Clinical Experience with Plasma Progranulin as a Biomarker in a Dementia Cohort
Received: 22-Jun-2019 / Accepted Date: 31-Jul-2019 / Published Date: 07-Aug-2019 DOI: 10.4172/2161-0460.1000472
Abstract
Background: Decreased plasma progranulin levels are a specific marker in screening for Frontotemporal Lobar Degeneration (FTLD) caused by mutations in the GRN gene.
Objective: To describe the performance of this biomarker in clinical practice.
Methods: Using a commercial kit (AdipoGen Inc), we analysed progranulin plasma levels in 436 samples from 240 cases with FTLD phenotypes, 122 with Alzheimer type dementia, 20 with Lewy body type dementia, 15 with a psychiatric phenotype and frontotemporal atrophy, plus 39 cognitively-preserved elderly controls. The GRN gene was sequenced in cases with lower plasma levels. Clinical variables were correlated with plasma levels in cases with GRN mutations.
Results: Eight probands with FTLD phenotypes (3% of all FTLD cases, 12% of FTLD with autosomal dominant family history) and plasma levels below 70 ng/ml were found to carry seven different GRN mutations. The frequency of mutation-positive cases increased to 6% when considering only FTLD cases with asymmetric atrophy, though applying this criterion would have omitted two diagnosed cases. Few false positive cases (n=5) were due to technical errors, and no false negatives (70 to 90 ng/ml) were detected. In the 60 to 70 ng/ml interval, both carriers and non-carriers were found when pulling all procedures together, although they never overlapped in a single assay. None of the cases with non-FTLD
phenotypes had GRN mutations. Plasma levels did not correlate with mutation type, age of onset, or disease stage. Asymptomatic carriers had low levels even decades before the expected age at disease onset.
Conclusion: Progranulin plasma levels are a reliable biomarker to detect the small percentage of FTLD GRNmutation carriers in our cohort, though they are not useful for clinical follow-up.
Keywords: Biological markers; Frontotemporal dementia; Plasma; Progranulin levels; GRN mutations
Introduction
Mutations in the GRN gene are one of the three major genetic causes of familial Frontotemporal Lobar Degeneration (FTLD), and the only such gene with an available peripheral biomarker [1]. Physiopathologically, GRN mutations act through a haploinsufficiency mechanism, causing a 35% loss of functional protein, which is reflected in low levels of serum progranulin, even in individuals who are still asymptomatic [2,3-5]. Over the last decade, several studies have reported plasma and serum progranulin levels to be a reliable marker to screen for the presence of GRN mutations [3-8]. However, use of this biomarker remains mostly limited to research settings and it is not commonly available in routine clinical practice.
We report our experience of analyzing this biomarker on a regular basis over the past eight years (2010-2018) for clinical diagnosis of a cohort of cases with dementia. We studied well-defined FTLD phenotypes, but also a wide sample of patients presenting disease that more closely fit an Alzheimer´s Type (DAT) or Lewy Body Dementia (LBD) diagnosis, but nonetheless displayed some frontotemporal features and/or had a strong family history of dementia.
Patients and Methods
This study presents data from a cohort of dementia patients recruited at the Dementia Clinic, Fundación Jiménez Díaz (Madrid, Spain) between 2006 and 2018, all of whom gave informed consent for the study of genetic and biological markers. A majority of cases had been recruited because of a positive family history of dementia or due to presenile onset (<65 years). The population studied (n=436) is summarized in Table 1. Patients with FTLD had been diagnosed according to published standards, following the criteria of Neary et al. before 2011, and afterwards following the revised criteria of Rascovsky et al. for behavioral FTD (FTDbv) and the criteria published by Gorno- Tempini et al. for Primary Progressive Aphasias (PPA) [9-11]. For cases with non-FTLD diagnosis the decision was made to have the progranulin biomarker analysed based on some clinical frontotemporal features (such as predominant aphasia, predominant frontal/temporal or asymmetric atrophy), a strong family history of dementia, and/or because of early onset dementia. Control samples were from cognitivelypreserved individuals, and included a subgroup of nonagenarians (n=29) and ten cases with ages between 42 and 80 years. The study was approved by the hospital research ethics committee.
| Clinical phenotype | Number of cases n= 436 | Age onset, year (mean ± SD) | Plasma Pgrn levels (ng/ml) | % with APOE4 | N* with GRN mutations |
|---|---|---|---|---|---|
| Frontotemporal dementia | |||||
| Non-fluent aphasia | 59 | 65 ± 8 | 145,05 ± 63,58 | 18 | 4 |
| Semantic aphasia | 44 | 62 ± 6 | 169,33 ± 55,20 | 34 | - |
| Behavioral variant | 115 | 66 ± 7 | 149,68 ± 67,38 | 43 | 4 |
| Behavioral variant with ALS | 8 | 60 ± 4 | 136,02 ± 58,69 | 17 | - |
| Progressive supranuclear palsy | 9 | 71 ± 7 | 136,37 ± 62,26 | 33 | 1 |
| Corticobasal degeneration | 5 | 70 ± 6 | 165,75 ± 65,49 | 40 | 0 |
| Non-FTD dementias | |||||
| Alzheimer type | 122 | 61 ± 7 | 169,40 ± 75,24 | 45 | - |
| Lewy body type | 20 | 70 ± 7 | 179,26 ± 72,80 | 37 | - |
| Psychiatric disease | 15 | 63 ± 7 | 159,13 ± 47,17 | 43 | - |
| Controls | 39 | 85 ± 14 | 132,68 ± 59,12 | 5 | - |
| ALS: amyotrophic lateral sclerosis. *Includes eight probands and one affected sibling of case 3 | |||||
Table 1: Population studied for progranulin plasma levels.
Plasma samples had been kept at -80°C. Progranulin levels were analysed by ELISA using a commercial kit (AdipoGen Inc, Seoul, South Korea) and following the standardized protocol. Samples were analysed in duplicate, and two positive control samples were included in every procedure. From 2010 to 2015, the commercial kit also included a quality control sample for each assay. Cases within the lower progranulin levels underwent GRN gene sequencing, which was performed by Secugen S.L. (Madrid, Spain). One family (Trp2* mutation carriers) was genetically diagnosed by whole exome sequencing conducted by the EO EUD Consortium at the VIB-UAntwerp Center for Molecular Neurology (Antwerp, Belgium), and this family has been reported recently in greater depth [12]. Plasma samples from mutation-positive cases were re-analysed all together in a single procedure to compare quantitative values.
An ANOVA test was used to compare plasma progranulin levels among FTD groups, and the Student t test was used to compare the FTD group with DAT, LBD, and controls. We observed relationships between plasma levels and mutation type (stop vs. missense, and among cases with the same mutation), and between clinical status (affected versus asymptomatic carriers). Finally, we searched for correlations (Pearson coefficient) between plasma progranulin levels and age at onset or years of evolution in GRN-mutation carriers, and correlations between plasma progranulin levels and age at which the sample was taken in controls.
Results
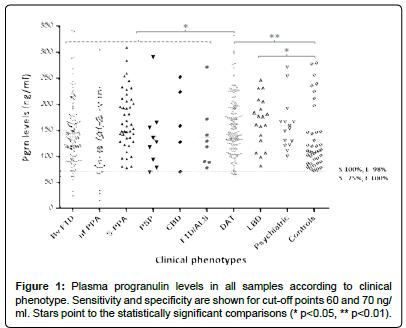
Plasma progranulin levels in all samples (except 10 outliers with levels between 350 and 450 ng/ml) are shown in Figure 1. A total of 14 ELISA analyses were conducted. We observed a wide range of values across samples, although in each individual assay there was a gap between cases with low levels which turned out to be mutation carriers and genetic-negative cases. The commercial kits maintained homogeneous accuracy and range of values over time, except for the first kit, in which the range of values was shifted significantly upwards compared to the subsequent thirteen assays.
Forty-two cases with progranulin levels <90 ng/ml underwent GRN gene sequencing. Eight cases (three with FTD bv, four with nonfluent PPA, and one with progressive supranuclear palsy) with progranulin levels ≤ 70 ng/ml were found to be carriers of seven different mutations (Table 2). Two unrelated cases carried the same M1V mutation. Six of the GRN mutation carriers had plasma levels ≤ 60 ng/ml, while another two had progranulin levels of 67 ng/ml (case 4) and 69 ng/ml (case 6), respectively. Sequencing of the gene was negative in another five cases (three FTLD, one DAT, and one psychiatric cases) with very low levels (<30 ng/ml), but these false positive cases were due to technical errors (probably incomplete sample loading during the first procedure) because re-analyses in these samples produced levels over 100 ng/ml.
| Case number c.DNA mutation NM_002087.3 | Mutation protein | Age of onset/ current age (gender) | Family history* | Clinical phenotype | Parkinsonism | Atrophy on neuroimaging |
|---|---|---|---|---|---|---|
| 1. c.1A>G | M1V | 55/61^ (F) | 1 | Nf-PPA | Right | FT Left predom |
| 2. c.1A>G | MIV | 59/67 (F) | 1 | Mixed PPA | Axial apraxia | Left Fr-Parietal |
| 3.A c.5G>A | Trp2* | 65/75^ (M) | 1 | Bv variant | Bilateral | FT Left predom |
| 3.B c.5G>A | 50/57^ (F) | Nf- PPA | Corticobasal | FT Left predom | ||
| 4. c.264+2T>C | A89VfsX41 | 53/59^ (M) | 3.5 | Nf-PPA | Corticobasal | Left perisilvian |
| 5. c.359 C>A | S120Y | 65/69 (F) | 4 | Semantic PPA | No | Left perisilvian |
| 6. c.415T>C | C139R | 60/? (M) | 3 | PSP-like | Axial, apraxia | Diffuse |
| 7. c.759_760delTG | C253X | 70/77 (M) | 2 | Bv variant/Amnestic | Right predom | Frontal bilateral |
| 8. c.909delC | A303AfsX57 | 61/? (F) | 1 | Bv variant | Right | FT Left predom |
| ^Deceased; *Goldman scale: 1. Autosomal dominant. 2. Aggregation of three or more siblings 3. One affected sibling with dementia at <65 years of age. 3.5. One affected sibling with dementia after 65 years of age. | ||||||
Table 2: Genetic and clinical characteristics of cases diagnosed with GRN mutations.
The other 29 cases (with levels >60 ng/ml) did not carry pathogenic variants. Aside from the two GRN carriers, we found nine cases with plasma progranulin levels between 61 and 70 ng/ml (five with the FTLD spectrum phenotypes and four with DAT) in which sequencing of the gene was negative. This overlap of GRN-carriers and non-carriers in the range 61 ng/ml to 70 ng/ml was only observed when pulling together all the assays, but in none of the individual procedures. We further sequenced GRN in another 20 cases with levels between 71 ng/ ml and 90 ng/ml, with negative results. Cases with higher levels (>90 ng/ml) were not sequenced, as we considered it very unlikely to detect false negatives above this point. None of the cases with non-FTLD phenotypes had GRN mutations. Assuming that there were no other positive cases in the cohort (although cases with levels over 90 ng/ml were not sequenced), the 70 ng/ml cut-off point had 100% sensitivity and 98% specificity, while the 60 ng/ml threshold displayed 100% specificity though 75% sensitivity when detecting GRN carriers.
Identifying eight probands/families implied the genetic diagnosis of 3% of all FTLD cases, increasing to 6% when considering only FTLD cases with asymmetric atrophy (six positive cases out of 98). However, this approach would have left out two diagnosed cases. There were 33 cases with an autosomal dominant pattern of inheritance in the FTLD group, and of these, four turned out to be GRN mutation carriers (12%). The clinical characteristics of the eight probands with the seven different mutations are summarized in Table 2. All except one had a presenile age of onset (mean 59.8 years, range 51 to 70). All had a positive family history except for the carrier of the S120Y mutation. Development of parkinsonism and neuroimaging studies revealing asymmetric left predominant frontotemporal atrophy were frequent features.
Plasma progranulin levels were also analyzed in six siblings of the Trp2* mutation carrier (case 3A): one affected (case 3B), one unaffected, and four at-risk offspring. The sibling with dementia and two at-risk siblings had low progranulin levels and, as expected, they were mutation carriers. Progranulin levels in all mutation carriers (as analyzed in the same assay) are shown in Figure 2. The two mutations affecting the first amino acids were associated with the lowest plasma levels. However, there was not a clear pattern of stop mutations being associated with lower levels than missense mutations. There was no correlation between plasma levels and age at onset (R=0.03), the age at which the sample was taken (R=0.03), or years of evolution of the disease (R=0.14). In fact, the lowest levels were found in a 24-year-old asymptomatic carrier.
There were no significant differences in plasma progranulin levels across different FTLD phenotypes, or when comparing FTLD cases versus controls or LBD cases. FTLD cases had significantly lower levels than DAT cases (p<0.05) (Figure 1). Both, DAT and LBD groups had significantly higher levels than the control group (p=0.006, and p=0.01, respectively). However, we do not support strong conclusions regarding comparisons with the control group due to their specific profile (majority of cognitively preserved nonagenarians), and because most of the control samples were analysed in a single assay, which could have biased the range of values. There was no correlation between plasma progranulin levels and age in the control group (R=0.14).
Discussion and Conclusion
As evidenced in this study, plasma progranulin levels are an efficient biomarker to detect GRN mutation carriers, although the percentage of positive cases in our FTLD cohort is small (around 3%). Our data indicate that a) analyzing cases outside of the spectrum of FTLD phenotypes is unlikely to reveal mutations in the gene; b) the number of positive cases significantly increases among patients with autosomal dominant inheritance and/or asymmetric atrophy, but limiting analysis to individuals who meet these criteria may cause positive cases to be overlooked; c) plasma levels do not correlate with mutation type or clinical variables; and d) asymptomatic carriers can have very low levels of plasma progranulin decades before the predicted age of onset of the disease. In addition, the Adipogen Elisa kit was found to be a reliable tool, exhibiting good performance in all procedures, with the most efficient cut-off point in 70 ng/ml (sensitivity 100%, specificity 98%).
In this large series we did not find GRN mutation carriers outside the FTLD spectrum phenotypes, which are in agreement with our previous experience, and with the extensive collaborative study of Yu et al. [13,14]. In the first reported series, some GRN-positive cases were found among cases with clinical diagnosis of DAT or LBD [15,16]. However, later refinement of diagnostic criteria for FTLD, together with a comprehensive characterization of GRN-mutation carrier phenotypes in several studies, has likely made clinicians better equipped to identify these cases among the FTLD spectrum [10,11,17-19]. The fact that the clinical phenotype of GRN-mutation carriers seems to be more homogeneous than the phenotypes associated with, for example, C9ORF72 expansion, facilitates this task even further [20].
The number of GRN-positive cases increased when our analysis was limited to the subgroups of FTLD cases with the autosomal dominant pattern of inheritance (12%), and to those with asymmetric atrophy (6%). Asymmetric cortical atrophy is present in about 76% of FTLD cases with GRN mutations [16,17]. In our cohort, however, only a minority of cases with FTLD and asymmetric atrophy were associated with GRN mutations. Moreover, asymmetric atrophy in the context of non-FTLD phenotypes was not associated with GRN mutations in any case, as previously reported [13].
The GRN mutations found in our cohort are considered pathogenic, with the exception of the S120Y and C139R missense variants [12,13,21, AD & FTD Mutation Database]. The S120Y variant, currently considered as benign, has been reported in two independent European cases with ALS, though probably coming from a common ancestor [22,23]. Factors against its pathogenic impact are that Ser120 is not a highly conserved residue, the variant has also been found in one control sample, and in one carrier there were no effects on RNA or protein levels in peripheral blood [23,24]. Furthermore, ALS in not a usual phenotype associated with GRN mutations [18,22]. However, our S120Y carrier (case 5) has a PPA phenotype and her plasma progranulin levels were found to be consistently decreased (in two independent assays), although there was no known family history. As regards the C139R variant, which is included in the AD&FTD Mutation Database as “pathogenic nature unclear”, there are reports of families with FTLD carrying this change, as well as reported cases with a PSP phenotype resembling our case [8,25]. C139R carriers also showed low progranulin plasma levels in several studies, although the values were not as low as in cases with null mutations, suggesting a partial loss of function caused by this mutation [4,5,8,13]. Moreover, in vitro studies suggest that this mutation affects the function of full-length progranulin as well as elastase cleavage of progranulin into granulins, increasing susceptibility to neurodegeneration [26].
Finally, we did not find significant differences in progranulin levels by comparing different FTLD phenotypes. All GRN-mutation carriers had reduced levels to around one third for the mean of non-carriers, but the biomarker did not correlate with any disease measure such as clinical status, age at onset of the disease, age at which the sample was taken, or years of evolution of disease. It was clear from the family with the Trp2* mutation that asymptomatic carriers could have very low levels one to three decades before the expected age of onset. Other studies have also emphasized how asymptomatic carriers had the same reduction in progranulin levels as already symptomatic siblings [27,28]. That is, progranulin peripheral level is an efficient predictor of genetic status in population at risk but it is not a useful biomarker for clinical follow-up.
Acknowledgments
We thank G. Sánchez for collecting and processing of blood samples and Oliver Shaw for editing the manuscript.
Funding
This study was supported by grants SAF2010-18277 from the Ministry of Sciences and Technology (Spain), FIS14/00099 from Instituto de Investigación Carlos III, and FEDER funds, Spain.
References
- Benussi A, Padovani A, Borroni B (2015) Phenotypic heterogeneity of monogenic frontotemporal dementia. Front Aging Neurosci 7: 171.
- Gijselinck I, Van Broeckhoven C, Cruts M (2008) Granulin mutations associated with frontotemporal lobar degeneration and related disorders: an update. Human Mutation 29: 1373-1386.
- Ghidoni R, Benussi L, Glionna M, Franzoni M, Binetti G (2008) Low plasma progranulin levels predict progranulin mutations in frontotemporal lobar degeneration. Neurology 71: 1235-1239.
- Sleegers K, Brouwers N, Van Damme P, Engerlborghs S, Gijselinck I, t al. (2009) Serum biomarker for progranulin-associated frontotemporal lobar degeneration. Annals Neurol 65: 603-609.
- Finch N, Baker M, Crook R, Swanson K, Kuntz K, et al. (2009) Plasma progranulin levels predict progranulin mutation status in frontotemporal dementia patients and asymptomatic family members. Brain 132: 583-591.
- Almeida MR, Baldeiras I, Ribeiro MH, Santiago B, Machado C, et al. (2013) Progranulin peripheral levels as a screening tool for the identification of subjects with progranulin mutations in a Portuguese cohort. Neurodegener Dis 13: 214–223.
- Schofield EC, Halliday GM, Kwok J, Loy C, Double KL, et al. (2010) Low serum progranulin predicts the presence of mutations: a prospective study. J Alzheimers Dis 22: 981-984.
- Antonell A, Gil S, Sanchez-Valle R, Balasa M, Bosch B, et al. (2012) Serum progranulin levels in patients with frontotemporal lobar degeneration and Alzheimer’s disease: Detection of GRN mutations in a Spanish cohort. J Alzheimers Dis 31: 581-591.
- Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, et al. (1998) Frontotemporal lobar degeneration: A consensus on clinical diagnostic criteria. Neurology 51: 1546-1554.
- Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, et al. (2011) Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 134: 2456-2477.
- Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, et al. (2011) Classification of primary progressive aphasia and its variants. Neurology 6: 1006-1014.
- Gómez-Tortosa E, Baradaran-Heravi Y, González Alvarez V, Sainz MJ, Prieto-Jurczynska C, et al. (2019) Presence of tau astrogliopathy in frontotemporal dementia caused by a novel Grn nonsense (Trp2*) mutation. Neurobiol Aging 76 :214.e11-214.e15.
- Gómez-Tortosa E, Guerrero-López R, Gil-Neciga E, Franco E, Del Ser T, et al. (2013) Plasma progranulin levels in cortical dementia phenotypes with asymmetric perisylvian atrophy. Eur J Neurol 20: 1319-1324.
- Yu CE, Bird TD, Bekris LM, Montine TJ, Leverenz JB, et al. (2010) The spectrum of mutations in progranulin. A collaborative study screening 545 cases of neurodegeneration. Arch Neurolo 67: 161-170.
- Rademakers R, Baker M, Gass J, Adamson J, Huey ED, et al. (2007) Phenotypic variability associated with progranulin haploinsufficiency in patients with the common 1477C-->T (Arg493X) mutation: an international initiative. Lancet Neurol 6: 857-868.
- Le Ber I, Camuzat A, Hannequin D, Pasquier F, Guedj E, et al. (2008) Phenotype variability in progranulin mutation carriers: a clinical, neuropsychological, imaging and genetic study. Brain 131:732-746.
- Beck J, Rohrer JD, Campbell T, Isaacs A, Morrison KE, et al. (2008) A distinct clinical, neuropsychological and radiological phenotype is associated with progranulin gene mutations in a large UK series. Brain 131: 706-720.
- van Swieten JC, Heutink P (2008) Mutation in progranulin (GRN) within the spectrum of clinical and pathological phenotypes of frontotemporal dementia. Lancet Neurol 7: 965-974.
- Chen-Plotkin AS, Martinez-Lage M, Sleiman PM, Hu W, Green R, et al. (2011) Genetic and clinical features of progranulin-associated frontotemporal lobar degeneration. Arch Neurol 68: 488-497.
- Gómez-Tortosa E, Prieto-Jurczynska C, Serrano S, Franco-MacÃas E, Olivié L, et al. (2016) Diversity of cognitive phenotypes associated with C9ORF72 hexanucleotide expansion. J Alzheimers Dis 52: 25-31.
- Cruts M, Theuns J, Van Broeckhoven C (2012) Locus-specific mutation databases for neurodegenerative brain diseases. Human Mutation 33: 1340-1344.
- Schymick JC, Yang Y, Andersen PM, Vonsattel JP, Greenway M, et al. (2007) Progranulin mutations and amyotrophic lateral sclerosis or amyotrophic lateral sclerosis-frontotemporal dementia phenotypes. J Neurol Neurosurg Psychiatry 78: 754-756.
- Del Bo R, Corti S, Santoro D, Ghione I, Fenoglio C, et al. (2011) No major progranulin genetic variability contribution to disease etiopathogenesis in an ALS Italian cohort. Neurobiol Aging 32: 1157-1158.
- Guerreiro RJ, Washecka N, Hardy J, Singleton A (2010) A thorough assessment of benign genetic variability in GRN and MAPT. Human Mutation 31: E1126-40.
- Bernardi L, Tomaino C, Anfossi M, Gallo M, Geracitano S, et al. (2009) Novel PSEN1 and PGRN mutations in early-onset familial frontotemporal dementia. Neurobiol Aging 30: 1825-1833.
- Wang J, Van Damme P, Cruchaga C, Gitcho MA, Vidal JM, et al. (2010) Pathogenic cysteine mutations affect progranulin function and production of mature granulins. J Neurochem 112: 1305–1315.
- Galimberti D, Fumagalli GG, Fenoglio C, Cioffi SMG, Arighi A, et al. (2018) Progranulin plasma levels predict the presence of GRN mutations in asymptomatic subjects and do not correlate with brain atrophy: Results from the GENFI study. Neurobiol Aging 62:245.e9-245.e12.
- Benussi A, Gazzina S, Premi E, Cosseddu M, Archetti S, et al. (2019) Clinical and biomarker changes in presymptomatic genetic frontotemporal dementia. Neurobiol Aging 76: 133-140.
Citation: Gómez-Tortosa E, Ruggiero M, Agüero P, Gómez A, Prieto-Jurczynska C, et al. (2019) Clinical Experience with Plasma Progranulin as a Biomarker in a Dementia Cohort. J Alzheimers Dis Parkinsonism 9: 472. DOI: 10.4172/2161-0460.1000472
Copyright: © 2019 Gómez-Tortosa E, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.