Research Article Open Access
Clinical Training of Primary Health Care Physicians to Reduce False Positive Diagnoses of Pediatric Urinary Tract Infections
Urrutia-Herrera D1, Greiner F2, Tejada-Tayabas LM1 and Monárrez-Espino J1,2*1Master Program in Public Health, San Luis Potosi Autonomous University, San Luis Potosi, Mexico
2Master Program in Global Health, Department of Public Health Sciences, Karolinska Institutet, Stockholm, Sweden
- *Corresponding Author:
- Assoc. Prof. Joel Monárrez-Espino
Department of Public Health Sciences. Karolinska Institutet
Tomtebodavägen 18A, Floor 4. SE-17717
Stockholm, Sweden
Tel: +46 8 52483384
Fax: +46 8 311590
E-mail: joel.monarrez-espino@ki.se
Received date: Feb 15, 2016; Accepted date: April 04, 2016; Published date: April 15, 2016
Citation: Urrutia-Herrera D, Greiner F, Tejada-Tayabas LM, Monárrez-Espino J (2016) Clinical Training of Primary Health Care Physicians to Reduce False Positive Diagnoses of Pediatric Urinary Tract Infections. J Community Med Health 6:412. doi:10.4172/2161-0711.1000412
Copyright: © 2016 Urrutia-Herrera D, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Community Medicine & Health Education
Abstract
Background: Presumptive clinical diagnosis of pediatric urinary tract infections (UTI) remains in practice in many low- and middle-income countries in spite of its limited accuracy; improving its precision could be potentially useful until more accurate methods can be implemented in resource-limited locations.
Objective: To assess whether clinical training can result in a reduction of false positive diagnoses of pediatric UTIs.
Methods: A non-randomized pragmatic trial was conducted in six medical units. Each arm included doctors from two units. Those in the first (IG9, n=14) and second (IG20, n=14) group received 9 h and 20 h training, respectively; the control group (CG, n=17) received none. Training in the IG9 consisted of three sessions lasting 3 h each, one per week over three consecutive weeks, and for the IG20 training spread over five weeks with two 2 h sessions per week. Sessions were led by an expert pediatrician covering relevant UTI topics; focus was given on common signs and symptoms including fever of unknown origin, urinary urgency, hematuria, dysuria, fetid urine, and suprapubic pain. A total of 134 children between two months and nine years were diagnosed; 41, 44, and 49 from the CG, IG9 and IG20, respectively. The main measure of effect was the difference in the proportions of accurate positive clinical diagnoses between the trained groups and the control using urine culture as standard. Adjusted odds ratios (OR) from binary logistic regression were computed to estimate the probability of correctly diagnosing a UTI adjusting by physicians’ sex, age, years of experience, postgraduate education, and re-training knowledge.
Results: The proportion of accurate diagnoses was 39.0, 27.3 and 32.7% in the CG, IG9 and IG20, respectively. Doctors trained for 9 or 20 h had a non-significantly lower chance of a correct diagnosis (OR; 95% CI for IG9=0.57;0.21-1.5, IG20=0.55; 0.21-1.4).
Conclusion: Training did not reduce false positives diagnoses. Confirmatory methods are required to diagnose UTIs in children with symptomatology.
Keywords
Clinical diagnosis; Mexico; Training; Pediatric; Urinary tract infection
Abbreviations
CI: Confidence Interval, CG: Control Group, IIG9: Intervention Group with 9 h training, IIG20: Intervention Group with 20 h training, ICD: International Classification of Diseases, OR: Odds Ratio, ROC: Receiving Operating Curve, s.d.: Standard Deviation, SLP: San Luis Potosi, UTI: Urinary Tract Infections
Introduction
Urinary tract infection (UTI) is a common condition caused by growth of bacteria in the urinary tract. Pediatric UTIs are frequently diagnosed in primary health care consultation. In the United States, the prevalence among febrile children aged 0-24 months has been reported to be 7% (95% CI 5.5-8.4%), but variation exists depending on the sex, age, race, and male circumcision status [1]. Pediatric UTIs can be recurrent [2] and linked to more serious consequences such as renal scarring, chronic renal failure, and hypertension [3,4].
While UTI incidence in most developing countries is not well established, it is believed to be higher than in affluent societies, as risk factors tend to be more prevalent in poorer areas. In Mexico, according to the Ministry of Health, UTIs (ICD-10 N30, N34 and N39) ranked third in overall morbidity among children aged 0-9 years old with 373,795 new cases reported in 2013 [5]. In the central Mexican state of San Luis Potosi (SLP), where this study was conducted, the prevalence also ranked third. However, these estimates must be taken with caution, as many diagnoses in Mexico are based only on clinical symptomatology.
Accurate diagnosis in children is challenging as clinical signs and symptoms are not specific [6], and also due to practical difficulties in obtaining adequate urine specimens [7]. While presumptive clinical diagnosis of uncomplicated UTIs has been reported to be relatively adequate in adults [8], achieving a high accuracy in small children is more difficult, not only because signs can be less overt, but also due to the infants’ limitations to express symptoms explicitly. As a result, diagnoses based on clinical criteria have lower sensitivity and specificity, particularly when performed by poorly trained physicians [9]. For children, especially infants, it has been documented that proper diagnosis would require bacteriological methods such as dipstick urinalysis and urine culture, which are considered gold standards [6]. Using these techniques in deprived settings can be problematic mostly due to the lack of laboratory facilities and costs involved. Inadequate standardization of diagnostic procedures can also hamper accurate diagnoses (e.g., problems to obtain a noncontaminated urine specimen from small children). Misdiagnoses often result in inappropriate prescription of antibiotics leading to increased medical costs, bacterial resistance, and the potential side effects of these drugs [10-12].
In most high-income countries, presumptive clinical UTI diagnosis has been abandoned; instead they have started to use confirmative methods such as urine culture. However, in countries like Mexico this remains in practice, not least in pediatric populations. Improving the precision of clinical diagnoses is thus needed until urine cultures or dipstick urinalyses are implemented in resource-limited locations.
So far, physicians have been instructed to follow diagnostic guidelines, especially for infants, based on algorithms that begin with the occurrence of fever without a source of general or local symptoms [13]. However, proposed algorithms [6,13,14], as a rule, require a urine16 specimen collection for urinalysis or culture using a catheter, bag or suprapubic bladder aspiration to prevent contamination by perineal flora [15]. Some authors even advise the use of ultrasonographic guidance to further increase the success rate of obtaining a sterile sample [16,17]. Yet, the use of such algorithms is not feasible in poor settings due to the lack of equipment, laboratory supplies, and trained personnel needed to follow the guidelines.
For clinical diagnoses, authors have built models using multinomial logistic regression to improve decision making by increasing the sensitivity to detect infections in febrile children [18]; from the nearly 40 clinical symptoms and signs commonly elicited in febrile children suspected of having a serious bacterial infection, 26 were considered to have clinical validity and were thus selected. The final model produced an area under the ROC curve of 0.80 (95% CI 0.78-0.82) using urine culture as gold standard, but just slightly better than that of the clinical judgment of non-trained physicians (no estimates were reported).
In theory, training doctors to perform more accurate clinical UTI pediatric diagnoses could increase the diagnostic precision [19]. Yet, there is little evidence assessing whether training can lead to less false positive and negative diagnoses. In the present study we assess if clinical training of primary care physicians could result in a reduction of false positive diagnoses.
Material and Methods
Study design
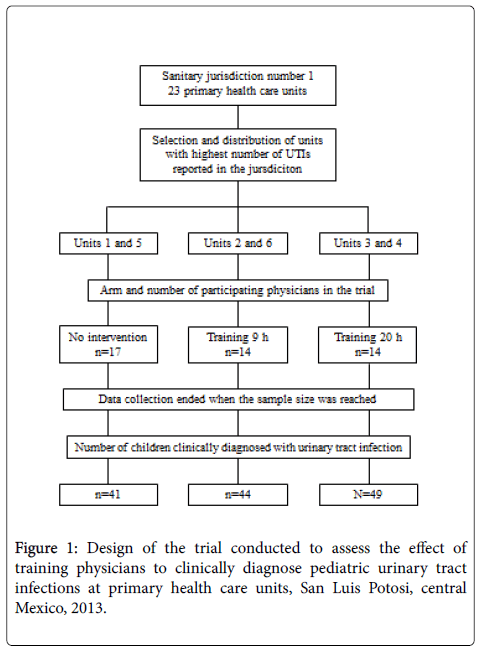
A non-randomized pragmatic clinical trial with three arms was carried out. The study was conducted in the largest sanitary jurisdiction of the state of SLP run by the Ministry of Health, which includes 23 primary health care units. Due to financial and logistic constraints, it was not possible to conduct the study in all 23 units. Hence, six units located within the city limits of SLP, the state capital, were used. These were chosen because they had the highest number of reported cases of pediatric UTIs in the year prior to the trial, and because they had appropriate infrastructure to collect urine specimens and keep them refrigerated until analysis.
The six units were divided up so that each of the three arms in the trial included doctors from two health units. In this way, crosscontamination of the interventions was avoided, as all participating physicians from the same unit belonged to the same study arm. Physicians in the first (IG9) and second (IG20) intervention groups received 9 and 20 hours of training, respectively, while those in the control group (CG) received no training at all.
A total of 48 physicians working at these units participated in the study. However, three physicians had to be excluded, one from IG9 (quit the job), and two from CG (one quit the job and the other went into retirement), leaving a remaining 45 physicians in the trial. The final allocation included 14 physicians in both IG9 and IG20, and 17 in CG (Figure 1).
Physicians were instructed to obtain an adequate urine specimen for urine culture when they presumptively diagnosed a UTI based on clinical signs and symptoms. Data was collected for ten months until the sample size for each group was reached. In the end, a total of 134 children between the age of two months and nine years were identified by the physicians as having a UTI; 41, 44, and 49 children from the CG, IG9 and IG20, respectively. Excluded were children who had received antibiotics seven days before the urine culture, those with a diagnosed anatomic or functional abnormality of the genitourinary tract, and those whose caretaker did not consent to participate in the study.
The main measure of effect was the difference in the proportions of accurate positive presumptive clinical diagnoses between the trained groups and the control using urine culture as gold standard.
Pre and post training assessment
The physicians’ clinical knowledge and skills concerning pediatric UTIs were assessed using a non-validated paper-based questionnaire administered prior to and after the training intervention. It contained relevant data to characterize each participant physician (e.g., sex, age, formal education, and work experience). The questionnaire was designed by an experienced, trained, and certified pediatric specialist based on a thorough literature review of the UTI topic. The instrument consisted of five simulated clinical cases with questions addressing both knowledge and clinical skills such as recognition of risk factors, signs and symptoms, differential diagnosis, and the use of diagnostic and therapeutic resources. Most answers were coded either “true”, “false”, or “don’t know”. The correct answers added one point, the wrong ones deducted one point, and “don’t know” or unanswered items did neither. The purpose of the assessment was to measure the impact of the training in terms of UTI knowledge and skills.
Intervention
The training was implemented only with physicians belonging to the intervention groups. It was decided that two lengths of training would be used: IG9 was a short 9 h course, and IG20 was a lengthier 20 h course. The purpose behind this decision was to potentially reduce time and costs if the shorter course could prove to be as effective as the longer one. IG9 consisted of three sessions lasting 3 h each, with one session per week over three consecutive weeks. IG20 spread over five weeks, with two 2 h sessions per week. All sessions were led by the expert pediatrician mentioned above.
The training covered the following topics: UTI definition, epidemiology, etiology, pathogenesis, classification, risk and protective factors, diagnosis, treatment, and health promotion. Teaching strategies entailed lectures, readings, group-work, clinical role-playing, and presentation of simulated clinical cases. While both interventions covered the same topics, the 20 h training included more diversified simulated clinical cases and allowed for more in-depth discussions.
Special focus was given to common signs and symptoms associated with clinical UTI pediatric diagnosis [20] such as fever of unknown origin, history of prior UTI, enuresis, urinary urgency, hematuria, dysuria, pollakiuria, fetid or cloudy urine, abdominal and back pain, suprapubic tenderness, among others.
Sample size
The sample size was computed based on an expected minimum absolute difference of 30% in the proportion of accurate positive clinical diagnoses (i.e. total UTIs based on a positive urine culture divided by all UTIs diagnosed by the physician in each group) between the CG (expected accurate diagnoses 30%) and the IG20 (expected accurate diagnoses 60%) groups. Using a confidence level of 95% and a statistical power of 80%, the calculated sample size for a two-sided test was 42 urine samples per group. The data collection ended when all three groups had at least the required sample.
Presumptive UTI diagnosis and urine culture
The ultimate decision of whether a child had a presumptive UTI or not relied on the physician’s judgment based on his/her full clinical assessment of the patient.
Urine was collected from the midstream and placed on a sterile container when children had sphincter control; otherwise, a special bag was used to collect non-contaminated urine. Samples were kept in refrigeration until urine cultures were performed by an experienced chemist in a microbiology laboratory following standard procedures.
Patient information
A general questionnaire was administered by the physician to obtain personal and clinical data for each participating child. Identification data included the patient's sex, age, health unit, consultation date, and treating physician. The questionnaire was organized into three domains: 1) identification of risk and protective factors such as the use of exclusive breastfeeding for six months and the history of male circumcision, 2) clinical signs and symptoms reported, including fever, fetid or cloudy urine, hematuria, dysuria, abdominal or back pain, pollakiuria, urinary urgency or incontinence, irritability, decreased appetite, and vomiting, among various others, and 3) physical examination involving the measurement of the child’s weight in kg, height in m, and temperature in °C, and the assessment of suprapubic pain at palpation.
Statistical analysis
Mean ± standard deviations (s.d.) were calculated for continuous variables such as age, years of medical experience, and pre-training assessment score. Statistical differences were assessed using Pearson Chi2 and Fisher’s exact tests for nominal data. Non-parametric Kruskal-Wallis tests with pairwise comparisons were used to identify differences between groups, as continuous data was not normally distributed. The techniques used to collect urine samples and the isolated bacteria were also compared using Chi2 tests to identify differences in proportions between groups. Statistical differences were set at p < 0.05.
Crude and adjusted odds ratios (OR) with 95% CI from binary logistic regression were computed to estimate the probability of correctly diagnosing a UTI (i.e. positive urine culture). Adjusted covariates included the physicians’ sex, age, years of experience after graduation, postgraduate education, and pre-training score. Physicians in the CG served as reference category. Analyses were stratified by age group; children were categorized as small infants (2-23 months) and older children (2-9 years), as age could be differentially linked to the possibility of under and over diagnoses.
A logistic model was run to identify risk and protective factors potentially associated with a pediatric UTI. Factors with p-values ≤ 0.20 in crude analyses were entered in the adjusted model, but the final model included only significant variables (p < 0.05) and the children’s age.
Ethical considerations
The study protocol was revised and approved by the Research and Ethics Committee of the Faculty of Nursing and Nutrition at SLP Autonomous University. Informed consent was obtained from all participants involved, including children’s caretakers and physicians. Their identity was not revealed, as they were identified with a number only.
Doctors did not necessarily wait for confirmatory diagnosis to give treatment; some decided to prescribe antibiotics right after consultation, as this was the institution’s operational norm at the time when the study was conducted.
Results
Table 1 compares selected characteristics of the physicians participating in the study stratifying by intervention group. While no statistical differences in proportions and means were identified across groups, there was a larger proportion of female doctors in the IG9 and IG20 compared with those in the CG. Also, these two groups had a lower mean age and less mean years of professional experience. About 80% of the medical doctors in the IG20 finished medical school in a university outside SLP state, in contrast with the other two groups where nearly half completed their medical degree at the local state university. Most physicians did not have any further formal specialization beyond their medical degree, especially those in the IG9. Mean pre-training scores were similar across groups, ranging from 27 in the IG20 to 33 points in the IG9. The difference between the mean pre- and post-training scores showed no improvement in the CG, in contrast with both IG9 and IG20, which significantly increased their baseline mean score by nearly 10 points (p < 0.05).
| Characteristics | Measure | Intervention group1 | ||
|---|---|---|---|---|
| None | 9 h | 20 h | ||
| Sex, n (%) | Male | 10 (58.8) | 6 (42.9) | 5 (35.7) |
| Female | 7 (41.2) | 8 (57.1) | 9 (64.3) | |
| Age in years | Mean ± s.d | 48 ± 15 | 44 ± 11 | 39 ± 9 |
| Graduation institution, n (%) | UASLP2 | 8 (47.1) | 8 (57.1) | 3 (21.4) |
| Other | 9 (52.9) | 6 (42.9) | 11 (78.6) | |
| Years of experience3 | Mean ± s.d | 22 ± 14 | 17 ± 10 | 13 ± 9 |
| Post-graduate education, n (%) | None | 11 (64.7) | 12 (85.7) | 11 (78.6) |
| Masters | 4 (23.5) | 1 (7.1) | - | |
| Specialty | 2 (11.8) | 1 (7.1) | 3 (21.4) | |
| Working shift, n (%) | Morning | 10 (58.8) | 10 (71.4) | 7 (50.0) |
| Afternoon | 7 (41.2) | 4 (28.6) | 7 (50.0) | |
| Pre-training assessment score4 | Mean ± s.d | 31 ± 9 | 33 ± 12 | 27 ± 10 |
| Difference pre vs. post score | Mean± s.d | 0.8 ± 6a | 13.9 ± 10b | 9.6 ± 12b |
1Statistical differences were tested: Pearson Chi square or Fisher tests were used for nominal data (no significant differences were seen at p<0.05) and nonparametric Kruskal-Wallis tests to compare distributions for continuous data (superscript letters indicate differences between pairwise comparisons)
2Refers to the university where the physician obtained his/her medical degree (UASLP is the Spanish acronym of the local university, San Luis Potosi Autonomous University)
3Refers to the total years working as physician after finishing the medical degree
4Refers to the total score obtained in the test administered to measure the physician’s knowledge and skills about urinary tract infections prior to the intervention
a,bThe values with different superscripts differs significantly
Table 1: Comparison of selected background characteristics of the physicians participating in the training intervention, San Luis Potosi trial, Mexico, 2013.
Table 2 presents the probability of correctly diagnosing a UTI (i.e. positive urine culture) by intervention arm and age group. For the whole sample, the proportion of correct diagnoses out of those made clinically by the physicians in the CG, IG9, and IG20 was 39, 27.3 and 32.7%, respectively. Doctors trained for either 9 or 20 h had a nonsignificantly lower chance of getting the correct diagnosis compared with those in the control group (IG9: adj. OR 0.57, 95% CI 0.21-1.55; IG20: adj. OR 0.55, 95% CI 0.21-1.42). The chance of getting a correct positive UTI diagnosis was smaller in small infants than in older children irrespective of intervention arm compared with children in the non-intervention group; yet, neither adjusted ORs reached statistical significance.
| Age group andintervention arm | Urine culture, n (%) | Diagnosed by physicians | OR (95% CI) | ||
|---|---|---|---|---|---|
| Positive | Negative | Crude | Adjusted1 | ||
| 2-23 months | |||||
| No intervention | 9 (50.0) | 9 (50.0) | 18 | 1.0 | 1.0 |
| Training, 9 h | 4 (40.0) | 6 (60.0) | 10 | 0.66 (0.13-3.19) | 0.54 (0.08-3.61) |
| Training, 20 h | 7 (35.0) | 13 (65.0) | 20 | 0.53 (0.14-1.98) | 0.23 (0.04-1.36) |
| 2-9 years | |||||
| No intervention | 7 (30.4) | 16 (69.6) | 23 | 1.0 | 1.0 |
| Training, 9 h | 8 (23.5) | 26 (76.5) | 34 | 0.70 (0.21-2.31) | 0.66 (0.17-2.49) |
| Training, 20 h | 9 (31.0) | 20 (69.0) | 29 | 1.02 (0.31-3.36) | 0.72 (0.20-2.63) |
| All children | |||||
| No intervention | 16 (39.0) | 25 (61.0) | 41 | 1.0 | 1.0 |
| Training, 9 h | 12 (27.3) | 32 (72.7) | 44 | 0.58 (0.23-1.46) | 0.57 (0.21-1.55) |
| Training, 20 h | 16 (32.7) | 33 (67.3) | 49 | 0.75 (0.31-1.80) | 0.55 (0.21-1.42) |
1Adjusted by the physicians’ sex, age, years of experience after graduation, postgraduate education, and pre-assessment score
Table 2: Crude and adjusted odds ratios (OR) with 95% confidence intervals (CI) from logistic regression for the probability of correctly diagnosing a urinary tract infection (positive urine culture) among children aged ≤9 years at primary health care centers by intervention arm (physicians’ training) and age group, San Luis Potosi trial, Mexico, 2013.
The most relevant clinical findings associated with a positive urine culture are presented in Table 3. Although various signs and symptoms had a p-value of less than 0.20 in bivariate analyses, only fever, undernutrition and suprapubic pain remained statistically significant in the adjusted model (p < 0.05). While fever and undernutrition were associated with a lower chance of having an infection, suprapubic pain was the only sign predictive of a positive culture (adj. OR 4.32, 95% CI 1.52-12.2).
| Clinical findings1 | Prevalence in % | OR (95% CI) | |||
|---|---|---|---|---|---|
| Crude | p | Adjusted2 | p | ||
| Referred by caretaker | |||||
| Fever | 56.7 | 0.49 (0.23-1.06) | 0.07 | 0.44 (0.19-1.02) | 0.05 |
| Frequent urination | 60.4 | 2.91 (0.86-9.75) | 0.08 | - | - |
| Urinary urgency | 60.2 | 2.49 (0.85-7.24) | 0.09 | - | - |
| Blood in urine | 14.2 | 2.10 (0.78-5.64) | 0.14 | - | - |
| Fetid urine | 35.1 | 1.63 (0.76-3.52) | 0.20 | - | - |
| Assessed in physical exam | |||||
| Suprapubic pain | 17.2 | 2.65 (1.06-6.64) | 0.03 | 4.32 (1.52-12.2) | <0.01 |
| Undernutrition | 23.1 | 0.31 (0.11-0.89) | 0.02 | 0.22 (0.07-0.72) | 0.01 |
1Only factors with p-values <0.20 in crude analyses were entered in the adjusted model; the following were excluded: mixed and exclusive breastfeeding during the first 6 postpartum months, irritability, headache, constipation, hyporexia, vomiting, nausea, dysuria, abdominal pain, turbid urine, temperature, overweight, diarrhea, tenesmus, back pain, and vulvar erythema in girls
2Final adjusted model included significant variables and children’s age
Table 3: Crude and adjusted odds ratios (OR) with 95% confidence intervals (CI) from logistic regression to identify clinical findings recorded during consultation associated with pediatric UTIs (positive urine culture), San Luis Potosi trial, Mexico, 2012-13.
Table 4 shows the technique used to collect urine samples as well as the bacteria isolated by intervention group. Of the two collection techniques, midstream was used in over half the children in all three groups, but mostly by physicians of the IG9 (72.7%). The most common bacteria found was Escherichia coli, ranging from 20.4% in the IG20 to 34.1% in the CG.
| Variable | Intervention group, n (%)1 | Total | ||
|---|---|---|---|---|
| None | 9 h | 20 h | ||
| Collection technique | ||||
| Perineal bag | 18 (43.9) | 12 (27.3) | 21 (42.9) | 51 (38.1) |
| Middle stream | 23 (56.1) | 32 (72.7) | 28 (57.1) | 83 (61.9) |
| Isolated bacteria | ||||
| None | 25 (61.0) | 32 (72.7) | 33 (67.3) | 90 (67.2) |
| Escherichia coli | 14 (34.1) | 11 (25.0) | 10 (20.4) | 35 (26.1) |
| Other | 2 (4.9) | 1 (2.4) | 6 (12.2) | 9 (6.7) |
1No statistical differences between proportions were seen (Pearson Chi square and Fisher tests were used)
Table 4: Technique used to collect urine samples and bacteria isolated by intervention group, San Luis Potosi trial, Mexico, 2012-2013.
Discussion
We conducted a study to assess whether training primary health care physicians could reduce false positive pediatric UTI diagnoses based on clinical symptomatology. The results of this trial showed that training physicians for either 9 h or 20 h did not reduce false positive diagnoses compared with those not trained at all.
The results are unlikely explained by lack of effect of the intervention in terms of gaining UTI knowledge, as physicians who received the training improved their baseline scores significantly compared with those in the control group.
We purposely avoided using guidelines and algorithms to reach the diagnosis, as these were designed for relatively affluent settings where laboratory means and trained personnel are more readily available. Although we could have used and followed such tools to train doctors and assess compliance as other authors have done [14], and eventually determine whether training leads to less false positive diagnoses, such a result would not have been applicable to poor settings that lack facilities and resources to diagnose UTIs in practice. We wanted to follow a pragmatic approach that could actually be implemented if proven beneficial. Clinical training of doctors (without relying on any laboratory methods) appeared to be a worth-testing alternative in deprived locations, as previous research had shown that clinical models based on signs and symptoms had the potential to improve diagnostic accuracy [6,8,18,21].
Even though non-significant statistical differences were observed across the groups, training seems to have led to some degree of over diagnosing. Some authors have recently pointed out that over diagnosis may occur through different mechanisms in commonly found pediatric conditions, including UTIs, leading to potential harm to the children, and call for and education strategies to mitigate this problem [22]. However, in this study there are insufficient grounds to claim that the training was counterproductive, as various factors could have played a role in explaining the lack of a positive effect, including the training and methodology used. For instance, we now realize in retrospect that we should have made physicians aware of the possibility of over diagnosing; they were not explicitly requested to be stringent when diagnosing a UTI.
But even implementing clear diagnostic guidelines can be challenging. For instance, a recent study with infants and young children presenting with unexplained fever at an emergency department in The Netherlands showed that while implementing diagnostic guidelines can lead to substantial improvement in compliance, the assessment was still not accurate in almost half of the children studied [14]. A review of physicians’ guideline adherence highlighted several barriers such as lack of awareness, familiarity, agreement, self-efficacy, and outcome, inertia of previous practice, and various external barriers [23] that might also play a role when following guidelines to diagnose UTIs. Some of these factors could have well played a role in our study.
Some relevant background characteristics of the physicians could have potentially led to selection bias had not these been adjusted for in the analyses. For instance, physicians in the CG were, on average, 4-9 years older, had 5-9 more years of experience, and had somewhat higher specialized education compared with those trained, potentially leading to a greater likelihood of accurately diagnosing a UTI, and thus to an underestimation of the effect. However, as already mentioned, these factors were adjusted for, so it is unlikely that our results are due to differences in physicians’ characteristics.
The only clinical feature positively associated with a UTI was suprapubic pain. Children with this sign had over four times significantly higher chance of having a UTI. This finding goes in line with a review to assess the diagnostic accuracy of signs and symptoms for pediatric UTIs [6]. The authors reported that suprapubic tenderness was one of the most useful findings associated with a UTI diagnosis among infants. High temperature was also found to be positively related in this review, contrasting with our findings where fever was associated with a lower risk. The reason behind this difference is unclear, but could relate to the subjective assessment of fever, as it was reported by the caretaker. In our study, temperature measured at the physician’s office was not statistically associated with the likelihood of UTI. In our sample, 42.5% of the 132 children had fever according to the caretaker, but only 11.4% had temperature of 38°C or more in the physical exam.
The review also reported that when children could express symptomatology, the presence of abdominal and back pain, dysuria, urinary frequency, and incontinence increased the likelihood of a UTI [6]. However, in our study, none of these remained significant in the adjusted analyses, as it has been the case with more recent studies, where clinical features had not been significantly associated with UTIs [24].
UTI is an entity that can be both under and over diagnosed. Such scenarios could be related to the child’s age. While the unspecific nature of the symptoms among infants could lead to the possibility of overlooking a UTI, in older children symptoms related to the urinary tract may have other determinants increasing the chance of over diagnosis. In our study we were unable to assess under diagnoses, as we did not screen all children receiving consultation. However, for over diagnoses, we observed that doctors receiving training had a nonstatistically significant higher chance of getting a false positive diagnosis when assessing infants aged 2-23 months than when looking at those aged 2-9 years. This could be related to the efforts of the trained physicians to avoid missing UTIs in smaller children.
Our results then confirm the poor sensitivity and specificity of signs and symptoms to diagnose a pediatric UTI. If a urine culture is not available, physicians should at least perform a urinalysis before initiating treatment with antibiotics [25]. In fact, the clinical model produced by Craig and colleagues [19] produced an area under the ROC curve still lower than that of the dipstick results (0.80 vs. 0.89; p<0.05).
The current study has some limitations that ought to be mentioned. Excluding children not presumptively diagnosed with a UTI from urine culture limited the possibility of a complete assessment of the physicians’ diagnostic accuracy, as false negatives could not be detected; this was due to logistic and financial constraints, and we had to rely only on a partial appraisal of the diagnostic accuracy, namely, that of false positive diagnosis. Not being able to use better techniques to collect sterile urine specimens (e.g., catheter or suprapubic bladder aspiration) could have also led to more false positives as result of contamination [15]. Also, the very training strategy that purposely requested physicians to use their clinical judgement to diagnose a UTI prevented standardization across doctors potentially leading to differences in the criteria used to identify UTIs. Finally, the expected difference of 30% in the proportion of accurate positive diagnosis between the groups, used to estimate the required sample size, needs to be revised when using similar training interventions, as this fraction was clearly overestimated; a more conservative difference should be expected that leads to larger group samples and narrower CIs.
This study could also illustrate how training medical professionals does not always result in better outcomes, as it has been reported in a systematic review on the effectiveness of educational strategies to change physician’s performance in health care outcomes. In this review, it was documented that only half of the interventions that aimed at improving health outcomes resulted in a positive change [26]. However, our results must not discourage health authorities to use educational activities to train medical personnel. Instead, they should try to test new educational technologies that are easier, faster, and cheaper to implement (e.g., online training) that could eventually lead to better results.
For now, we believe that institutional efforts should be put in place so that children with relevant symptomatology can be tested for a UTI using a urine culture, as it is the best tool to accurately establish the right diagnosis [27,28].
Acknowledgements
The authors wish to thank the physicians, as well as the children and their families for their participation in this study. This study was funded by SLP Ministry of Health.
References
- Shaikh N, Morone N, Bost J, Farrell M (2008) Prevalence of Urinary Tract Infection in Childhood: A Meta-Analysis. Pediatr Infect Dis J 27: 302-308.
- Conway P, Cnaan A, Zaoutis T, Henry B, Grundmeier R, et al. (2007) Recurrent Urinary Tract Infections in Children: Risk Factors and Association With Prophylactic Antimicrobials. JAMA 298: 179-186.
- Shaikh N, Ewing AL, Bhatnagar S, Hoberman A (2010) Risk of renal scarring in children with a first urinary tract infection: a systematic review. Pediatrics 126: 1084-1091.
- Toffolo A, Ammenti A, Montini G (2012) Long-term clinical consequences of urinary tract infections during childhood: a review. ActaPaediatr 101: 1018-1031.
- Ministry of Health (2013) Yearbooks morbidity : Twenty leading causes of illness by age group , United Mexican States 2013 general population. Ministry of Health.
- Shaikh N, Morone NE, Lopez J, Chianese J, Sangvai S, et al. (2007) Does this child have a urinary tract infection? JAMA 298: 2895-2904.
- Tullus K (2011) Difficulties in diagnosing urinary tract infections in small children. PediatrNephrol 26: 1923-1926.
- Schmiemann G, Kniehl E, Gebhardt K, Matejczyk MM, Hummers-Pradier E (2010) The diagnosis of urinary tract infection: a systematic review. DtschArzteblInt 107: 361-367.
- Brkic S, Mustafic S, Nuhbegovic S, Ljuca F, Gavran L (2010) Clinical and epidemiology characteristics of urinary tract infections in childhood. Med Arh 64: 135-138.
- Cheng Chi, Tsai M, Huang Y, Su L, Tsau Y, et al. (2008) Antibiotic resistance patterns of community-acquired urinary tract infections in children with vesicoureteral reflux receiving prophylactic antibiotic therapy. Pediatrics 122: 1212-1217.
- Segovia M, Gil García M (2002) Rational use of drugs in the treatment of urinary tract infections in the area of Talavera de la Reina. AtenPrimaria 29: 481-485.
- Chakupurakal R, Ahmed M, Sobithadevi DN, Chinnappan S, Reynolds T (2010)Urinary tract pathogens and resistance pattern. J ClinPathol 63: 652-654.
- Subcommittee on Urinary Tract Infection, Steering Committee on Quality Improvement and Management, Roberts KB (2011) Urinary tract infection: clinical practice guideline for the diagnosis and management of the initial UTI in febrile infants and children 2 to 24 months. Pediatrics 128: 595-610.
- Geurts DHF, Vos W, Moll HA, Oostenbrink R (2014) Impact analysis of an evidence-based guideline on diagnosis of urinary tract infection in infants and young children with unexplained fever. Eur J Pediatr 173: 463-468.
- Pollack CV, Jr Pollack ES, Andrew ME (1994) Suprapubic bladder aspiration versus urethral catheterization in ill infants: Success, efficiency and complication rates. Ann Emerg Med 23: 225-230.
- Chu RW, Wong YC, Luk SH, Wong SN (2002) Comparing suprapubic urine aspiration under real-time ultrasound guidance with conventional blind aspiration. ActaPaediatr 91: 512-516.
- Buntsma D, Stock A, Bevan C, Babl FE (2012) Success rate of Bladder Scan-assisted suprapubic aspiration. Emerg Med Australas 24: 647-651
- Craig JC, Williams GJ, Jones M, Codarini M, Macaskill P, et al. (2010) The accuracy of clinical symptoms and signs for the diagnosis of serious bacterial infection in young febrile children: prospective cohort study of 15 781 febrile illnesses. BMJ 340: 1594.
- Bloom BS (2005) Effects of continuing medical education on improving physician clinical care and patient health: a review of systematic reviews. Int J Technol Assess Health Care 21: 380-385.
- Bitsori M, Galanakis E (2012) Pediatric urinary tract infections: diagnosis and treatment. Expert Rev Anti Infect Ther 2012 10: 1153-1164.
- Hay AD, Whiting P, Butler CC (2011) How best to diagnose urinary tract infection in preschool children in primary care? BMJ 343: 6316.
- Coon ER, Quinonez RA, Moyer VA, Schroeder AR (2014) Overdiagnosis: how our compulsion for diagnosis may be harming children. Pediatrics 134: 1013-1023.
- Cabana MD, Rand CS, Powe NR, Wu AW, Wilson MH, et al. (1999) Why Don’t Physicians Follow Clinical Practice Guidelines? A Framework for Improvement. JAMA 282: 1458-1465.
- Ibeneme CA, Oguonu T, Okafor HU, Ikefuna AN, Ozumba UC (2014) Urinary tract infection in febrile under five children in Enugu, South Eastern Nigeria. Niger J ClinPract 17: 624-628.
- Robinson JL, Finlay JC, Lang ME, Bortolussi R (2014) Urinary tract infections in infants and children: Diagnosis and management. Paediatr Child Health 19: 315-325.
- Davis DA, Thomson MA, Oxman AD, Haynes RB (1995) Changing physician performance. A systematic review of the effect of continuing medical education strategies. JAMA 274: 700-705.
- National Institute for Health and Care Excellence (2007) Urinary tract infection in children: Diagnosis, treatment and long-term management. National Institute for Health and Care Excellence 1-36.
- Roberts KB (2012) Revised AAP Guideline on UTI in Febrile Infants and Young Children. Am FamPhys 86: 940-946.
Relevant Topics
- Addiction
- Adolescence
- Children Care
- Communicable Diseases
- Community Occupational Medicine
- Disorders and Treatments
- Education
- Infections
- Mental Health Education
- Mortality Rate
- Nutrition Education
- Occupational Therapy Education
- Population Health
- Prevalence
- Sexual Violence
- Social & Preventive Medicine
- Women's Healthcare
Recommended Journals
Article Tools
Article Usage
- Total views: 11633
- [From(publication date):
April-2016 - Apr 04, 2025] - Breakdown by view type
- HTML page views : 10685
- PDF downloads : 948