Clinical Risk Factors and Duration of Infectiousness of SARS-CoV-2 Among Symptomatic and Asymptomatic Patients
Received: 22-Mar-2021 / Accepted Date: 05-Apr-2021 / Published Date: 12-Apr-2021
Abstract
We have investigated the clinical characteristics, duration of infectiousness from onset of symptoms and viral kinetics among moderate, mild and asymptomatic cases. For 181 cases, clinical features that were statistically significant between moderate and mild cases are fever (p-value 0.028), cough (p-value 0.352), runny nose (p-value 0.016), nausea (p-value 0.039), loss of smell and taste (p-value 0.02 each), fatigue (p-value 0.008), muscle ache (p-value 0.004), loss of appetite (p-value 0.041) and contact history (p-value 0.010). Logistic regression showed fever (OR, 4.35; p-value 0.006), cough (OR, 12.38; p-value 0.0001), expectoration (OR, 7.54; 0.003), sore throat (OR, 8.50; 0.0001), SOB (OR, 3.02; p-value 0.034), chills (OR 3.50; p-value 0.0005), diarrhea (OR 2.09; p-value 0.034), headache (OR, 14.80, p-value 0.0001) and loss of appetite (OR, 3.79, p-value 0.034) to be associated with disease severity in moderate cases as compared to mild cases. Median duration of viral shedding from symptoms onset to diagnosis was 6 days (IQ 4-8 days; range 2-10 days) while for the mild cases it was 8 days (IQ 6-10 days; range 4-11 days). The mean Ct value for moderate cases (median 25.03, 95% CI 23.83-26.81) was significantly lower than mild (median 35.93, 95%CI 35.57-36.23) and asymptomatic cases (median 38.92, 95% CI 38.96-40.07) (p-value <0.001) and mild cases from asymptomatic cases (p-value <0.001). The median infectious period for moderate cases was 15 days while for mild and asymptomatic cases it was 10 days each. The estimated mean time from symptom onset to two negative RT-PCR tests for moderate cases was 17.59 days (95% CI 16.39-18.80), mild cases 12.67 days (95% CI 11.11-14.22) and for asymptomatic cases 12.21 days (95% CI 9.40-15.01). Moderate cases took longer time as compared to mild and asymptomatic cases. When stratified by gender, female patients took significantly longer time (p-value <0.0001) to negative RT-PCR test.
Keywords: SARS-CoV-2; RT-PCR; Cycle threshold; Viral kinetics
Introduction
The pandemic of infectious novel coronavirus disease (COVID-19) is an international emergency that has caused substantial damage to public health [1]. Globally, by 27th January 2021, 99.7 million cases have been reported as COVID 19, including 2.14 million deaths to World Health Organization (WHO) [2]. Till date, 536 thousand cases have been reported in Pakistan with 11,376 deaths.
The clinical spectrum of COVID-19 infection is very wide ranging from asymptomatic infection, mild upper respiratory tract illness to severe viral pneumonia with respiratory failure, irreversible lung damage and even death [3-5]. The spectrum of this disease is usually characterized by various respiratory symptoms including asymptomatic to mild infection (e.g. dry cough and fever) and moderate to severe infection (e.g. pneumonia) [3,6]. It has been reported that the clinical outcomes and symptomatic severity is highly correlated with age, gender and various comorbidities. The absence of immunity in the human population is due to novelty of the pathogen and its infection and there is no effective treatment or vaccination approaches at present to cope with the emerging situation. The route of transmission of the virus and detection of high SARS-CoV-2 viral loads in respiratory samples from asymptomatic or pauci-symptomatic is unavoidable [7-10].
There is a need to investigate several epidemiological parameters of the infection including incubation period and duration of infectiousness. This is important for the monitoring, surveillance and control of infectious disease in the population [11,12]. It is important to understand the extent of infectiousness in positive cases to develop an evidence-based and effective public health policies on quarantine, duration of quarantine, tracing of contacts and return to work. The widely used diagnostic method for SARS-CoV-2 is the viral detection by Reverse Transcription-PCR (RT-PCR) from respiratory and other tissue or body fluid samples. RT-PCR is also used to monitor SARSCoV- 2 infection, its progression to infectivity of an individual [13]. Various studies have been performed to study the extent of virus detection in asymptomatic or pre-symptomatic patients with estimates found to vary widely [14-16]. The course of SARS-CoV-2 infection is typically determined by the incubation period of the virus, the symptomatic phase, an asymptomatic phase until virus detection becomes negative, and by observing the features of disease activity on chest CT scans. The course of disease illness vary remarkably in various stages of infection [17].
Several cases have been reported as positive that have no obvious symptoms (asymptomatic) or develop symptoms late after the exposure or infection. The reason for varied immune response of the individuals need to be investigated in detail [18,19]. Furthermore, asymptomatic/ pre-symptomatic) cases have been reported as secondary cases and are often infectious posing a hidden threat in the spread of the infection [20,21]. However, it is very difficult to measure the duration of this infectious period with certainty therefore it is important to gain an understanding on this key variable affecting COVID-19 disease transmission [22].
As moderate and mild cases represent the absolute majority of cases, the present study was aimed to investigate the clinical characteristics, duration of infectiousness from onset of symptoms and viral kinetics among moderate, mild and asymptomatic cases in comparison to onset of symptoms.
Materials and Methods
Study design and study population
This was a retrospective study conducted on Outdoor Patient Department (OPD) of Jinnah hospital from May 2020 to July 2020. Jinnah Hospital, Lahore is a tertiary care hospital in the capital of Punjab, the largest province of Pakistan. It is the pioneer hospital which is conducting large scale screening and diagnosis of COVID-19 infection by using RT-PCR in specialized healthcare. The samples collected from here can be considered as representative of the large population. A total of 181 adult patients of >15 years of age with laboratory confirmed COVID-19 were included. Demographic, clinical and laboratory data including serial samples of viral RNA detection were taken.
Patient characterization
Patients were characterized into symptomatic (moderate and mild) and asymptomatic following the criteria.
1) Individuals who were tested positive for RT-PCR for SARS- CoV-2, but who have no symptoms that are consistent with COVID-19 were categorized as asymptomatic or pre-symptomatic.
2) Mild illness was categorized as presence of various signs and symptoms of COVID-19 that include fever, cough, sore throat, malaise, headache, muscle pain, nausea, vomiting, diarrhea, loss of taste and smell but who do not have shortness of breath, dyspnea, or abnormal chest imaging. Additionally, evidence of lower respiratory disease during clinical assessment or imaging and saturation of oxygen (SpO2) ≥ 94% on room air at sea level was categorized as moderate illness.
Sample collection
Nasopharyngeal swab samples were collected according to the Standard Operating Procedures (SOPs) established by National Institute of Health (NIH) and WHO [23]. Nasopharyngeal samples were collected by using a plastic swab with a polypropylene fiber head and is commercially available. The swab was gently passed to the posterior wall of the nasopharynx through nostrils and held for approximately 3 seconds, rotated 2 or 3 times, withdrawn gently, and placed in 3 mL of virus transfer medium. Samples were immediately stored at 4-8˚C and transported on ice to the laboratory where they were analyzed for PCR within 24 hours of collection.
Data collection
Clinical, epidemiological, demographic, laboratory and disease outcome data were taken using a standardized questionnaire which was a modified version of the WHO/International Severe Acute Respiratory and Emerging Infection Consortium cases record form for severe acute respiratory infections. Demographic data of patients including age and gender was taken. Information regarding clinical symptoms such as fever, cough, expectoration, runny nose, sore throat, chills, vomiting, nausea, diarrhea, headache, fatigue, muscle ache, loss of smell, loss of taste, loss of appetite, Shortness Of Breath (SOB), co-morbid condition and contact with COVID-19 patients was noted.
RNA extraction and RT-PCR .
The viral RNA was extracted from nasopharyngeal swab samples was performed through automated extraction kit (Xiamen Zeesan Biotech, China. Catalog no. 606104) using Lab-Aid 808/824/824s instrument according to manufacturer’s protocol. Briefly, 1-2 ml of the sample was transferred into a tube containing 5-10 ul Poly A solution (lysis buffer), followed by the addition of binding buffer. Lysates were thoroughly mixed with magnetic beads to allow optimal adsorption of RNA to the silica surface. After binding, containments were efficiently washed away during a sequence of washing steps using wash buffer R1 and R2. Highly pure RNA was eluted in the elution buffer and subsequently used for PCR reaction.
Qualitative RT-PCR was performed using Novel Coronavirus (2019-nCoV) Nucleic Acid Diagnostic Kit (PCR-Fluorescence Probing) (Sansure biotech, China, Catalog no. S3102E) according to manufacturer’s protocol. Briefly, for one sample 30 uL of 2019-nCoV- PCR master mix was prepared by mixing 26 μL of 2019-nCoV-PCR mix and 4 μL 2019- nCoV-PCR-Enzyme mix. 30 μL of master mix was added into each well. The wells were covered the plate was transferred to the sample processing area. 20 μL of the extracted RNA was added to the well pre-filled with reagent mix in the following order: 2019-nCoV- PCR-Negative Control, patient specimen (s), and 2019-nCoV-PCR- Positive Control. Each well was covered followed by centrifugation at 2000 rpm for 10 seconds, and place into Applied Biosystems ABI. PCR conditions were one cycle each for reverse transcription at 50˚C for 30 min and melting of cDNA at 95˚C for 1 min. 45 cycles of PCR were performed, each including denaturation (95˚C for 15 sec), annealing, extension and fluorescence collection (60˚C for 1 min). At the end of PCR, device was cooled at 25˚C for 10 sec.
FAM (ORF-1 ab region) and ROX (N gene) channels were selected to test 2019-nCoV nucleic acid. Start value was set between 3-15, and end value was between 5-20. Amplification curve of negative control was adjusted to be flat or below threshold. Results were analyzed as positive and negative based on Ct value. Sample was considered as positive if there was typical S-shape amplification curve detected at FAM and/or ROX channel, and the amplification curve which is detected at CY5 channel, Ct ≤ 40. For negative sample, there was no typical S-shape amplification curve (No Ct) or Ct >40 detected at FAM and ROX channel, and the amplification curve which is detected at CY5 channel, Ct ≤ 40. For each sample Ct value was also noted for quantification of the RNA.
Statistical analysis
Continuous and categorical variables were presented as mean, median (IQR), 95% Confidence Interval and n (%), respectively. To compare the differences between the two groups, mean values and percentages were compared between the two groups using the Student t-test and Mann-Whitney U-test. One-way ANOVA was used to compare differences between moderate and mild symptomatic and asymptomatic patients where appropriate. All the analysis was done on Graph Pad Prism (Prism 8.0, Graph Pad; SPSS 25.0, IBM). A two-sided α (p-value) of less than 0.05 was considered statistically significant.
Ethical approval
This study was approved by Institutional Ethical Review Committee of Jinnah Hospital, Lahore (Ref No: 2/24/11/2020/S2 ERB). The objectives of the study were explained to all study participants in their native language. A written consent was obtained from all those participants who agreed to participate in the study. The individuals who didn’t give consent, were not included in the study but this decline of consent had no effect on their diagnosis and treatment regimen. After the written consent, nasopharyngeal swab samples were obtained according to the Standard Operating Procedures (SOPs) approved by the ethical review board of the institution.
Results
Patient characteristics
A total of 181 cases were recruited that had follow up samples with negative results. The median age of the patients was 30 years (IQ, 25- 37 years; range 16-75 years). The proportion of male cases was 66% (n=120) with median age of 30 years (IQ, 26-43 years; range 16-71 years) and that of female patients was 34% (n=61) with median age of 30 years (IQ, 25-35 years, range 19-72 years). Disease was categorized as moderate and mild based on the clinical signs and symptoms. Out of 181, 101(56%) cases were characterized as moderate, 51(28%) as mild and 29 (16%) were asymptomatic. All cases were RT-PCR positive and were self-quarantined.
Clinical characteristics of symptomatic patients
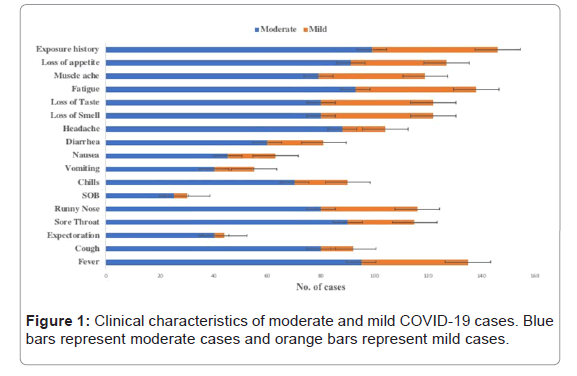
Among moderate cases, 63/101 (62%) were male whereas 38/101 (38%) were female. Among mild cases, 35/51 (68%) were male and 16/51 (32%) were female. On the other hand, 22/29 (75%) male and 7/29 (25%) female cases were found to be asymptomatic. All the symptomatic cases were experiencing many clinical signs of the disease included fever (92%), cough (60%), expectoration (28%), sore throat (75%), runny nose (76%), SOB (19%), chills (60%), vomiting (36%), nausea (34%), diarrhea (53%), headache (68%), loss of smell and taste (80% each), fatigue (90%), muscle ache (78%), loss of appetite (76%) and contact history (96%) (Figure 1).
Mann-Whitney test (non-parametric t-test) was performed to see the difference of clinical features between moderate and mild cases. Clinical features that were statistically significant between moderate and mild cases are fever (p-value 0.028), cough (p-value 0.352), runny nose (p-value 0.016), nausea (p-value 0.039), loss of smell and taste (p-value 0.02 each), fatigue (p-value 0.008), muscle ache (p-value 0.004), loss of appetite (p-value 0.041) and contact history (p-value 0.010). Expectoration, sore throat, SOB, chills, vomiting, and diarrhea were statistically insignificant between two groups (Table 1).
| Symptoms | Moderate (n=101) | Mild (n= 51) | p-value | ||
|---|---|---|---|---|---|
| Yes (%) | No (%) | Yes (%) | No (%) | ||
| Fever | 95 (94) | 6 (6) | 40 (78) | 11(22) | 0.028 |
| Cough | 80 (79) | 21 (21) | 12 (23) | 39 (77) | 0.352 |
| Expectoration | 40 (39) | 61 (61) | 04 (7) | 47 (93) | 0.44 |
| Sore Throat | 90 (89) | 11 (11) | 25 (49) | 26 (51) | 0.153 |
| Runny Nose | 80 (79) | 21 (21) | 36 (70) | 15 (30) | 0.016 |
| SOB | 25 (24) | 76 (76) | 05 (9) | 46 (91) | 0.267 |
| Chills | 70 (69) | 31 (31) | 20 (39) | 31 (61) | 0.143 |
| Vomiting | 40 (39) | 61 (61) | 15 (29) | 36 (71) | 0.055 |
| Nausea | 45 (44) | 56 (56) | 18 (35) | 33 (65) | 0.039 |
| Diarrhea | 60 (59) | 41 (41) | 21 (41) | 30 (59) | 0.075 |
| Headache | 88 (87) | 13 (13) | 16 (31) | 35 (69) | 0.267 |
| Loss of Smell | 80 (79) | 21 (21) | 42 (82) | 9 (18) | 0.002 |
| Loss of Taste | 80 (79) | 21 (21) | 42 (82) | 9 (18) | 0.002 |
| Fatigue | 93 (92) | 8 (8) | 45 (88) | 6 (12) | 0.008 |
| Muscle ache | 79 (78) | 22 (22) | 40 (78) | 11 (22) | 0.004 |
| Loss of appetite | 91 (90) | 10 (10) | 36 (70) | 15 (30) | 0.041 |
| Exposure history | 99 (98) | 2 (2) | 47 (92) | 4 (8) | 0.010 |
Table 1: Clinical characteristics of patients.
Radiological characteristics of symptomatic and asymptomatic patients
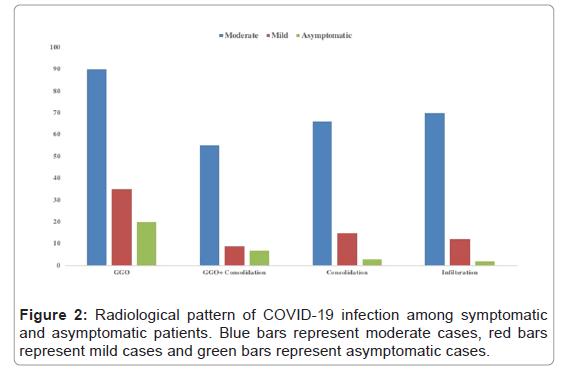
Radiological parameters included consolidation, infiltration and Ground Glass Opacity (GGO) with unilateral or bilateral involvement of the lungs. In 88/181 cases, the pattern of infection was unilateral whereas 93/181 cases showed bilateral lung involvement. Among moderate cases, 30% cases were with unilateral pattern and 70% cases were with bilateral pattern. Mild and asymptomatic cases with unilateral disease pattern were 72% and 68% respectively, while with bilateral pattern were 28% and 32% respectively. Ground Glass Opacity (GGO) was the most frequent and typical abnormality of COVID-19 infection observed in 90%, 69% and 68% among moderate, mild and asymptomatic cases respectively (Figure 2).
Risk factors associated with COVID-19 infection
Logistic regression analysis was performed to estimate the clinical features that are highly associated with the disease severity. Individuals that had moderate infection were at higher risk of disease severity as compared to mildly infected cases. Clinical symptoms like fever (OR, 4.35; p-value 0.006), cough (OR, 12.38; p-value 0.0001), expectoration (OR, 7.54; 0.003), sore throat (OR, 8.50; 0.0001), SOB (OR, 3.02; p-value 0.034), chills (OR 3.50; p-value 0.0005), diarrhea (OR 2.09; p-value 0.034), headache (OR, 14.80, p-value 0.0001) and loss of appetite (OR, 3.79, p-value 0.034) were associated with disease severity in moderate cases as compared to mild cases (Table 2).
| Risk factors | Odds ratio | 95 % CI (lower-upper) | p-value |
|---|---|---|---|
| Fever | 4.35 | 1.50-12.58 | 0.006 |
| Cough | 12.38 | 5.53-27.71 | 0.0001 |
| Expectoration | 7.54 | 2.51-22.58 | 0.003 |
| Sore Throat | 8.5 | 3.70-19.56 | 0.0001 |
| Runny Nose | 1.58 | 0.73-3.43 | 0.239 |
| Shortness of Breadth | 3.02 | 1.08-8.45 | 0.034 |
| Chills | 3.5 | 1.73-7.07 | 0.0005 |
| Vomiting | 1.57 | 0.76-3.24 | 0.218 |
| Nausea | 1.47 | 0.73-2.95 | 0.274 |
| Diarrhea | 2.09 | 1.05-4.14 | 0.034 |
| Headache | 14.8 | 6.45-33.96 | 0.0001 |
| Loss of Smell | 0.81 | 0.34-1.93 | 0.645 |
| Loss of Taste | 0.81 | 0.34-1.93 | 0.645 |
| Fatigue | 1.55 | 0.50-4.70 | 0.441 |
| Muscle ache | 0.98 | 0.43-2.23 | 0.975 |
| Loss of appetite | 3.79 | 1.55-9.21 | 0.003 |
| Exposure history | 4.21 | 0.74-23.82 | 0.103 |
Table 2: Risk factors analysis for COVID-19 cases.
Viral kinetics of symptomatic and asymptomatic cases
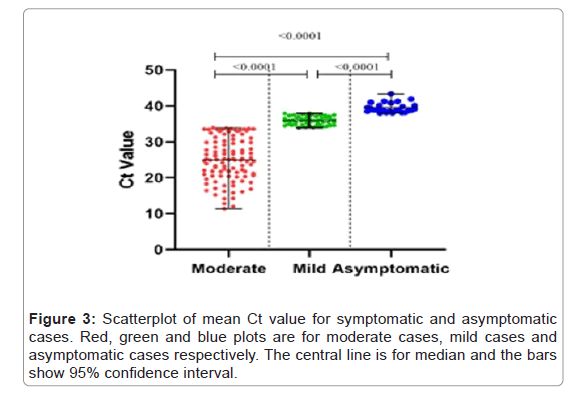
To observe the difference between the viral load of moderate, mild and asymptomatic cases, scatter plot was plotted for the Ct values. The mean Ct value for moderate cases (median 25.03, 95% CI 23.83- 26.81) was significantly lower than that of mild (median 35.93, 95%CI 35.57-36.23) and asymptomatic cases (median 38.92, 95% CI 38.96- 40.07) (p-value <0.001). Similarly, the mean Ct value of mild cases was significantly lower than asymptomatic cases (p-value <0.001). As Ct value is inversely proportional to the viral load, this data shows that viral load was directly related to disease severity as the viral load of moderate case was observed to be significantly higher than that of mild and asymptomatic cases (Figure 3).
Relationship between ‘symptoms to test’ interval and virus detection
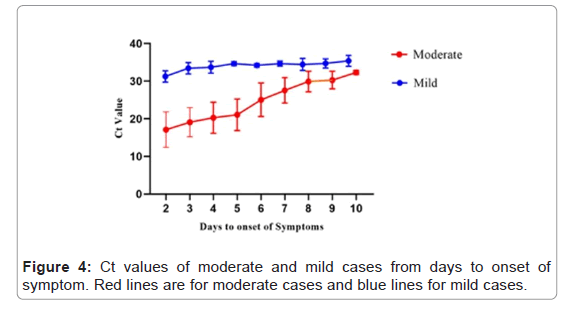
There were 152 symptomatic cases for which the dates of onset of symptoms and diagnosis were known. Median duration of viral shedding from symptoms onset to diagnosis was 6 days (IQ 4-8 days; range 2-10 days) while for the mild cases median was 8 days (IQ 6-10 days; range 4-11 days). Viral load was statistically higher in first week than second week in moderate cases as compared to mild cases. 29 cases that were asymptomatic at the time of sampling developed the symptoms within 14 days (Figure 4).
Factors associated with duration of virus and viral load
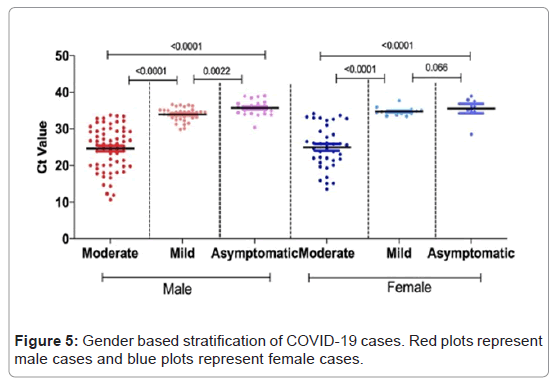
Patients of different disease stages were stratified by gender, male patients had significantly higher viral load (p-value <0.0001) as compared to the female patients. Among both male and female patients, the viral load of moderate cases was higher as compared to mild (p-value <0.0001) and asymptomatic cases (p-value <0.0001). Viral load of mild and asymptomatic cases was also significantly different among male (p-value 0.0022) but no significant difference was observed in female patients (p-value 0.066), (Figure 5).
Duration of infectious period of SARS-CoV-2
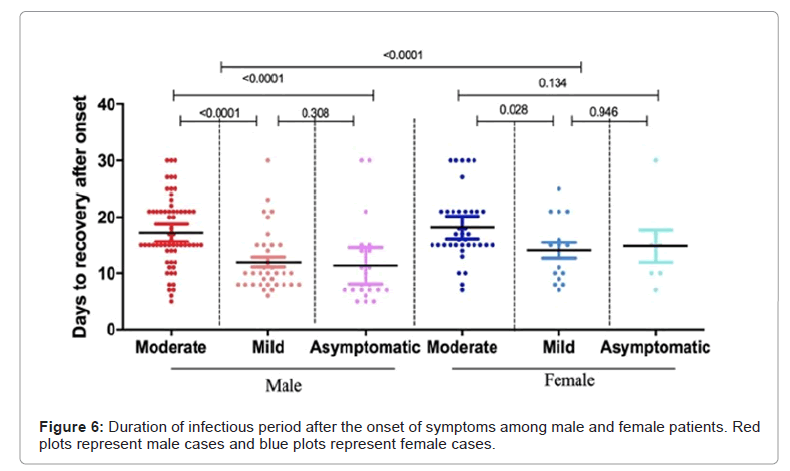
Depending upon the state of the disease, inferred duration of infectious period of the virus varies substantially. The median infectious period for moderate cases was 15 days while for mild and asymptomatic cases it was 10 days each. The estimated mean time from symptom onset to two negative RT-PCR tests for moderate cases was 17.59 days (95% CI 16.39-18.80), mild cases 12.67 days (95% CI 11.11-14.22) and for asymptomatic cases 12.21 days (95% CI 9.40-15.01). Moderate cases took longer time as compared to mild and asymptomatic cases. When stratified by gender, female patients took significantly longer time (p-value <0.0001) to negative RT-PCR test (Figure 6 and Table 3 and Table 3).
| Male | Female | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean | Median | 95% CI Lower-upper | p-value | N | Mean | Median | 95% CI Lower-upper | p-value | |
| Moderate vs. mild | ||||||||||
| Moderate | 63 | 24.66 | 25.61 | 23.16-26.16 | <0.0001 | 38 | 24.99 | 25.18 | 23.08-26.90 | <0.0001 |
| Mild | 35 | 33. 96 | 34.26 | 33.35-34.56 | 16 | 34.76 | 34.18 | 34.21-35.31 | ||
| Moderate vs. Asymptomatic | ||||||||||
| Moderate | 63 | 24.66 | 25.61 | 23.16-26.16 | <0.0001 | 38 | 24.99 | 25.18 | 23.08-26.90 | <0.0001 |
| Asymptomatic | 22 | 35.74 | 35.365 | 34.81-36.67 | 7 | 35.56 | 36.04 | 32.35-38.77 | ||
| Mild vs. Asymptomatic | ||||||||||
| Mild | 35 | 33.96 | 34.26 | 33.35-34.56 | 0.0022 | 16 | 34.76 | 34.18 | 34.21-35.31 | 0.066 |
| Asymptomatic | 22 | 22 | 35.74 | 35.365 | 7 | 35.56 | 36.04 | 32.35-38.77 | ||
Table 3: Gender based viral kinetics of moderate, mild and asymptomatic cases.
Discussion
COVID-19 pandemic is a major issue in public health since the emergence of the novel virus in 2019. Most patients reported throughout the world are observed to be of mild to moderate severity [24]. In this study, we have comprehensively studied the relationship between disease severity and clinical indicators. We included 150 cases with moderate and mild disease and 29 cases without any symptoms at the time of diagnosis but developed the symptoms after an average of 14 days. As expected, there were more male than female cases in all three categories. Clinical symptoms that were significantly related to the disease severity were fever, cough, expectoration, runny nose, nausea, loss of smell, loss of taste, fatigue, muscle ache, loss of appetite and exposure history. Among radiological parameters, GGOs, consolidation and infiltration were the most common abnormalities with bilateral distribution in majority of the cases. These findings are in consistent with previously published studies [25-27].
Various diagnostic studies have been performed that provide useful information about the duration of infection, but it is limited by the gap between the time of diagnosis and infectiousness. Other factors that can affect are the sampling procedure and site of the sample such as upper respiratory, lower respiratory, urine, blood or other body fluid [28]. Viral load studies have shown a peak in viral load close to onset of symptoms (typically, -1 to 7 days of onset); however, there is uncertainty whether this typically occurs prior to, on or after onset. High viral loads, measured as Ct values, have been recorded to range from 1 to 3 weeks after the onset of symptoms followed by a general decreasing trend with time [29]. We also observed the similar data in this study, as the virus remained detectable for up to 15 days in both moderate and mild cases. Viral load (estimated ion the basis of Ct value) of moderate cases and male patients was significantly higher (p-value <0.0001) as compared to mild and female cases independently.
The viral load was statistically higher in first week than second week in moderate cases as compared to mild cases. Characterization of viral detection and kinetics is important for decision making about self-isolation.
The duration of infectiousness and severity of the infection caused by virus replication are important factors to understand the pathogenesis of the disease and to estimate the risk of transmission. This is important for making decisions regarding isolation and treatment of patients. A number of studies have used qualitative or quantitative viral RNA tests as a potential diagnostic tool for infectious coronavirus because RNA detection is more sensitive than virus isolation. For SARS-CoV-2, viral RNA was detected up to 4 weeks of onset of symptoms in respiratory samples in one third of patient population [30]. We observed that median duration of viral shedding from symptoms onset to diagnosis was 6 days (IQ 4-8 days; range 2-10 days) and for mild cases median was 8 days (IQ 6-10 days; range 4-11 days). The mean time from symptom onset to two negative RT-PCR tests for moderate cases was 17.59 days (95% CI 16.39-18.80), mild cases 12.67 days (95% CI 11.11- 14.22) and for asymptomatic cases 12.21 days (95% CI 9.40-15.01). The course of COVID-19 is dependent on multiple factors including onset of symptoms, the duration of exposure, incubation and detection time, the symptomatic phase and an asymptomatic phase until virus becomes undetectable. Despite the low severity of the described cases, the course of the disease was remarkably long [17].
Conclusion
The mean time from symptom onset to two negative RT-PCR tests for moderate cases was 17.59 days (95% CI 16.39-18.80), mild cases 12.67 days (95% CI 11.11-14.22) and for asymptomatic cases 12.21 days (95% CI 9.40-15.01). The course of COVID-19 is dependent on multiple factors including onset of symptoms, the duration of exposure, incubation and detection time, the symptomatic phase and an asymptomatic phase until virus becomes undetectable. Despite the low severity of the described cases, the course of the disease was remarkably long.
Acknowledgment
The authors would like to acknowledge Muhammad Tajjamul, Muhammad Asim for helping in data acquisition.
Conflict of Interest
None
Funding
This study received no formal funding for data acquisition and interpretation.
Autor Contribution
Concepftuaflffizaftffion: AK, FA, HA, SS
Data Curation: AK, FA, HA, ST, SJ
Formafl Anaflysffis: AK, HA, SS
Invesftffigaftffion: AK, FA, HA, ST, SJ, SS
Methodology: AK, FA, HA, ST, SJ
Project Administration: AK, FA, HA, ST, SJ
Resources: AK, FA, HA, ST, SJ
Supervision: AK, FA
Validation: AK, FA
Visualization: AK, FA
Wrffiftffing-orffigffinafl draft: AK, FA, HA, SS
Writing review and editing: AK, FA
References
- Dong E, Du H, Gardner L (2020) An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis 20: 533-534.
- WHO (2020) Coronavirus Disease (COVID-19) Dashboard.
- Huang C, Wang Y, Li X, Ren L, Zhao J, et al. (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet Infect Dis 395: 15-21.
- Wang D, Hu B, Hu C, Zhu F, Liu X, et al. (2020) Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 323: 1061-1069.
- Chen N, Zhou M, Dong X, Qu J, Gong F, et al. (2020) Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 395: 507-513.
- Pan Y, Zhang D, Yang P, Poon LLM, Wang Q, et al. (2020) Viral load of SARS-CoV-2 in clinical samples. Lancet Infect Dis 20: 411-412.
- Rabi FA, Zoubi MSA, Kasasbeh GA, Salameh DM, Al-Nasser AD, et al. (2020) SARS-CoV-2 and coronavirus disease 2019: What we know so far. Pathogens 9: 231.
- Casey M, Griffin J, McAloon CG, Byrne AW, Madden JM, et al. (2020) Pre-symptomatic transmission of SARS-CoV-2 infection: A secondary analysis using published data. MedRxiv 2020: 1-6.
- Murchu EO, Byrne, Walsh PKA, Carty PG, Connolly M, et al. (2021) Immune response following infection with SARS‐CoV‐2 and other coronaviruses: A rapid review. Rev Med Virol 31 e2162.
- Liu Y, Gayle AA, Wilder-Smith A, Rocklov J (2020) The reproductive number of COVID-19 is higher compared to SARS coronavirus. J Travel Med 27: taaa021.
- Backer JA, Klinkenberg D, Wallinga J (2020) Incubation period of 2019 novel coronavirus (2019-nCoV) infections among travellers from Wuhan, China, 20-28 January 2020. Euro Surveill 25: 2000062.
- Jiang X, Rayner S, Luo M (2020) Does SARS-CoV-2 has a longer incubation period than SARS and MERS?. J Med Virol 92: 476-478.
- Singanayagam A, Patel M, Charlett A, Bernal JLP, Saliba V, et al. (2020) Duration of infectiousness and correlation with RT-PCR cycle threshold values in cases of COVID-19, England, January to May 2020. Euro Surveill 25: 2001483
- Le TQM, Takemura T, Moi ML, Nabeshima T, Nguyen LKH, et al. (2020) Severe acute respiratory syndrome coronavirus 2 shedding by travelers, vietnam, 2020. Emerg Infect Dis 26: 1624-1626.
- Qiu H, Wu J, Hong L, Luo Y, Song Q, et al. (2020) Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China: An observational cohort study. The Lancet Infect Dis 20: 689-696.
- To KK, Tsang OT, Leung W, Tam AR, Wu T, et al. (2020) Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis 20: 565-574.
- Xia L, Chen J, Friedemann T, Yang Z, Ling Y, et al. (2020) The course of mild and moderate covid-19 infections-the unexpected long-lasting challenge. Open Forum Infect Dis 23: 286.
- Hu Z, Song C, Xu C, Jin G, Chen Y, et al. (2020) Clinical characteristics of 24 asymptomatic infections with COVID-19 screened among close contacts in Nanjing, China. Sci China Life Sci. 65: 706-711.
- Ma S (2020) Epidemiological parameters of coronavirus disease 2019: A pooled analysis of publicly reported individual data of 1155 cases from seven countries. MedRxiv 2020: 1-7.
- Rothe C, Schunk M, Sothmann P, Bretzel G, Froesch G. (2020) Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N Engl J Med 382 970-971.
- Bai Y, Yao L, Wei T, Tian F, Jin D, et al. (2020) Presumed asymptomatic carrier transmission of COVID-19. JAMA 323: 1406-1407.
- Zhou F, Yu T, Du R, Fan G, Liu Y, et al. (2020) Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 395: 1054-1062.
- National Institute of Health (2020) Protocol for nasophrangeal swab smaples for COVID-19.
- Wang D, Li R, Wang J, Jiang Q, Gao C, et al. (2020) Correlation analysis between disease severity and clinical and biochemical characteristics of 143 cases of COVID-19 in Wuhan, China: A descriptive study. BMC Infect Dis 20: 519.
- Shabrawishi M, Al-Gethamy MM, Naser AY, Ghazawi MA, Alsharif GF, et al. (2020) Clinical, radiological and therapeutic characteristics of patients with COVID-19 in Saudi Arabia. PLoS One 15: e0237130.
- Guan W, Ni Z, Yu Hu, Liang W, Ou C, et al. (2020) Clinical characteristics of coronavirus disease 2019 in China. NEJM 328: 1708-1720.
- Yang W, Cao Q, Qin L, Wang X, Cheng Z, et al. (2020) Clinical characteristics and imaging manifestations of the 2019 novel coronavirus disease (COVID-19): A multi-center study in Wenzhou city, Zhejiang, China. J Infect 80: 388-393.
- Wölfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, et al. (2020) Virological assessment of hospitalized patients with COVID-2019. Nature 581: 465-469.
- Byrne AW, McEvoy D, Collins AB, Hunt K, Casey M, et al. (2020) Inferred duration of infectious period of SARS-CoV-2: rapid scoping review and analysis of available evidence for asymptomatic and symptomatic COVID-19 cases. BMJ Open 10: e039856.
- Xu D, Zhang Z, Jin L, Chu F, Mao Y, et al. (2005) Persistent shedding of viable SARS-CoV in urine and stool of SARS patients during the convalescent phase. Eur J Clin Microbiol Infect Dis 24: 165-171.
Citation: Khaliq A, Ashraf F, Tahir S, Javed S, Abbas H, et al. (2021) Clinical Risk Factors and Duration of Infectiousness of SARS-CoV-2 Among Symptomatic and Asymptomatic Patients. Epidemiol Sci 11: 404.
Copyright: © 2021 Khaliq A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 2085
- [From(publication date): 0-2021 - Nov 05, 2025]
- Breakdown by view type
- HTML page views: 1223
- PDF downloads: 862