Research Article Open Access
Clinical Features of Cerebral Cortex Malformations in Children: A Study in Upper Egypt
| Abdelrahim A Sadek1, Alzahraa E Ahmed Sharaf2, Elsayed M Abdelkreem3 and Saad R Abdul Wahed4* | |
| 1Lecturer of Pediatrics, Neurology Unit, Department of Pediatrics, Sohag University, Egypt | |
| 2Assistant Professor of Pediatrics, Department of Pediatrics, Sohag University, Egypt | |
| 3Assistant lecturer of Pediatrics, Department of Pediatrics, Sohag University, Egypt | |
| 4Assistant professor of Radiology, Department of Radiology, Al Azhar University, Assuit Branch, Egypt | |
| Corresponding Author : | Saad R Abdul Wahed Assistant professor of Radiology Department of Radiology, Al Azhar University Assuit Branch, Egypt E-mail: srage73@yahoo.com |
| Received May 16, 2013; Accepted May 21, 2013; Published May 27, 2013 | |
| Citation: Sadek AA, Ahmed Sharaf AE, Abdelkreem EM, Abdul Wahed SR (2013) Clinical Features of Cerebral Cortex Malformations in Children: A Study in Upper Egypt. OMICS J Radiology 2:123. doi: 10.4172/2167-7964.1000123 | |
| Copyright: © 2013 Sadek AA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Radiology
Abstract
Malformation of Cortical Development (MCD) corresponds to a broad spectrum of cerebral lesions resulting from cortical development abnormalities during embryogenesis. MCD are increasingly recognized as an important cause of epilepsy, especially refractory ones. Aims: Evaluation of pattern and clinical spectrum of MCD.
Patients and methods: The data of children presented to the Pediatric Department, or Pediatric Neurology Clinic, Sohag University Hospital whom Computed Tomography (CT) or Magnetic Resonance Imaging (MRI) brain showing MCD were collected retrospectively regarding their history, examinations, and developmental parameters, Electroencephalography (EEG), Intelligence Quotient (IQ). Results: during the study periods we reviewed the data of 50 Child with MCD, the mean age of the study group was 3.7 years with age range from 4 days to 15 years. The majority of the patients (50%) belonged to the age group 1-5 years old. Convulsions were the main complaint in the majority of children 21 (42%) out of 50 child, followed by global developmental delay in 20 out of 50 child. Seizures generally were present in (82%). Concerning type of seizures, multiple seizure types were found in 16 (39%) out of 41 child, partial seizures found in 13 (31.7%), generalized tonic in 6 (14.6%), infantile spasm in 4 (9.8%), and generalized tonic-clonic in 2 (4.8%). Types of malformation of cortical development in the studied children as defined by neuro-imaging, (42%) had lissencephaly, (16%) had bilateral Schizencephaly, (12%) had unilateral schizencephaly, (12%) had polymicrogyria, (10%) had focal cortical dysplasia, (6%) had periventricular heterotopia, and one child with subcortical laminar heterotopia.
Conclusion: The common cortical malformations found in Sohag University Hospital were lissencephaly, bilateral Schizencephaly, unilateral schizencephaly. Also the most common clinical features of MCDs in our community are refractory seizures and/or global developmental delay, and the most valuable tool in diagnosis is brain MRI. Recommendations: Informing pediatricians to raise their clinical suspicion to MCDs in children presented by frequent, intractable, early onset, and partial or multiple types’ seizures and/or global developmental delay, and that MRI brain must be done then.
| Keywords |
| Maformation of cortical development (MCD); Seizures; Global developmental delay (GDD) |
| Introduction |
| Malformation of cortical development (MCD) corresponds to a broad spectrum of cerebral lesions resulting from cortical development abnormalities during embryogenesis. The cerebral cortex is a modular structure [1-3] modules of neurons are induced in a neuroepithelial sheet and subsequently differentiate, migrate and organize into a functioning cerebral cortex. Neuronal induction results from a combination of graded extracellular signals and transcription factor gradients that operate across several fields of neocortical progenitor cells [4]. This process is regulated by interplay between intrinsic genetic mechanisms and extrinsic information relayed to cortex by thalamo cortical input and other, largely unknown, factors [5,6]. |
| They are characterized by abnormalities in the volume, location, or architecture of cerebral gray and white matter. Updates of the classification relied more heavily on genetics, and noted that the classification likely would never be finalized because of ongoing discoveries [7,8]. Many new syndromes have been described, and many new genes and mutations of known genes have been identified. A new classification has been proposed for Focal Cortical Dysplasias (FCDs) [9]. MCD have characteristic histopathological features and recognizable appearance on MRI. Some authors report greater degrees of disability with more extensive lesions, while other authors find no such association. Clearly, the overall relationship of extent of CNS malformation with clinical disability across the spectrum of these disorders has been incomplete [10]. |
| While clinical symptoms, past medical and family history are important in evaluating patients with cortical malformations, neuroimaging findings, especially, those seen with Magnetic Resonance Imaging (MRI), are the most common non-invasive means of arriving at a diagnosis. Diagnosis of MCD has greatly improved with the progress in contemporary imaging techniques [8]. Malformations of Cortical Development (MCDs) are increasingly recognized as important cause of epilepsy, especially refractory epilepsy. They were earlier identified only at post mortem examination of subjects with gross developmental disorders or with severe childhood epilepsy. Now the scenario has changed due to the availability of newer and sophisticated imaging techniques such as Computed Tomography (CT) and high-resolution Magnetic Resonance Imaging (MRI). Many of the cases initially considered as idiopathic or cryptogenic epilepsy are now found to be secondary to developmental malformations of the cortex [11]. Several studies have now shown that malformations of cortical development are the cause of 23% to 26% of intractable epilepsies in children and young adults [12,13]. This number emphasizes the point that cortical malformations must be ruled out in essentially every pediatric patient with developmental delay or epilepsy. Moreover, it is becoming increasingly clear that many malformations of cortical development are caused by chromosomal mutations; thus, the presence of cortical malformations is important for counseling of parents of affected children [14]. |
| Therefore, the objective of this study was performing a clinical analysis and reviewing the data of 50 children having MCD considering the type of MCD by neuroradiological findings (CT or MRI Brain or Both) with special consideration for the presence of epilepsy and its characteristics. |
| Patients and Methods |
| Study design |
| Retrospective Cohort study, Place of the study: Neuropediatric outpatient Clinic, and Pediatric Department at Sohag University Hospital, Upper Egypt. Study Period: one year (from 1-1-2011 to 31- 12-2011). A written consent was taken from the parents and the study was approved by Faculty of Medicine, Sohag University Hospital Ethics Committee. |
| Patients |
| Inclusion criteria: Children from birth to 15 years old who diagnosed as having definite malformations of cortical development by neuroradiological studies (CT, MRI Brain, or both) presented to the Neuropediatric outpatients Clinic or inpatients of Pediatric Department during the period of the study. |
| Exclusion criteria: 1) Children with history suggestive of definite perinatal asphyxia. 2) Other congenital malformations of the nervous system. |
| Methods of the Study |
| Detailed clinical data of the patients were retrospectively collected with special reference to 1. The antenatal, perinatal and postnatal events. 2. Developmental history. 3. The presenting symptoms focusing on nervous system as convulsions , abnormal movements, motor symptoms, sensory symptoms, sphincteric disorders, abnormal behavior. 4. Family history of neurological problems. 5. Type and frequency of seizures, duration, response to treatment. |
| Data of patients examination focusing on neurological system as conscious level, mental status, gait, abnormal features, other congenital anomalies, motor system, sensory system, and cranial nerves examination. |
| Neuroradiological (CT, MRI Brain or both), Neurophysiological data as electroencephalography (EEG), and IQ testing. |
| Statistical Analysis |
| Both clinical, neuroradiological, EEG, IQ data were entered into the SPSS 10, software package for descriptive statistics. Results of the study are expressed as mean with standard deviation and range, for continuous variables and as percentages for discrete variables. |
| Results |
| During the period of the study from 1st of January 2011 to 31st December 2011, we reviewed the data of 50 child who diagnosed as having definite malformations of cortical development by neuroradiological studies (CT and/or MRI Brain) presented to the Neuropediatric outpatients clinic or inpatients of Pediatric Department. |
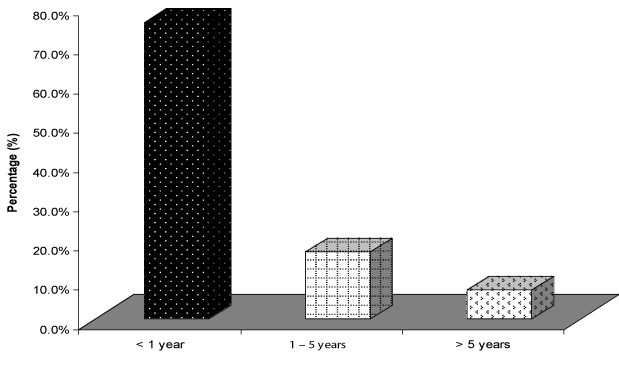
| The mean age of the study group was 3.7 years with age range from 4 days to 15 years (Figure 1). We noticed that the majority of the patients 25 child (50%) belonged to the age group 1-5 years old. It is also to be noticed that 14 children (28%) of patients were less than one year while only two children (4%) aged more than 10 years old. Concerning the sex distribution of the studied children, there was no significant difference, 23 (46%) child were males and 27 (54%) were females. There was no history of any antenatal maternal infection or drug exposure. 42 (84%) patients had normal vaginal delivery while remaining eight had lower segment caesarean section. Delayed motor milestones were noted in 43 (86%) patients. Delayed speech and social development were noted in 41 (82%) and 40 (80%) patients respectively. Family history of consanguinity was present in 30 (60%) patients and history of similar disease (seizure and/or severe developmental delay) was present in six (12%) patients. Head circumference of the studied children was below 5th percentile in 21 (42%) child, and in the other 29 (58%) was between 5th–95th percentiles. The weight and length were below 5th percentile in 18 (36%) child. Head and neck examination was normal in 25 (50%) child, microcephaly in 21 (42%), dysmorphic features in 2 (4%), microphthalmia in 2 (4%), squint in 1 (2%) and ptosis in 1 (2%) child. During cranial nerve examination, we noticed that three children were blind, and that one child had dysarthria and abnormal tongue movement. Motor system examination in the studied group showed that the majority 26 (52%) had Generalized Hypotonia, 6 (12%) were normal, 2 (4%) had Monoparesis, 9 (18%) had hemiparesis, and spastic quadriparesis was found in 7 (14%) children. |
| It is clear from Table 1 that convulsions were the main complaint in the majority of children 21 (42%), followed by global developmental delay in 20 (40%) child, hemiparesis in three, small sized head in three, delayed motor development in two and one child with delayed language development. |
| Seizures generally were present in 41 children (82%), (They were the main presenting complaint in only 21 children (42%), from which 20 child were males. The majority of children 31 (75.6%) developed seizures before completing their first year of life, seven children (17.1%) between 1-5 years, and only three had late onset seizures after 5 years of life (Figure 2). |
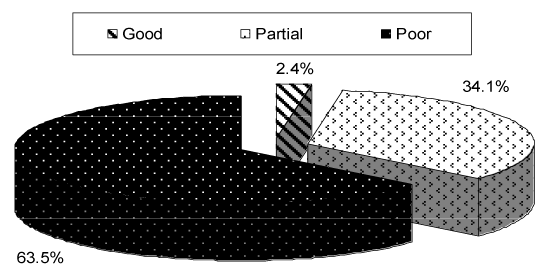
| Concerning type of seizures, as shown in Table 2, partial seizures found in 13 (31.7%) out of 41 child, generalized tonic in 6 (14.6%), infantile spasm in 4 (9.8%), generalized tonic-clonic in 2 (4.8%) while multiple seizure types were found in 16 (39%) child. Majority had very frequent, uncontrolled seizures with 36 (87.8%) patients having a seizure frequency daily from which 12 (29.3%) had more than one per day. four (9.8%) patients had seizure frequency of one per week, while one patient had seizure frequency of one per month (Table 2). From Figure 3, It is clear that only one patient had a good response to antiepileptic drugs, 14 (34.1%) child had partial response , while poor response found in the majority 26 (63.5%). |
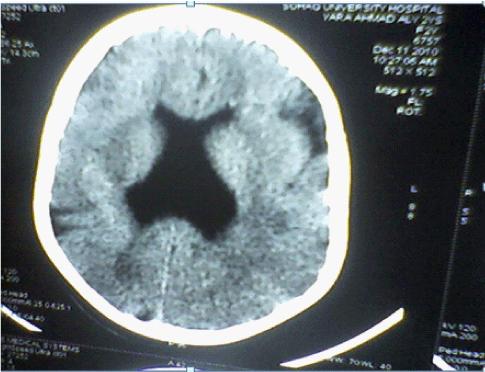
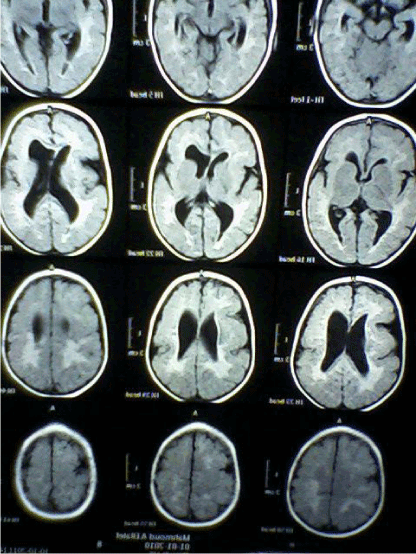
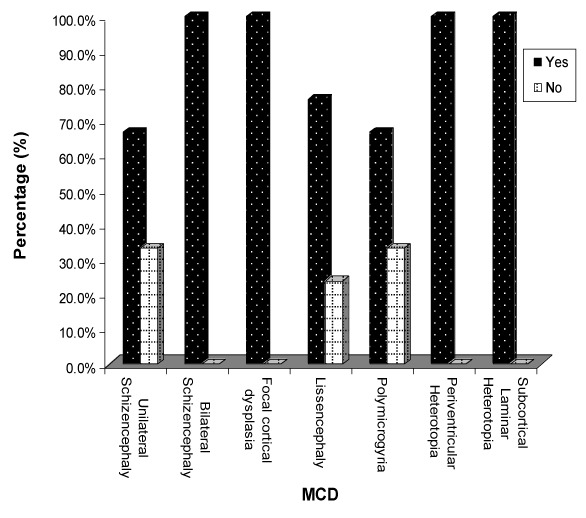
| CT Brain imaging was available in all patients, MRI Brain obtained in 45 (90%) patients. In the five patients not had MRI Brain two of them diagnosed as unilateral Schizencephaly, two as bilateral Schizencephaly, and one as lissencephaly. Table 3 and Figures 4-7 showed the Types of malformation of cortical development in the studied children as defined by neuro-imaging, It is clear from the table and the figure that 21 (42%) child had lissencephaly, eight (16%) had bilateral Schizencephaly, six (12%) had unilateral schizencephaly, six (12%) had polymicrogyria, five (10%) had focal cortical dysplasia, three (6%) had periventricular heterotopia, and one child with subcortical laminar heterotopia. |
| From Table 4 that shows the main complaint in different types of MCDs in the studied group, we can see that not all MCDs presented by the same way, lissencephaly presented mainly by global developmental delay (52.4%) followed by convulsions (33.3%) and microcephaly (14.3%), unilateral schizencephaly presented mainly by convulsions (50%) and hemiparesis (33.3), bilateral schizencephaly presented mainly by global developmental delay (87.5%) and convulsions (12.5%), FCD and periventricular heterotopia presented mainly by convulsions (80%) and (100%) respectively, polymicrogyria by convulsions (50%) and motor developmental delay (33.3), and subcortical laminar heterotopia presented mainly by global developmental delay (100%). |
| Focusing on seizures Figure 8 showed seizures in different types of MCDs, we noticed that seizures found in all children with bilateral schizencephaly, focal cortical dysplasia and periventricular heterotopia. Seizures found in two thirds of children with subcortical laminar heterotopia and unilateral schizencephaly, and in three quarters in those with lissencephaly. |
| Scalp EEG was available in 46 (92%) patients, 43 (93.5%) of them had abnormal EEG, 39 out of the 43 had clinical convulsions, while 4 had no clinical convulsions, thus EEG was abnormal in 66.7% (4/6) of the cases without clinical convulsions (note that the total cases without convulsions are nine, but three of them not had EEG). Among this, 25 (50%) patients had generalized epileptic form discharges 12 (48%) with lissencephaly, 7 (28%) with bilateral schizencephaly, 3 (12%) with polymicrogyria, 2 (8%) with periventricular heterotopia and 1 (4%) with subcortical laminar heterotopia). Of the 14 (28%) patients that had focal epileptic form discharges, 6 (42.9%) of them with unilateral schizencephaly, 5 (35.7%) with FCD, 2 (14.3%) with polymicrogyria, and 1 (7.1%) with periventricular heterotopia. 4 (8%) of patients had hypsarrhthmia abnormality, all of them had lissencephaly. EEG was normal in three patients, two with lissencephaly and one with polymicrogyria. In the four cases not had EEG, three of them had lissencephaly and one had polymicrogyria. Of the 46 cases that had EEG, It showed bilateral synchronous and diffuse epileptic form discharges in 57.1% of patients with lissencephaly. Patients with unilateral schizencephaly and FCD had mostly focal epileptic form discharges. Patients with bilateral schizencephaly and subcortical laminar heterotopia had mostly generalized epileptic form discharges. 66.6% of cases with periventricular heterotopia and 50% of those with polymicrogyria had generalized epileptiform discharges (Table 5). |
| Discussion |
| Malformation of Cortical Development (MCD) corresponds to a broad spectrum of cerebral lesions resulting from cortical development abnormalities during embryogenesis [7]. Malformations of Cortical Development (MCDs) are increasingly recognized as important cause of epilepsy, especially refractory epilepsy. |
| The present study performed a clinical analysis and reviewing the data of 50 children having MCD regarding the type of MCD by neuroradiological findings (CT or MRI Brain or Both) with special consideration for the presence of epilepsy and its characteristics, analysis of neuro developmental status. Concerning family history, consanguinity was present in 60% of patients, results much higher than that of Thomas Mathew (12.9%) [15], this can be explained by the fact that relatives marriage traditionally present in high ranges in our country especially in Upper Egypt where we made our study. History of similar disease (seizure and/or severe developmental delay) was present in 12% of patients, results lower than that of Thomas Mathew (26.5%) [15] and that of Raymond (20%) [16]. |
| Neuroimaging, especially high-resolution MRI imaging, is of paramount importance in detecting MCDs. Gross abnormalities such as lissencephaly or schizencephaly may be identified on CT scan [8]. Diagnosis of cortical malformation was established by CT brain imaging in all patients, and MRI brain in 90% of patients. In the 10% (five) of patients not had MRI Brain, two of them diagnosed as unilateral Schizencephaly, two as bilateral Schizencephaly, and one as lissencephaly. Similarly, in Thomas Mathew study, where MRI imaging was available in 93.5% of patients [15]. Majority of the patients (42%) had lissencephaly followed by schizencephaly (28%), polymicrogyria (12%), FCDs (10%), periventricular heterotopia (6%) then subcortical laminar heterotopia (2%). In Thomas Mathew study majority of the patients (31%) had multiple abnormalities, the commonest being a combination of pachygyria and heterotopia [15]. In Güngör study 53.5% were diagnosed with polymicrogyria, 22.8% lissencephaly, 11.9% with schizencephaly, and 11.9% with heterotopia [17]. In the study of MCDs, among intractable focal epilepsy by Janszky, nine patients had focal cortical dysplasia, followed by heterotopia in six patients and pachygyria in six patients [18]. Heterotopias were the commonest abnormality in the series of Raymond [16], while schizencephaly being the commonest finding in the series of Brodtkorb [19]. Differences between different case series probably reflect selection bias rather than true frequencies. |
| We found that the most common clinical features of MCDs are refractory seizures and/or global developmental delay, and the most valuable tool in diagnosis is brain MRI. Therefore one should do MRI brain to any child with refractory seizures and/or global developmental delay. Seizures found in 82% of the study group (these were the main presenting complaint in 42%) with no significant risk factor as regarding sex distribution, this was slightly higher than results of Leventer who found seizures in 75% of patients with MCDs [20], and that of Güngör that found seizures in 71.3% [17]. According to our study, seizures due to MCDs are of early onset, frequent, intractable and mostly of partial and multiple seizure type. The mean age of onset of epilepsy was about one year old, and this was lower compared to 2.7 years in the study of Güngör et al. [17] and 5 years in study done by Mathew et al. [15]. This findings could be explained on the basis that our study included children from birth to fifteen years old while that of Güngör included those between one month and nineteen years and that of Thomas Mathew included those between one month and twenty-seven years, so in our study there were cases below one month and no cases above fifteen years unlike that of Güngör et al. and Thomas Mathew et al. [17,15] this led to decrease in the mean age. Also lissencephaly found in 42% of children in our study compared to 22.8% in Güngör study, and because the onset of epilepsy tend to be younger in patients with lissencephaly and older in patients with heterotopias [17,21,22], this led to more decrease in the mean age. |
| Concerning response to antiepileptic drugs, forty out of fortyone (97.6%) patients had refractory/difficult to treat epilepsy and were on two or more antiepileptic drugs. Twenty six (63.5%) of them received three or more medications, indicating the refractoriness of seizures in this class of patients. These results agree with that of Thomas Mathew, who found that twenty-seven out of 34 (79.4%) patients had refractory/difficult to treat epilepsy and were on two or more antiepileptic drugs. Nearly three-fourth of them received three or more medications [15]. MCDs are known for their intrinsic epileptogenicity and refractoriness. Aronica and Spalice reported that there is high expression of metabotropic glutamate receptors (mGluRs) especially mGluR1a and mGluR5 in the dysplastic neurons, suggesting a possible contribution of glutamate receptors in the intrinsic and high epileptogenicity of dysplastic cortical regions [23,24]. This point is very important in counseling of parents of these patients. Concerning type of seizures, the most common were partial and multiple seizure types forming more than two thirds (70.7%) of children with seizures (partial seizures found in 31.7% and multiple seizure types were found in 39%). For other types generalized tonic found in 14.6%, infantile spasm in 9.8%, and generalized tonic-clonic in 4.8%. This agree with the study of Thomas Mathew, the most common were also partial and multiple seizure types forming more than two thirds (79.4%) of children with seizures, but partial Seizures found in 55.9% and multiple types of seizures in 23.5%. For other types, generalized tonic found in 5.9%, myoclonic seizure in 2.9% and generalized tonic-clonic in 11.7% [15]. In the study of Güngör, 32.7% of patients had generalized seizures, and 25.7% had complex partial seizures [17]. This difference may be due to that types of MCDs not present in the same percentage in every study. Therefore we should suspect that child has MCDs if presented with frequent, intractable, and early onset seizures, and especially if partial or multiple types of seizures. |
| EEG was obtained in only 46 children as four families refused to conduct EEG thinking that it is harmful for their children. Half of cases had generalized epileptiform discharges, we disagree with Thomas Mathew who found that focal epileptiform discharges were the commonest EEG abnormality [15], but there was no specific pattern consistent with the findings of other studies. This disagreement can be explained by that types of MCDs not present in the same percentage in the two studies (lissencephaly represent majority of cases (42%) in our study, but in that of Thomas Mathew the commonest being a combination of pachygyria and heterotopia). EEG should be seen as an investigational tool, but not one that defines the condition. But this along with video EEG could be used while planning for surgical mode of treatment of MCDs. We do not have video EEG data in this study as this facility was not available. Epilepsy is the most common problem in MCD. Epilepsy and EEG findings of patients with MCD are variable and seem to be correlated with the extent of cortical involvement [25]. |
| Global developmental delay was the main complaint in 40% of children, delayed motor milestones were noted in 86% of patients, while delayed speech and social development were noted in 82% and 80% of patients respectively, these results are higher than that of Thomas Mathew in which delayed motor and mental milestones were present in 44.1% of patients [15], and the series of Raymond where delayed milestones were present in only 10% of patients [16]. This may be explained that in countries with limited resource settings like Egypt, the facilities for sophisticated imaging are not easily accessible to all, and that neuro-imaging is done only to severe cases leaving large number of mild cases undiagnosed. |
| Conclusion |
| Our study included the following MCDs: lissencephaly, bilateral Schizencephaly, unilateral schizencephaly, polymicrogyria, focal cortical dysplasia, periventricular heterotopia, and subcortical laminar heterotopia. We found that the most common clinical features of MCDs in our community are refractory seizures and/or global developmental delay, and the most valuable tool in diagnosis is brain MRI. These findings are very important in the diagnosis; management and counseling of patients with MCDs. |
| Based on this study, we recommend informing pediatricians to raise their clinical suspicion to MCDs in children presented by frequent, intractable, early onset, and partial or multiple types’ seizures and/or global developmental delay, and that MRI brain must be done then. Malformations of cortical development are an area of active research especially in the field of imaging and genetics and there are rapid developments. In future we may have better diagnostic and therapeutic options for this group of patients with MCDs. |
| Conflicts of interests |
| The authors declare that there were no conflicts of interests. |
| Acknowledgement |
| The authors thank all parents and children participated in the study for their cooperation. |
References |
|
--
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 | Table 4 | Table 5 |
Figures at a glance
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
 |
 |
 |
 |
| Figure 5 | Figure 6 | Figure 7 | Figure 8 |
Relevant Topics
- Abdominal Radiology
- AI in Radiology
- Breast Imaging
- Cardiovascular Radiology
- Chest Radiology
- Clinical Radiology
- CT Imaging
- Diagnostic Radiology
- Emergency Radiology
- Fluoroscopy Radiology
- General Radiology
- Genitourinary Radiology
- Interventional Radiology Techniques
- Mammography
- Minimal Invasive surgery
- Musculoskeletal Radiology
- Neuroradiology
- Neuroradiology Advances
- Oral and Maxillofacial Radiology
- Radiography
- Radiology Imaging
- Surgical Radiology
- Tele Radiology
- Therapeutic Radiology
Recommended Journals
Article Tools
Article Usage
- Total views: 14715
- [From(publication date):
July-2013 - Jul 05, 2025] - Breakdown by view type
- HTML page views : 10017
- PDF downloads : 4698
