Clinical Assessment of Feasibility and Safety of an Adapted Diving Mask as an Autonomous Respiratory Support in Healthy Volunteer
Received: 06-Dec-2021 / Accepted Date: 20-Dec-2021 / Published Date: 27-Dec-2021
Abstract
Background: The scenario of global health crisis due to SARS-CoV-2 pandemic combined with the shortage of resources in many countries has justified numerous studies to design easily replicable and economic respiratory support devices. We have developed an Adapted Diving Mask (ADM) starting from Decathlon Easy breath™ diving mask which were donated to be used as an autonomous and safe respiratory support. The aim of the present study was to prove a minimum Positive End Expiratory Pressure (PEEP) with the ADM as an autonomous respiratory support without any adverse events in healthy volunteer.
Methods: The clinical assessment was done in 22 healthy volunteers recruited among Banco Santander employees with our ADM prototype. The 3 hour monitoring was carried out in the medical instalations of Banco Santander, previous informed consent, and tutored by a physician and a nurse during all the assesment. Expiratoryinspiratory flow and pressure were registered with a pneumotachograph apart from arterial blood analysis before and after the intervention as well as the transcutaneous capnography during the whole monitoring using the ADM prototype. Any deleterious effect during the monitoring was registered in the sing-in patient sheet.
Results: There were no statistically significant differences in the baseline analysis results and after therapy, except in pO2. Mean PEEP measured was 8.2 ± 4.2 cm H2O with a peak measured pressure of 20 cm H2O. No deleterious effect was observed during the monitoring.
Conclusion: The ADM has shown good tolerance and a therapeutic maintaining PEEP with no evidence of any deleterious effect or hypercapnia with its continuous use.
Keywords: Non-invasive ventilation; COVID; Non-electrical device; Mask; High flow oxygen
Introduction
In February 2020, the World Health Organization (WHO) designated the disease caused by the new coronavirus, SARS-CoV-2, originating from Wuhan, China, as COVID-19. It was declared a public health emergency in January 2020 and a pandemic in March 2020, currently exceeding the four million confirmed cases globally. This pandemic has spread over the entire territory of Spain, being one of the countries with the highest number of confirmed cases in the COVID first wave. This exponential growth of cases caused the saturation of many health systems all over the world, mainly of Intensive Care Units (ICU), causing the shortage of approved respiratory support devices (invasive and non-invasive ventilators) and stimulating the search for other effective and safe available alternatives [1]. Despite having spent one year since the health world emergency began, the expansion of the pandemic continues including new strains hitting equally developing countries and first world, unfortunately faster than the administration of the vaccine. [2]
The interstitial pneumonia is the main lung affectation due to SARS-CoV-2, causing in 41.8% of patients an Acute Respiratory Distress (ARDS) that will require of Orotracheal Intubation (OTI) or Non-Invasive Ventilation (NIV) in 19% of them. The mortality of ARSDS in coronavirus disease (COVID) exceeds 50% independently of ICU admission [3-6]. The administration of high flow oxygen with positive Pressure at the End of Expiration (PEEP), represents the minimum rescue respiratory support for many patients in a situation prior to admission to the ICU or even not the minimum but the only [7]. Although the clinical applicability of homologated PEEPgenerating devices also called Continuous Positive Airways Pressure (CPAP) devices are not very versatile, in many cases they have managed to reduce mortality and OTI. Administering a sufficient PEEP level will be able to avoid alveolar collapse and greater blood oxygenation is achieved meanwhile we save time for the patient’s recovery or until the possibility of admission to the ICU is feasible [8,9]. Approved non electronic devices have been highly demanded in the situation of sanitary crisis by SARS-CoV-2, as Pulmodyne™. This homologated autonomous device has inspired us to develop a respiratory support starting from a Decathlon diving mask, having tested previously with our monitoring machines. Desired mean pressure achieved as it was with Pulmodyne, was 5 cm H2O with a mandatory connection to high flow oxygen (>15 lpm -liters per minute).
Our clinical experience in the first wave in the emergency ward from Hospital Universitario Infanta Leonor have been recently published and the combined experience with Standford University (le tocaría el número 10 al PLOS ONE) in its lung function laboratory has accomplished the help needed in developing countries which this prototype could be the last chance to face the COVID-19 pandemic [1].
Materials and Methods
Aim of the study
The main objective of this study is to demonstrate the safety, in the context of the COVID-19 pandemic, of an alternative and inexpensive prototype of respiratory support with an Adapted Diving Mask (ADM) achieving a minimum PEEP of at least 4 cm H2O. The safety will be considered proven if no hypercapnia (pCO2>45 mmHg) was demonstrated in arterial blood of the volunteers and through continuous monitoring of transcutaneous capnography during the continuous use of the prototype during 3 hours. If appeared, any deleterious effect, apart from a possible hypercapnia, would be collected and referred in the volunteer sign-in sheet.
Human resources
Sample size for this study was calculated for a population of 310.000 inhabitants with a 95% of confidence interval and error of 20%. The clinical assessment was done in 22 healthy volunteers recruited among Banco Santander employees after clinically ruling out SARS-CoV-2 infection. Volunteers with chronic controlled diseases were not excluded for the study. All volunteers were Caucasian with a mean age of 42.45 ± 8.03 years with 86.4% males and a mean Body Mass Index (BMI) of 25.67 kg/m2 ± 4.82. Only one of them had diabetes and in no case was there a pulmonary disease.
The minimum lapse time to observe either positive or negative effect in a therapeutic conventional use ranges from one to six hours so; we decided to maintain three hours of monitoring in order to adapt human resources to the Banco Santander facilities. The 3 hour monitoring was carried out in the medical installations of Banco Santander in Madrid, with a previously signed informed consent for the monitoring with the ADM and an arterial blood gas analysis before initiating the use of ADM and at the end of the monitoring just before taking it off. Expiratory-inspiratory flow and pressure were registered with a pneumotachograph. The levels of Carbon dioxide (CO2) were registered by a transcutaneous capnography during the whole test in each volunteer during the use of the ADM. All the monitoring was tutored by a physician and a nurse. Every volunteer had its own signin sheet with the inform consent, the starting and ending time, blood gases analysis and any event or deleterious effect during the monitoring patient sheet. After the test all volunteers referred how comfortable ADM was during the monitoring.
Technical resources
Monitoring: The Aisys CS2 respirator with monitor (Datex- Ohmeda of General Electrics) was used to collect the flow and pressure signals, connecting to its side stream oxygen (O2) and CO2 sensor incorporated in the system connected in the upper connection. The record of the pressure and flow waves was carried out thanks to the iCollect software installed on an external computer. Oximetry monitoring and transcutaneous capnography (Digital monitor SENTEC Biolyne supply® with the sensor V-Sign2™ and V-STATS data processing software) were added, placing the sensor in the supraclavicular space of all patients with signal monitoring for the entire duration of the recording. Their automatic reports were generated through the V-stats software.
A portable gasometer (Epoc Blood analysis system, Siemens Healthcare®) was used for the arterial blood analysis. The first sample was extracted with patient breathing ambient air and the second sample, before removing the prototype having been at least 3 hours breathing air with a 100% of oxygen by the mask. The values of pH, pCO2, PO2, HCO3 and stO2 were recorded.
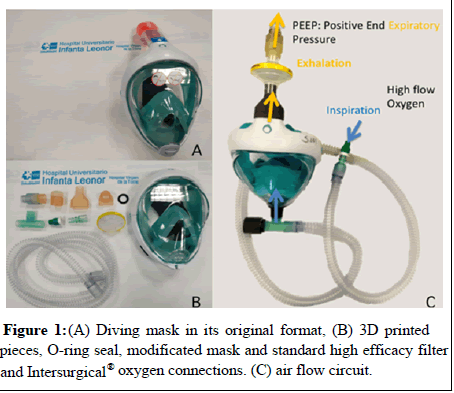
Materials: Our prototype respiratory support device starts from a diving mask from Decathlon. The Easybreath™ diving mask from 2015 (Figure 1A) was the model we modified to connect to a PEEP valve and a high flow oxygen input. A total of four 3D printed pieces were developed for this purpose by a multidisciplinary team that includes pulmonologists, emergency physicians, orthopedic surgery at the Hospital Universitario Infanta Leonor and engineers from Airbus and CT. Two of these pieces have a connector function. The remaining two are a PEEP valve with the help of a spring adjusted to the maximum pressure given and an anti-suffocation valve with the sum of a membrane already present in the original mask. The mean PEEP settled reached with the maximum clousure of the valve was 8 cmH2O. These printed pieces comply with the geometric characteristics described in UNE-EN ISO 5356-1: 2015 apt. 3 and 4. The necessary software for printing is freely accessible and distributed in standard STL format. To direct the oxygen inside the mask were assembled four Intersurgical® approved connector pieces as described below (Figures 1B and 1C).
• T-piece 22 F-22 M-22 M: references I1982, I1986 or I1985
• 22 M-15 M I1943 connector
• Estomeric connector for I1702 flow meter
• Loose high flow branches I5018.
A non-return valve was placed in the front inhalation port of the ADM, reversing the direction of the membrane that by default is in the original mask and serves as an expiratory seal in operation as a diving mask.
The indicates the direction of the inspiratory and expiratory flow of the patient, indicating the entrance of the oxygen flow with the arrangement of all the elements of the previously proposed system.
Healthy volunteers were placed in sitting position with the monitoring previously mentioned and the mask system connected to a high flow oxygen source (in this study was a 50 liter of liquid oxygen bottle with daily refill each two volunteers) through a flowmeter adjusted to more than 15 lpm (achieving a 100% of oxygen concentration measured inside the ADM).
The four pieces printed have been by Stereolithography (SLA) with the Form 2 machine (Formlabs) using a biocompatible Class IIA (I) resin (Dental LT5 Clear) with high resistance to fracture and wear, following the protocols indicated by the manufacturer including cleaning after printing in isopropanol to remove excess unpolymerized resin and a post-cure step at temperature to ensure complete conversion of the resin. With this protocol it is achieved that there are no residual monomers/oligomers that can leave the material. The resins used comply with the ISO 10993-5: 2009 Not Cytotoxic, ISO 10993-10: 2010/(R) 2014 Non Irritation, ISO 10993-10: 2010/(R) 2014 Not a sensitizer, ISO 13485: 2016 Medical Devices (Quality Management Systems-Requirements for Regulatory Purposes) and ISO 14971: 2012 Medical Devices-Application of Risk Management to Medical Devices).
Statistics
All the data collected have been processed with version 22 SPSS. A descriptive study was done to the data collected. The quantitative variables are expressed as means ± standard deviation. Categorical variables are expressed as percentages. A value of p<0.05 was considered statistically significant when studying the variation of pCO2, pO2, pH and stO2 from the start until the end of the monitoring using the ADM.
Results
The safety was proved with no adverse event registered, maintaining therapeutic pressure during the study and with no deleterious effect shown in blood gases tests or in capnography. All volunteers felt comfortable with the ADM with no complaints except if the oxygen bottle needed replacement or the oxygen input was not opened when placed on the volunteer.
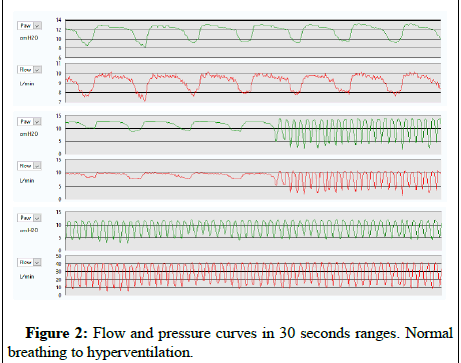
The transcutaneous capnography records showed a mean CO2 of 34.71 ± 3.55 mm Hg, (if pCO2>45 mm Hg was considered as hypercapnia and a possible deleterious effect could be appear if maintained). The mean StO2 was 96.72 ± 3.41% with no desaturation registered. There were no statistically significant differences in the baseline blood gas analysis results before and at the end of the therapy, except in pO2 which improved even starting from a normal stO2 (Table1). The printed PEEP valve was kept adjusted to its maximum closure, reaching throughout the whole sample monitored a mean PEEP of 8.2 ± 4.2 cm H2O. Figure 2 shows the evolution of the flow and pressure measured in a volunteer with ADM at baseline respiratory rate. Only for registering how high the peak pressure could be, the same volunteer was asked to cough with ADM, measuring a peak pressure of 20 cm H2O and observing a leak around the ADM with no adverse event registered. The same volunteer was asked to breathe at 30 breaths per minute during 30 seconds and the pressure did not fall under therapeutic range (<4 cm H2O) as other autonomous homologated devices behave (Figure 2).
| pH | pCO2 (mmHg) | pO2 (mmHg) | HCO3 (mmHg) | stO2 (%) | |
|---|---|---|---|---|---|
|
Baseline ABG |
7.42 | 38.43 | 91.68 | 25.26 | 96.75 |
|
ABG with ADM |
7.42 | 41.22 | 520.99 | 26.97 | 99.94 |
|
P |
0.63 | 0.51 | 0.00 | 0.51 | 0.98 |
Table 1: Baseline Arterial Blood Gases (ABG) values and previous withdrawal of ADM.
Discussion
In this manuscript we report the test results in healthy volunteers of a new respiratory device created as a result of shortness of conventional CPAP devices in the context of COVID-19 pandemic. Our prototype has met the standard of respiratory support as it was designed to, presenting the most relevant characteristics of previously approved CPAP devices (easy to use and low resource requirements as Pulmodyne®) with no deleterious effect observed, besides being reusable due to their sterilisable materials and except for the highefficiency filters used already homologated.
One of the main strengths of this prototype is its biosecurity. For most of the homologated devices used in NIV, the exhaled air through the expiratory leak is not filter or it is necessary to modify the circuit to avoid aerosolization of little particles into the environment and favoring the possibility of rebreathing depending on the dead space of One of the main strengths of this prototype is its biosecurity. For most of the homologated devices used in NIV, the exhaled air through the expiratory leak is not filter or it is necessary to modify the circuit to avoid aerosolization of little particles into the environment and favoring the possibility of rebreathing depending on the dead space of Citation : Page 3 of 6 J Respir Med, an open access journal Volume 3 • Issue 6 • 1000119 Arias-Arcos B, Collado-Escudero C, Candelario-Cáceres A, Buendia-García MJ, Abad-Gurumeta A, et al. ( 2 021) Clinical Assessment of Feasibility and Safety of an Adapted Diving Mask as an Autonomous Respiratory Support in Healthy Volunteer. J Respir Med 3:119. the mask used. With the use of the ADM, the air flow enters the mask directly to the mouth and goes across the mask “pushing” the inner air to get all the expired air out entirely filtered through the expiratory upper port where a high-efficiency electrostatic filter is adapted and avoiding the rebreathing [10,11].
All the respiratory support, regardless of the type of therapy, presents pressure fluctuation in situations of tachypnea (>25 breathing per minute) or respiratory drive increment, due to the high demand for support by the patient. If this happen, the device could not be able to provide the adequate respiratory support, even worsening the respiratory mechanic of the subject. This problem must be kept in mind in the designs of PEEP devices, although in not autonomous devices a better compensation will be expected.
In our clinical tests in healthy volunteers no drop of pressure was observed during the monitoring even in abrupt inhalation with no significant decrease in O2 concentration measured inside the mask. This is due because the pressure in our system depends in a major part of the high flow oxygen supplied, that must not be less than 15 liters per minute. The lack of interconnection leaks of the printed parts and their assembly is another cause to explain the stable pressure found in ADM.
In any non-invasive respiratory therapy, the adaptation of the interface to the patient's physiognomy is the main leaks generator, being a higher pressure the main risk factor in their appearance. In our prototype, pressures greater than 10 cm H2O were associated with around-mask leaks appeared that caused a bad tolerability and a suboptimal therapy. Due to this, the tolerable therapeutic pressure with the minimum amount of leaks was the range of 4-10 cm H2O. Fluctuation in PEEP and maximum peak pressure in the range to consider dangerous to produce lung damage was not achieved (Peak Pressure-PEEP>15 cm H2O) [12-15]. The respirator used was not able to quantify the around-mask leaks, as it is mainly for invasive use.
There were no statistically significant differences in the variation of the gasometric parameters at baseline and after therapy, except in pO2, as a consequence of hyperoxygenation caused by the exposure to continuous high flow oxygen (concentration of 100% of the air supplied). This situation is called hyperoxia and surveillance is mandatory due to the possibility of causing secondary hypercapnia and promoting alveolar collapse [16]. Hyperoxia due to external supply of oxygen can decrease the hyperventilation reflex, in patients with a tendency to retain carbon dioxide, promoting the appearance of respiratory acidosis. This is why the limitation of use for 4-5 hour shifts with intermediate breaks with less oxygen supply and a close monitoring of CO2 levels are necessary, especially in high risk patients.
The monitoring of pCO2 in our sample did not show elevation above the maximum pathological threshold (pCO2>45), ruling out hypercapnia during the registration. A tendency to mild hypocapnia (<35 mm Hg) without having detected any side effect. The choice of a minimum of 3 hours-monitoring was made based on the nonstandardized indication of non-invasive respiratory support therapy in the hospital ward, usually indicated to let the patient have breakfast, lunch and dinner once the first acute phase with 24-72 hours of uninterrupted NIV is overcame. Alveolar collapse is another adverse event of hyper oxygenation but neither was detected in our patient.
Mental status of candidates to receive NIV may vary from wide awake to the unconsciousness. In the last group re-breathing and bronchoaspiration can appear. Parenteral treatment and nutrition is recommended to avoid bronchoaspiration. In patients with conscious preserved, maintaining intravenous treatment is not mandatory, but advisable, complemented with liquid diet and might keep tight surveillance of hypercapnia. Re-breathing occurs if the patient breathes in his own previously exhaled air with a high carbonic concentration. This would take place if the circuit does not have a release valve where the expired air can escape or if the arrangement of the system eases its recirculation. In the ADM prototype, the release valve is placed at the top of the mask, where the air flow will exit through the high-efficiency electrostatic filter and the PEEP valve. The location of an anti-return membrane in the outside-in direction is directed to avoid the recirculation of the exhaled air inside the ADM, permitting the high flow oxygen to penetrate into the mask. This way, we will prevent the patient exhaled air from drifting back to the oxygen connection. Thereby, the continuous flow of oxygen will ease the upward direction of the circulating air inside the mask, finally leaving through the upper exhalation port, as was mentioned before (Figure 1C). To allow the patient normal breathing if the flow system fails, our prototype has an anti-suffocation valve to take in air from the ambient if it is necessary.
Ventilator-Induced Lung Injury (VILI) can occur in ARDS ventilated patients. COVID-19 patients with ARSD generates a high inspiratory demand in a normally compliant lung. This high demand can generate peak inspiratory pressures greater than 25 mmHg, which can be damaging to the lung causing barotrauma (pneumomediastinum and pneumothorax), volutrauma (if higher flow is provided) and atelectrauma. Also, the presence of high peak pressures in normally compliant lungs and in a situation in which the patient has a high respiratory drive, can generate alveolar shear forces that end up generating lung injury due to the wide fluctuation of pressures between the granted and the demanded by the patient. Despite in our registry there were no Peak Pressure-PEEP>15 cm H2O, it is important to mention that the study population profile is not that expected in the patients usually subsidiary to this respiratory support [12-15].
A limiting factor in the use of PEEP autonomous devices is the need of a high flow oxygen supply through a flow meter that supplies more than 15 liters per minute. This is due because with a lower flow, a sufficient pressure is not reached for the autonomous PEEP valve to be functional. It is estimated that for the continuous treatment for 4 hours, a 50-liter cylinder of liquid oxygen would be consumed. In a hospital with access to a wall-mounted oxygen output, this is not a problem. However, in circumstances of scarce resources or in developing countries this can limit the use of these devices. Nowadays our prototype has not solved this problem but our team is working on it with a new project [17-23].
Conclusion
The respiratory support of choice in patients with ARDS is invasive mechanical ventilation with OTI. This is not different in COVID patients. However, in a situation of global health crisis (COVID or not), the shortage of resources and hospitals with overflowing ICUs, respiratory support with NIV is mandatory pending the patient's own favorable evolution or the possibility of admission to the ICU later. In places without access to first level health resources, the possibility of having autonomous respiratory support devices becomes more necessary and becomes important the possibility of being sterilisable and reusable as our ADM prototype is. Our aim was not to validate this design to use in day by day hospital circumstances, but to validate the safety of our ADM as an alternative to other CPAP devices, at exceptional times and in a context of shortages of other homologated devices. It does not require electronic equipment which provides added value for exports to developing countries, in which the existences of autonomous and reusable devices can bring hope to patients who do not even have an option to be admitted to an ICU understanding and working on its main limitation which is the high flow oxygen needed.
What is known
• SARS-CoV-2 pandemic has caused a shortage of resources worldwide and have justified numerous studies to design easily replicable, reusable and economic respiratory support devices. • We have developed an Adapted Diving Mask (ADM) starting from Decathlon Easybreath™ diving mask to be used as an autonomous and safe respiratory support already used in the first wave and which experience have been already published. We have completed the safety study of this prototype evaluating a minimum Positive End Expiratory Pressure (PEEP) with the ADM as an autonomous respiratory support without any adverse events in healthy volunteer.
Conflicts of Interest
The authors certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
Funding
The study was financed and carried out thanks to Banco Santander and the assignment of its staff and facilities. Decathlon Spain gave the Easybreath masks to carry out the entire project. The design and study of materials for the parts adapted for the prototype was carried out by AIRBUS and CT engineers Printed pieces used were thanks to CSIC (ICTP-CSIC).
Authors' Contributions
Beatriz Arias-Arcos analysed and interpreted the patients’ data. Carlos Collado-Escudero collected the data. Ariela Candelario- Cáceres helped to review the bibliography. Maria J. Buendía supervised the manuscript. Alfredo Abad-Gurumeta facilitated the machines to collect the data; Jose M. Mendiguren-Santiago facilitate the installations to carry out the tests and supervise them, Ricardo Larrainzar-Garijo coordinated the tests. All authors read and approved the final version of the manuscript.
Acknowledgements
Contributors
The authors acknowledge Banco Santander for the financing of medical material needed for the monitoring, recruitment of the healthy volunteers among its employees and the availability of its facilities to carry out the study. The authors acknoledge Decathlon Spain who altruistically donated the Easybreath diving masks needed to carry out the entire project.
Participating investigator
The authors acknowledge Juan Manuel Canalejo and Alberto Molina who served as scientific advisors and designers of the adapted parts for the prototype (AIRBUS and CT ingineers). Printed pieces used were thanks to Juan Rodriguez Hernández (ICTP-CSIC).
References
- Bibiano-Guillen C, Arias-Arcos B, Collado-Escudero C, Mir-Montero M, Corella-Montoya F, et al. (2021) Adapted Diving Mask (ADM) device as respiratory support with oxygen output during COVID-19 pandemic. Am J Emerg Med 39: 42-47.
- Ministerio de Sanidad. España. Informe nº 31. Situación de COVID-19 en España a 14 de mayo de 2020. Equipo COVID-19. RENAVE. CNE. CNM (ISCIII).
- Chaomin Wu, Chen X, Cai Y, Xia J, Xing Zhou, et al. (2019) Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease Pneumonia in Wuhan, China JAMA Intern Med 7: 934-943.
- Â Bellani G, Laffey JG, Pham T (2016) Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA 315: 788-800.
- Bellani G, Laffey JG, Pham T (2016) Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA 315: 788-800.
- Yang X, Yu Y, Xu J (2020) Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 8:475-481.
- Zhou F, Yu T, Du R (2020) Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395: 1054-62.
- Cinesi Gómez C, Ó. Peñuelas RodrÃguez, Torné M l, Santaolalla C E, Jiménez J.F.M, et al. (2020) Recomendaciones de consenso respecto al soporte respiratorio no invasivo en el paciente adulto con insuficiencia respiratoria aguda secundaria a infección por SARS-CoV-2. Rev Esp Anestesiol Reanim 67: 261-270.
- Farré R, Montserrat JM, Solana G, Gozal D, Navajas D, et al. (2019) Easy-to-build and affordable continuous positive airway pressure CPAP device for adult patients in low-income countries. Eur Respir J 53: 1802290.
- Kroo L, Kothari A, Hannebelle M, Herring G, Pollina T, et al. (2021) Modified full-face snorkel masks as reusable personal protective equipment for hospital personnel. PLoS One 6: e0244422.
- Olivieri C, Costa R, Conti G, Navalesi P (2012) Bench studies evaluating devices for non-invasive ventilation: critical analysis and future perspectives. Intensive Care Med 38: 160-7.
- Simonds AK, Hanak A, Chatwin M, Morrell M, Hall A, et al. (2010) Evaluation of droplet dispersion during non-invasive ventilation, oxygen therapy, nebuliser treatment and chest physiotherapy in clinical practice: implications for management of pandemic influenza and other airborne infections. Health Technol Assess 46: 131-72.
- Cressoni M, Gotti M, Chiurazzi C, Massari D, Algieri I, et al. (2016) Mechanical power and development of ventilator-induced lung injury. Anesthesiology 124: 1100-1108.
- Silva PL, Ball L, Rocco PRM, Pelosi P (2019) Power to mechanical power to reduce ventilator induced lung injury? Intensive Care Med Exp 7: 38.
- Tonetti T, Vasques F, Rapetti F, Maiolo G, Collino F, et al. (2017) Driving pressure and mechanical power: new targets for VILI prevention. Ann Transl Med 5: 286.
- Marini JJ (2019) How I optimize power to avoid VILI. Crit Care 23: 326.
- Brugniaux JV, Coombs GB, Barak OF, Dujic Z, Sekhon MS, et al. (2018) Highs and lows of hyperoxia: physiological, performance, and clinical aspects. Am J Physiol Regul Integr Comp Physiol 315: R1-R27.
- Rochwerg B, Brochard L, Elliott MW, Hess D, Hill NS, et al. (2017) Official ERS/ATS clinical practice guidelines: noninvasive ventilation for acute respiratory failure. Eur Respir J 50: 1602426.
- Alhazzani W, Møller MH, Arabi YM, Loeb M, Gong MN, et al. (2019) Surviving Sepsis Campaign: Guidelines on the Management of Critically Ill Adults with Coronavirus Disease (COVID-19). Crit Care Med 28: 1-34.
- Ji Y, Ma Z, Peppelenbosch MP, Pan Q Potential association between COVID-19 mortality and health-care resource availability. Lancet Glob Health 8: e480.
- Truog RD, Mitchell C, Daley GQ (2020) The Toughest Triage - Allocating Ventilators in a Pandemic. N Engl J Med 382: 1973-1975.
- Murthy S, Leligdowicz A, Adhikari NKJ (2015) Intensive Care Unit Capacity in Low-Income Countries: A Systematic Review. Azevedo LCP, editor. PLoS One 10: e0116949.
Citation: Arias-Arcos B, Collado-Escudero C, Candelario-Cáceres A, Buendia-García MJ, Abad-Gurumeta A, et al. ( 2 021) Clinical Assessment of Feasibility and Safety of an Adapted Diving Mask as an Autonomous Respiratory Support in Healthy Volunteer. J Respir Med 3:119.
Copyright: © 2021 Arias-Arcos B, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 2088
- [From(publication date): 0-2021 - Nov 26, 2025]
- Breakdown by view type
- HTML page views: 1442
- PDF downloads: 646