein, smooth muscle actin (SMA), CD34, epithelial membrane antigen (EMA), neuron specific enolase (NSE), and/or GLUT1. The most commonly utilized stain was S-100, which was performed on 12 cases (86% of stained cases) and was negative in all 11 cases (91.7%) deemed evaluable. EMA was utilized five times (36% of stained cases) and was positive in four of these cases (80%) (Figure 2).
Discussion
IPs are an often under-recognized mesenchymal proliferation occurring in the lamina propria of the colon, and often found in association with a serrated epithelial polyp [2-10]. The histologic features of IPs are quite unique and uniform and can typically be diagnosed without the use of immunohistochemical stains or other ancillary studies. IPs are characterized by a bland spindle cell proliferation limited to the lamina propria that distorts the crypt architecture. The spindled cells have eosinophilic cytoplasm, indistinct cell borders, and oval to fusiform nuclei with small, inconspicuous nucleoli. The cells lack pleomorphism and significant mitotic activity [2-10].
Similar to prior studies approximately half of the IPs identified in the current cohort were located in the distal colon. IPs can be associated with serrated epithelial proliferations, and more than half of the IPs in this study was associated with a hyperplastic polyp. One third of our cases of IPs were identified in association of SSP/As, a finding similar to previous studies. The average age was in the sixth decade, which is also similar to previous studies [2-10]. There was however only a slight female predominance identified within our clinical cohort. Unlike the prior studies,we had two intestinal perineuriomas in one patient, which has not been reported before, to our knowledge. The majority of IPs were identified based on morphology alone, while immunohistochemical markers were more often utilized to exclude other mesenchymal polyps, primarily mucosal Schwann cell hamartoma [11]. The exact pathogenesis of IPs is not entirely clear, but recent evidence suggests that they may harbor BRAF mutations and there may be a mesenchymal-epithelial interaction which causes the pericryptal fibroblasts to become modified and proliferate [9-10].
While originally termed benign fibroblastic polyp of the colon, these polyps were found to express markers of perineural differentiation including GLUT1, EMA, and claudin , which essentially led to their re-classificationas intestinal perineurioma. Four of five of the cases in our study identified with prior EMA staining were EMA positive, but it is well known that this marker can demonstrate very focal and weak staining. The staining may also depend on the antibody which is used, the methods of antigen retrieval, and the antibody concentration [7,8]. In practice, this marker is too unreliable to be routinely used for diagnosis. GLUT1 is also commonly positive in IPs; it was only utilized once in our series and was positive. Similar to previous studies, S-100 protein was uniformly negative in all evaluable cases (eleven cases overall) in our series. This stain does allow separation from other neural proliferations, especially mucosal Schwann cell hamartoma, as discussed below [11]. Interestingly, one of four of our cases demonstrated CD34 positivity, which has been reported as rarely occurring in IPs [4,5].
The histologic differential diagnosis of IPs includes other spindle cell proliferations that may present as mucosal polyps within the gastrointestinal tract, including leiomyomas, ganglioneuromas, and mucosal Schwann cell hamartomas. Leiomyomas of the muscularis mucosae of the colon and rectum have a predilection for the descending colon, sigmoid colon, and rectum. These lesions arise from the muscularis mucosae, whereas IPs are seen within the lamina propria of the mucosa. Cytologically, leiomyomas are composed of spindled cells with deeply eosinophilic cytoplasm and cigar-shaped nuclei arranged in intersecting fascicles. If necessary, immunohistochemical stains for desmin or smooth muscle actin may be utilized in the differential, as they will be strongly and diffusely positive in leiomyomas and negative in IPs [12]. Ganglioneuromas may present as solitary or multiple diminutive mucosal polyps from the stomach to the colon. The key distinguishing feature is the presence of ganglion cells in a Schwannian stroma. If there is a paucity of ganglion cells, S-100 protein will highlight the spindled, Schwannian stroma in a ganglioneuroma and will not be expressed in an IP [13]. Perhaps the most challenging differential diagnosis for IP is mucosal Schwann cell hamartoma, which is a relatively recently described benign mesenchymal polyp of the colon. These lesions also tend to occur in the lamina propria and are incidentally identified at screening colonoscopy as mucosal polyps, without any known association with any known inherited syndromes. In comparison with IPs, these are not associated with serrated epithelial lesions and are uniformly S-100 protein positive [11]. Although gastrointestinal stromal tumor (GIST) is a spindled cell lesion in the gastrointestinal tract, they tend to be mural based lesions, are uncommon in the colon, and are not typically identified on screening colonoscopy as polyps within the colorectum. Additionally, GIST is uniformly positive for CD117 and/or DOG1 while IPs are uniformly negative for these markers [14,15].
Conclusions
Our large case series confirms that IPs are most commonly located in the left colon, associated with hyperplastic polyps, and have a slight female predominance. Based on our institutional experience, immunostains were rarely performed, are more often used to exclude other mesenchymal polyps, and are not necessary to make this diagnosis as compared with other mesenchymal polyps. If a pathologist is aware of this entity or has seen examples of IPs, the diagnosis is typically straightforward and does not require ancillary testing or staining. While benign in nature, heightened awareness of their existence allows pathologists to include mesenchymal tumors as part of their differential diagnosis for intestinal polyps. Educationally, these polyps highlight to residents and pathologists alike the importance of examining the underlying stroma of an epithelial serrated polyp for an additional spindle cell proliferation or mesenchymal component.
References
- Harewood GC, Lieberman DA (2004) Colonoscopy practice patterns since introduction of medicare coverage for average risk screening. Clin Gastroenterol Hepatol 2: 72-77.
- Hornick JL, Fletcher CD (2005) Intestinal perineuriomas: clinicopathologic definition of a new anatomic subset in a series of 10 cases. American J Surg Pathol 29: 859-865.
- Huber AR, Shikle JF (2009) Benign fibroblastic polyps of the colon. Arch Pathol Lab Med 133: 1872-1876.
- Eslami-Varzaneh F, Washington K, Robert ME, Kashgarian M, Goldblum JR, et al. (2004) Benign fibroblastic polyps of the colon: A histologic, immunohistochemical, and ultrastructural study. Am J Surg Pathol 28: 374-378.
- Zamecnik M, Chlumska A (2004) Fibroblastic polyp of the colon shares features with vanek tumor. Am J Surg Pathol 28: 1397-1398
- Groisman GM, Polak-Charcon S, Appelman HD (2006) Fibroblastic polyp of the colon: Clinicopathological analysis of 10 cases with emphasis on its common association with serrated crypts. Histopathology 48: 431-437.
- Groisman GM, Polak-Charcon S (2008) Fibroblastic polyp of the colon and colonic perineurioma: 2 names for a single entity? Am J Surg Pathol 32: 1088-1094.
- Zamecnik M, Chlumska A (2006) Perineurioma versus fibroblastic polyp of the colon. Am J Surg Pathol 30: 1337-1339.
- Agaimy A, Stoehr R, Vieth M, Hartmann A (2010) Benign serrated colorectal fibroblastic polyps/intramucosal perineuriomas are true mixed epithelial-stromal polyps (hybrid hyperplastic polyp/mucosal perineurioma) with frequent BRAF mutations. Am J Surg Pathol 11: 1663-1671.
- Pai RK, Mojtahed A, Rouse RV, Soetikno RM, Kaltenbach T, et al. Histologic and molecular analyses of colonic perineurial-like proliferations in serrated polyps: Perineurial-like stromal proliferations are seen in sessile serrated adenomas. Am J Surg Pathol 35: 1373-1380.
- Gibson JA, Hornick JL (2009) Mucosal Schwann cell “hamartomaâ€: Clinicopathologic study of 26 neural colorectal polyps distinct from neurofibromas and neuromas. Am J Surg Pathol 33: 781-787.
- Miettinen M, Sarlomo-Rikala M, Sobin LH (2001) Mesenchymal tumors of muscularis mucosae of colon and rectum are benign leiomyomas that should be separated from gastrointestinal stromal tumors: A clinicopathologic and immunohistochemical study of eighty-eight cases. Mod Pathol 14: 950-958.
- d’Amore ES, Manivel JC, Pettinato G, Niehans GA, Snover D (1991) Intestinal ganglioneuromatosis: Mucosal and transmural types-a clinicopathologic and immunohistochemical study of six cases. Hum Pathol 22: 276-286.
- Miettinen M, Sarlomo-Rikala M, Sobin LH, Lasota J (2000) Gastrointestinal stromal tumors and leiomyosarcomas in the colon: A clinicopathologic, immunohistochemical, and molecular genetic study of 44 cases. Am J Surg Pathol 24: 1339-1352.
- Peterson BR, DeRoche TC, Huber AR (2012) Benign fibroblastic polyps of the colon and intestinal perineuriomas are the same entity: A report of 2 cases demonstrating variable epithelial membrane antigen staining. Arch Pathol Lab Med 136: 1020-1021.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 3690
- [From(publication date): 0-2018 - Dec 27, 2024]
- Breakdown by view type
- HTML page views: 3038
- PDF downloads: 652