Thesis Open Access
Clinical Analysis of 65 Patients with Pancreatic Fistula and Wound Infection after Pancreatoduodenectomy (PD) with Duct-to-Mucosa Pancreaticojejunostomy: A Single-Center Report
Mohammad Abdul Mazid, Zheng Hui Ye, Xiao-Ping Geng, Fu-Bao Liu, Yi-Jun Zhao, Fan Huang, Kun Xie and Hong-Chuan Zhao*
Department of Hepatobiliary, Pancreatic Surgery and Liver Transplantation, Anhui Medical University, China
- *Corresponding Author:
- Hong-Chuan Zhao
MD, Department of Hepatobiliary
Pancreatic Surgery and Liver Transplantation
The First Affiliated Hospital of Anhui medical University
Anhui Medical University, 81 Meishan Road, Sushan District
Hefei, 230032, Anhui
China
Tel: +8613856085670
E-mail: zhc0117@sina.com
Received date: November 28, 2016; Accepted date: December 06, 2016; Published date: December 14, 2016
Citation: Mazid MA, Ye ZH, Geng XP, Liu FB, Zhao YJ, et al. (2016) Clinical Analysis of 65 Patients with Pancreatic Fistula Wound Infection after Pancreatoduodenectomy (PD) with Duct-to-Mucosa Pancreaticojejunostomy: A Single-Center Report. J Gastrointest Dig Syst 6: 481. doi:10.4172/2161-069X.1000481
Copyright: © 2016 Mazid MA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Gastrointestinal & Digestive System
Abstract
Objective:The study sought to analyze Whipple procedure in 65 patients’ in-hospital evaluation of morbidity and mortality rate after pancreatoduodenectomy (PD) with adjusted duct-to mucosa pancreaticojejunostomy. Methods: A retrospective study of 65 consecutive patients that underwent (PD) at ‘The First Affiliated Hospital of Anhui Medical University teaching Hospital during the period of December 2008 to December 2015 was done. A two-layered duct-to-mucosa pancreaticojejunostomy over an internal transanastomotic stent was performed in all 65 patients. Results: The in-hospital morbidity and mortality rate in the study was 47.6% and 1.5%, respectively. One patient died as a consequence of mesenteric ischemia. Pancreatic fistula occurred in one patient (1.5%) and was treated conservatively with good results. The wound infection was the most common surgical complication (13/65; 20%) and occurred more often in patients who had a biliary stent inserted endoscopicallyprior to surgery (10/24; 41.7%), as compared to those without the stent (3/41; 7.3%; P<0.0001). Conclusions: The consequences of the present study recommend that a two-layered conduit to-mucosa pancreaticojejunostomy with inside trananastomotic stent is a sheltered anastomosis, connected with an okay of pancreatic fistula. The nearness of a biliary stent at the season of surgery speaks to a danger element for the improvement of postoperative injury contamination. In our information we have an aggregate number of 65 patients some of them we did endoscopy and some of them didn't. 24 patients were done endoscopy and in these cases 10 patients get wound contamination while 41 of our patients did not get endoscopy but rather 3 patients get wound disease. The summery of information is that the rate of wound contamination is high with patients is who get the endoscopy.
Keywords
Pancreatoduodenectomy; Duct-to-duct (DTD) pancreatic fistula; Pancreaticojejunostomy
Abbreviations
CT or CAT: Computed Tomography; MRCP: Magnetic Resonance Cholangiopancreatography; MRI: Magnetic Resonance Imaging; MRCP: Magnetic Resonance Cholangiopancreatography; PET: Positron Emission Tomography; IPMN: Intraductal papillary mucinous neoplasm; EUS: Endoscopic Ultrasound; PDAC: Pancreatic Ductal Adenocarcinoma; IPMNs: Intraductal Papillary Mucinous Neoplasms; PanIN: Pancreatic Intraepithelial Neoplasia; CEA: Carcinoembryonic Antigen; ERCP: Endoscopic Retrograde Cholangiopancreatography; PD: Pancreaticoduodenectomy; DNA: Deoxyribonucleic Acid
Introduction
Digestive organs
For the digestion of food, our body utilizes some organs chiefly, liver, gallbladder and pancreas. These organs produce and store enzymes and other material that helps in dissolving and digesting food.
Liver
The liver is an extensive organ located in the right upper quadrant of the abdomen, beside the stomach and behind the ribcage. Liver is an exceptional organ with double blood supply. Hepatic blood stream is around 1500 ml/min out of which portal vein contributes 80% and hepatic vein contributes 20%. One of its functional role is to manufacture a material called bile (made for the most part out of bilirubin, bile salts, and cholesterol) that is essential for fat digestion in the small intestine. The liver is separated into two flaps: a right lobe and a left lobe. The left lobbie’s further divided into other two lobes, the quadrate and the caudate lobe. Microscopically, each love comprises of hepatic cells. These cells make and drain bile into the bile channels, which convey bile to the gallbladder where it is put away until utilized by the small intestine.
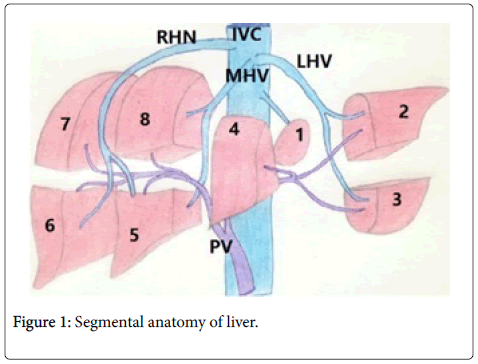
Segmental anatomy of liver
As see bib Figure 1, the liver can be divided into two segmental lobes, the right and left lobes, by a fissure that extends from the left of the gallbladder fossa to side of IVC. In the liver there are eight segments:
A) The left lobe portions are-I, II, III, and IV.
B) The right lobe portions are -V, VI, VII and VIII.
The section I is the caudate lobe and it has autonomous blood supply of bile portal vein and hepatic veins. This hepatic vein joins fuses with IVC. Right lobe has right hepatic conduit, right branch of portal vein and right hepatic vein. Left lobe has left hepatic duct, left central vein, left branch of bile duct as shown in Figure 1.
Gallbladder and bile ducts
The gallbladder is a pear-shaped, lies under the right lobe of the liver. The gallbladder stores and empties bile between at intervals. Bile is secreated at a constant rate by the liver, the capacity of gallbladder is 40-50 ml, and the size is 5-12 cm at its thickest part. The gallbladder conveys bile by the cystic, bile duct and releases bile into the duodenum at a rate of 1000 ml/day of bile.
It contains water (98%), bile salts, bile shades, unsaturated fats, lecithin, cholesterol and electrolytes (sodium, potassium, chloride, bicarbonate, calcium, magnesium) with a pH of >7.0. The portion of the bile duct that lies within the duodenum is thickened by layers of muscle called the sphincter of Oddi. Between meals, the sphincter of Oddi closes and keeps bile from entering the duodenum. During and after meals, this sphincter opens and permits bile to enter the duodenum.
Anatomy of the pancreas
Location of the pancreas
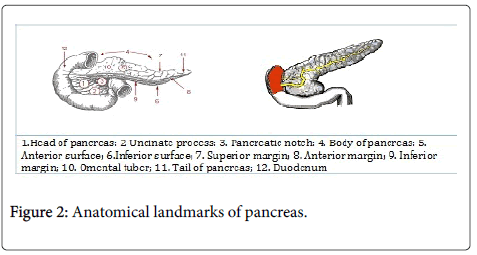
The pancreas is located behind the stomach in the left upper quandrat of the abdomen. It is secured in place by neighboring organs like the liver, spleen and intestine. It is long and like a level pear and on a level plane over the stomach area; it is divided into head, neck, body, and tail as seen in Figure 2.
The widest part of pancreas is called head and it lies in the concavity of the duodenum while the tail encroaches into the hilum of the spleen. The posterior surface of the neck of pancreas is identified with terminal piece of predominant mesenteric vein and the origin of portal vein. The pancreas comprises of exocrine tissue (95%) that produces pancreatic juice for digestion of food in the duodenum. The remaining tissue comprises of endocrine cells called Islets of Langerhans. These groups of cells look like grapes and produce hormones that maintain blood glucose levels and regulate pancreatic discharges.
Functions of the pancreas
The pancreas is an organ situated in the abdomen. The pancreas is a long, thin organ around 15-20 cm long that untruths on a level plane behind the stomach. It lies in front of L1-L2 vertebra. Exocrine and endocrine are the principle functions where exocrine function is concerned with processing the food while endocrine function controls glucose levels. A healthy pancreas produces the pancreatic juice in perfect quantities, at the correct time, to digest the foods we eat.
Exocrine part secretes pancreatic juice which helps in digestion of proteins, carbohydrates and fats. Endocrine part constitutes islets of pancreas which is distributed more numerous in tail of pancreas.
a) β-cells of islets secrete insulin.
b) α-Cell secretes glucagon.
Typical pancreatic juice is clear bicarbonate rich liquid containing around 15 gram of protein and 2.5 liters discharge per day. It alkalizes duodenal substances and helps in food processing. Latent proenzymes secreted into the duodenum in pancreatic juice are enacted by trypsin in duodenum. Amylase and lipase can likewise be secreted in dynamic structures. Basal secretion of these chemicals is very low; that can be quickly increased by hormonal and neural influences. The secretion of pancreatic juice is controlled by secretin (cAMP) and cholecystokinin (phospholipase C, calcium).
The protein content of the juice is secreted by acinar cells. The ductal cells secret liquid and electrolytes. Pancreatic secretion is low in resting stage. During eating, cephalic stage utilizes 10% of pancreatic secretion although incidental intervention by acetylcholine further animates 15% of pancreatic secretion through gastrin secretion and vagal excitement; During the fundamental intestinal stage, 75% of the pancreatic juice is secreted by the release of secretin due to duodenal fermentation, and by the release of bile and cholecystokinin after the passage of fat and proteins in duodenum.
Pathology of the pancreas
A) Pathology-non-malignant: In all tissues, the basic pathology is closely related to infections or inflammations. However, this concept is not always applicable to pancreatic pathology as it was in the year 1896, Prof. Hans Chiari, an Austrian, noticed that the inflamed pancreas did not demonstrate infectious agent and suggested that the inflammation of the pancreas was due to premature activation of its own digestive enzymes that lead to auto digestion of the pancreas.
His theory took almost hundred years to be verified as during 1996, Prof. Whitcomb and his associates discovered the cause of hereditary pancreatitis to be hereditary mutations in the genes coding for trypsinogen with over activity of enzyme trypsin.
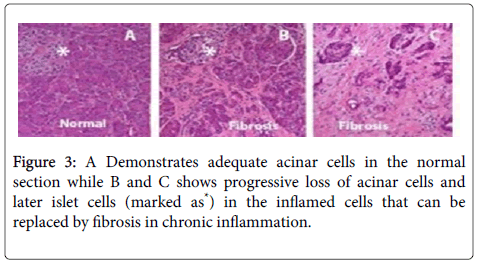
The most common non-malignant pathologies of the pancreas are acute inflammation that leads to acute pancreatitis or chronic inflammation that leads to chronic pancreatitis respectively. The progression of acute to chronic pancreatitis with resultant fibrosis (scarring) is depicted in Figure 3.
Chronic pancreatitis leads to loss of acinar cells leding to maldigestion of nutrients, inflammation and injury to nerves, activation of stellate cells that leads to fibrosis, sclerosis and distorted dilated ducts. Damage to the acinus in the islets leads to diabetes mellitus. Besides formation of calcium stones in the ducts or tissues can occur. Various forms of non-malignant pancreatic pathologies include genetic disorders (e.g. Shwachman-Precious stone disorder), pancreatic infarction, fatty replacement of pancreatic tissue, cyst growth, blockage of ducts and inflammation incided by autoimmune pathologies and infections (bacteria, viruses or parasites).
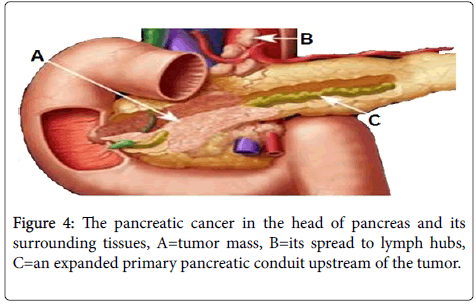
B) Pathology–malignant: The most common and severe form of malignant tumor is duct cell adenocarcinoma. Other forms are benign, borderline and malignant tumors. Figure 4 represents a pancreatic head adenocarcinoma.
Pancreatic cancer accounts for more than 35,000 in developed country USA and it continues to be one of the most dreaded cancers in the world. This cancer is difficult to detect early, early metastasis, treatment resistant and can cause death with lesser tumor burden.
The crux of combating pancreatic cancer is early detection and surgery, yet detection is prime. The role of chemotherapy is beneficial to some extent while radiation treatment remains questionable.
Risk factors of Pancreatic Cancer
The chief risk factors can be broadly divided into personal, environmental and inherited risk factors.
Personal risk factors
A) Age prevalence: After age 50 pancreatic cancer increases and thus this age is itself a risk factor. Most of the patients with pancreatic cancer range from 50-80 years.
B) Ethnicity prevalence: There is higher rate of pancreatic malignancy in Ashkenazi Jews, (ref) likely because of regular hereditary changes present in no less than 1% of people of this foundation. African Americans are likewise more prone to create pancreatic malignancy than are Asians, Hispanics, and Caucasians. These may be due to propensities like eating routine and cigarette smoking recurrence.
Environmental risk factors
A) Cigarette smoking: Smoking is the one of risk factor with increased incidence of pancreatic cancer in smokers compared to nonsmokers. The prevalence is estimated to be about 30% especially that are started before 10 years.
Health risk factors
A) Chronic pancreatitis: Inflammation of the pancreas is seen to be associated with pancreatic cancer development. Chronic pancreatitis are typically diagnosed at 35-45 years of age and can result from various variables including hereditary pancreatitis, malformations of pancreatic ducts, and injury to pancreas, or excessive consumption of alcohol for a long time.
B) Diabetes: Pancreatic cancer is seen to predominate to 2 times in patients with diabetes compared to non-diabetics, yet the underlying pathology to this occurrence is still at large. However one opinion can be that diabetes can result from impaired glucose tolerance that can be caused by pancreatic cancer.
C) Weight: A person with BMI more than 25 is considered as overweight and this feature is found to be associated with pancreatic cancer.
Inherited risk factors
A) Inherited risk: About 15% of pancreatic malignancy has been linked to familial genetic makeup. A person has 2-3 times more chances of developing pancreatic cancer if his immediate relatives (mom, father, kin, or child) have pancreatic cancer. There are several general responsible for such, moreover genes related to breast and ovarian cancer have related propensity too.
Classification of Pancreatic Tumors
The current day classification of pancreatic tumors is depicted in Table 1.
| Benign | Borderline | Malignant |
|---|---|---|
| Serous cyst adenoma | Mucinous cystic tumor with moderate dysplasia | Ductal adenocarcinoma |
| Mucinous cyst adenoma | Intraductal papillary mucinous neoplasm (IPMN) | Osteoclast-like giant cell tumor |
| Intraductal papillary mucinous adenoma | Solid-pseudopapillary tumor | Serouscystadenocarcinoma |
| Mature cystic teratoma | - | Mucinous cystadenocarcinoma |
| - | - | Intraductal papillary mucinous carcinoma |
| - | - | Acinar cell carcinoma |
| - | - | Pancreatoblastoma |
| - | - | Solid-pseudopapillarycarcinoma |
| - | - | Miscellaneous carcinoma |
Table 1: WHO classification of pancreatic tumors.
The pancreatic tumor
As known with any other tumors, the discrepancies to the control and differentiation as well as signals of cellular demise, give rise to a cancerous cell. Mutations in cell DNA can lead to uncontrolled and unnecessary cellular growth or may even prevent the natural programmed cell death phenomenon. The cause of these mutations is still under research studies. These cell growths are widely termed as tumors which can be either benign or malignant.
The difference between a benign tumor and cancer
The gross differences are presented in Table 2.
| Benign tumor | Malignant tumor |
|---|---|
| Remains in one place and stops growing | Tumor continues to grow and spread (metastasizes) |
| Does not invade other tissues or organs | Invades other tissues and organs |
| Not cancerous | Cancerous |
| Complications due to pressure effects to the nearby organs |
Table 2: Gross differences between benign and malignant tumors.
The pancreatic tumors can be either exocrine tumors (around 95% of pancreatic malignancies) or endocrine tumors (around 5% of pancreatic diseases).
The exocrine tumors
Pancreatic cancers arise in any part of the pancreas. Pancreatic adenocarcinoma, additionally called pancreatic ductal adenocarcinoma, or PDAC, is the most common type of pancreatic malignancy (95%). It originates from the cells of pancreatic duct. Other uncommon types of exocrine tumors are Acinar cell carcinoma, Adenosquamos carcinoma, and Mucinous cystadenocarcinoma.
The endocrine tumors
Endocrine tumors are growths that originate at the islet of Langerhans cells. As the tumor cells originate in the hormone producing cells, the clinical manifestations may also include signs and symptoms of excess or absent hormonal productions. These endocrine tumors constitute less than 5% of pancreatic tumors.
They are also called as neuroendocrine or islet cell tumors. Functional islet cell tumors also include insulinomas and glucagonomas, while VIPomas and somatostatinomas are less common. Nonfunctional tumors are less common and do not produce excess hormones
Diseases of the Pancreas
Clutters influencing the pancreas incorporate pancreatitis, precancerous conditions, for example, PanIN and IPMN, and pancreatic tumor. Every confusion may display distinctive side effects and requires diverse medicines.
Pancreatitis
Pancreatitis is defined as inflammation of pancreas, the results of which leads to increased secretion of pancreatic autolytic enzymes which begin to digest the gland itself. I can express as painful acute attacks or chronic form.
Precursors to pancreatic cancer
The etiology of pancreatic cancer is still obscure and we only know the risk factors. Cigarette smoking, family histories of pancreatic malignancy, chronic pancreatitis are some of the known risk factors.
Additionally pancreatic lesions like Intraductal Papillary Mucinous Neoplasms (IPMNs) and Pancreatic Intraepithelial Neoplasia (PanIN) are considered to be precursors of pancreatic cancer.
Pancreatic cancer
The most common pancreatic tumor is pancreatic adenocarcinoma, an exocrine tumor emerging from the cells covering the pancreatic duct while less than 5% are endocrine tumors
Diagnosis
Diagnosis chiefly utilizes the lab tests and imaging studies.
Lab tests
The lab tests are used as supportive to diagnose pancreatic cancers as there is no sole test that can give a clear cut confirmatory diagnosis of the disease.
Liver function test
A tumor that lies in the pathway of the bilirubin flow from the liver to the intestine can be assessed by determining the bilirubin level as it may increase. The normal range of bilirubin levels is between 0.3 and 1.3 mg/dl (milligrams per deciliter)
CA 19-9
It is a tumor marker and CA19-9 is very useful in the diagnosis of pancreatic cancer. The tumor marker CA19-9 normal range is between 0 and 37 U/ml but is raised in patients with pancreatic cancer. However all pancreatic cancer may not result in elevated levels of CA19-9 while some non-neoplastic conditions like pancreatitis and jaundice may have elevated levels of CA19-9. Thus clinical condition along with the markers levels should be correlated.
Besides, the CA19-9 levels are also used to see the effectiveness of therapy as post chemotherapy, if the levels decrease, then that indicates the treatment is effective. Conversely, if the levels rise, it may mean that there is tumor recurrence or that the chemotherapy is ineffective and needs a change.
Carcinoembryonic antigen (CEA)
CEA is also a tumor marker for pancreatic cancer. The normal amount of CEA in non-smokers is less than 2.5 ng/ml (nanograms per milliliter), and in smokers, less than 5.0 ng/ml. Like CA 19-9, CEA is used best to monitor progress and treatment response, rather than to establish a diagnosis.
Biopsy
It is a procedure where tissue from a suspected tumor is taken and observed under a microscope and studies its morphological characteristics and confirm the presence of cancer cells. Biopsies have been regarded as the gold standard while diagnosing neoplasms. There are several methods of performing biopsy that are:
Fine Needle Aspiration, where a thin needle is inserted into the pancreas. The needle can be inserted through an endoscope, or less commonly through the skin. Brush Biopsy, which involves using a small brush attached to an endoscope to gather cells in one of the ducts near the pancreas.
Laparoscopy, which is a form of surgery in which tissue of the tumor is collected through small incisions in the abdomen. Concomitant to the diagnosis, further treatment plan requires determining the correct clinical stage of the disease referring to whether, and how far, the cancer has spread in the pancreas and throughout the body. Determining the correct stage ensures the best treatment plan to extend survival and maintain quality of life.
Diagnostic imaging studies
Imaging studies provide important visual information about the pancreas and surrounding organs and blood vessels. They are crucial tools in diagnosing and monitoring pancreatic cancer. There are many types of imaging studies, each providing different information. Most imaging studies are non-invasive, but there are some that are invasive and require inserting an instrument into the body.
Depending on your symptoms, your doctor may ask that you undergo one or more of the imaging studies explained here. Each will help your physician visualize, diagnose, and monitor a pancreatic tumor.
A) Non-Invasive Tests
a) Abdominal Ultrasound
b) Computed Tomography (CT, CAT) Scan
c) Magnetic Resonance Imaging (MRI)
d) Magnetic Resonance Cholangiopancreatography (MRCP)
e) Positron Emission Tomography (PET) Scan
B) Invasive Tests
a) Endoscopic Ultrasound (EUS)
b) Endoscopic Retrograde Cholangiopancreatography (ERCP)
Non-Invasive tests
Abdominal ultrasound
Ultrasound of the abdomen is a non-intrusive test that deploys a guided sound wave to access at the body's interior organs, including the pancreas, gallbladder, liver, kidneys, spleen, stomach, and digestion tracts. These sound waves are of high frequency and can't be heard by the human ear. When the sound waves hit an inner organ they resound, making a picture called a sonogram. Since the sounds emitted by the tissue and tumors are diverse echoes, the span of the inner organs and the associated structural defects as well as the proximity of a tumor mass can be distinguished utilizing ultrasonography.
Computed tomography (CT, CAT) scan
CT is another important imaging where an X-ray machine is linked to a computer and takes a series of detailed cross-sectional pictures. These "slices" are then linked together to create a detailed 3- dimensional reconstruction of the body. It differs from a regular X-ray image as more detailed information about soft tissue and blood vessels are provided besides the bone. It can be of two types, a plain non contrast CT scan or often after the first set of pictures is taken, the patient may be asked to drink contrast dye, or may receive an intravenous (IV) line through which the dye is injected. This dye helps to better outline the body structures, show small pancreatic tumors, and to reveal whether the cancer has spread to any other organs.
Magnetic resonance imaging (MRI)
MRI use radio waves and powerful magnets to produce images of the body. Like a CT scan, an MRI can produce detailed 3-dimensional cross-sectional images of the body. The MRI can also produce image slices running the length of the body, providing an alternate view of the affected area.
Magnetic resonance cholangiopancreatography (MRCP)
MRCP is a specialized MRI scan that allows visualization of the bile and pancreatic ducts in a non-invasive way. Tumors originating in these ducts are the target of MRCP. Furthermore, the suitability of a surgical candidate for pancreatic procedures is assessed by MRCP.
Positron emission tomography (PET) scan
PET scans create images based on the metabolic activity of cells in the body. A small amount of radio labeled glucose is injected into the bloodstream. The glucose is taken up and metabolized by the tissues. The PET scanner reads the signal emitted by the radio labeled material to produce computer-generated images of your body. Since cancer cells metabolize more glucose than normal cells, they "light up" more brightly on the PET scans and can help your doctor pinpoint your disease.
PET scans may also be helpful in differentiating benign masses, such as cysts, from cancerous tumors. They may also help to identify small metastases to the liver and other surrounding organs that do not show up on CT or MRI scans. PET scans are now often done in conjunction with CT scans to provide a complete image of the body that including both molecular (PET) and anatomical (CT) visual information.
Invasive Tests
Endoscopic ultrasound (EUS)
EUS is one of the most useful imaging studies for diagnosing pancreatic cancer. It provides detailed images of the pancreas and surrounding tissues including the liver, blood vessels, and lymph nodes. Detailed pictures can be produced, and small tumors in the pancreas can be detected as the ultrasound probe to get very close to the pancreas and its surrounding organs.
Endoscopic retrograde cholangiopancreatography (ERCP)
ERCP is most often performed when a patient exhibits symptoms of jaundice, which can indicate presence of a mass narrowing or blocking the ducts. It visualizes the bile and pancreatic ducts clearly. It is an outpatient procedure where a catheter is threaded through the endoscope and inserted directly into the pancreatic and bile ducts. Dye is injected through the catheter and into the ducts and then an X-ray is taken. If a blockage, or stricture, is found, the physician can intervene by placing a stent into the obstructed duct. A stent is a device that helps hold the duct open to allow bile and pancreatic juices to flow properly.
Treatment
The best treatment of pancreatic disease depends on the spread and stage (0-IV) of the disease, although staging the pancreatic tumor is more accurate.
Stages of pancreatic cancer
Stage is used in the determination and treatment of pancreatic disease and to depict the degree of spread. The progression of disease/ pancreatic cancer progression can be broadly divided into 5 stages (0- IV) that are:
Stage 0: No spread. Pancreatic disease is limited to a solitary layer of cells in the pancreas. The pancreatic growth is not unmistakable on imaging tests or even to the naked eye.
Stage I: Neighborhood development. Pancreatic malignancy is restricted to the pancreas, [<2 cm cross-sections (stage IA) or >2 centimeters (stage IB)].
Stage II: Neighborhood spread. Pancreatic tumor has lies outside the pancreas, or has spread to close-by lymph nodes.
Stage III: More extensive spread. The tumor has ventured into closeby real veins or nerves however have not metastasized.
Stage IV: Affirmed spread. Pancreatic growth has spread to far off organs.
Deciding pancreatic growth's stage is frequently precarious. Imaging tests like CT scan and ultrasound can provide some data; yet knowing precisely how far the pancreatic disease has spread, normally requires surgery. Since surgery can lead to complications, a surgeon first figures out whether the pancreatic growth can be removed by surgery (resectable) or not.
Resectable: The disease hasn't spread (or if not far) on imaging studies, and if the specialist feels that it is removable. Around 10% of pancreatic tumors are viewed as resectable when initially analyzed.
Locally progressed (unrespectable): Pancreatic malignancy that has developed into vasculature on imaging studies, so that the tumor cannot be removed by surgery.
Metastatic: Pancreatic tumor has plainly spread to different organs, so surgery can't eliminate the disease.
Pancreaticoduodenectomy (PD)
A pancreatoduodenectomy, by Kausch in the pancreas surgery major surgical operation is the whipple strategy. Removal of the pancreas, duodenum, gallbladder and further different organs are included in the Whipple’s method of surgical operation. This operation is performed on carcinogenic tumors.
History of the pancreaticoduodenectomy (PD)
The first PD was described by Alessandro Codivilla in 1898, an Italian surgeon. The resection for a periampullary disease was firstly performed in 1909 by German surgeon Walther Kausch and was described in 1912 by Kausch. It is commonly called as ‘the Whipple’s procedure’, named after an American surgeon Allen Whipple who introduced and developed an enhanced variant of the surgery in (1934-1935). Since then, various refinements to his system and Whipple one-stage technique (1940) have/are being done.
Pancreatoduodenectomy (PD) is a method that employs major surgical strategy that includes both the pancreas and duodenum. This operation is typically performed to treat carcinogenic tumors on the head of the pancreas, malignant tumors of the bile duct, duodenal papilla, or duodenum that lies in close proximity to the pancreas and in some instances of pancreatitis. PD is a major procedure that demands surgical competency and a specialized care [1-5].
The chief reason that makes this procedure hypercritical is the occurrence of pancreatic fistula during pancreatic anastomosis creation. It has been found that even in standard specialized critical specialties the occurrence of pancreatic fistula ranges from 0-2% [3,6-8] to more than 20% in some cases [9-12].
Besides this dreaded complication that adds to significant morbidity and mortality, the anastomosis reconstruction procedure may lead to local abscess formation, sepsis, delayed gastric emptying and postoperative intra-stomach bleeding due to autolytic activity of the pancreatic juice [1-5].
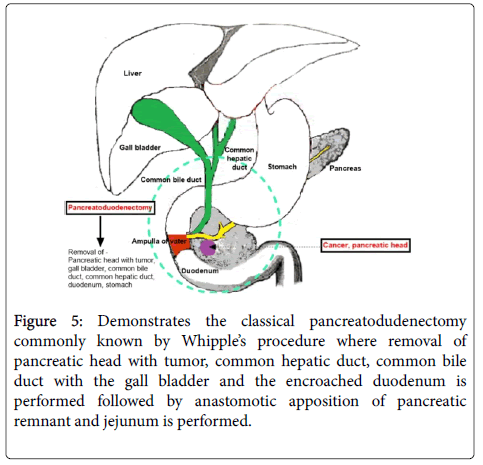
Basically during PD as shown in Figure 5, the common hepatic duct, the common bile duct along with the gall bladder, the cancerous pancreatic head as well as the encroached duodenum is excised. A direct anastomosis between the remaining pancreatic remnant and the jejunum is made.
Figure 5: Demonstrates the classical pancreatodudenectomy commonly known by Whipple’s procedure where removal of pancreatic head with tumor, common hepatic duct, common bile duct with the gall bladder and the encroached duodenum is performed followed by anastomotic apposition of pancreatic remnant and jejunum is performed.
Different centers deploy various methods of pancreatic anastomosis and there is no single consensus as to the best possible approach and strategy [13-15]. Simple classical pancreaticojejunostomy have been the most widely well-known approach that comprises of either invagination technique or duct to mucosa pancreaticojejunostomy. These procedures further, may or may not deploy a stent between the anastomosis. In this study, we report our experience with the use of the classical procedure by creating an anastomosis in a single layered duct to mucosa pancreaticojejunostomy and highlight its consequences in terms of mortality and occurrence of pancreatic fistula.
Methods and Materials
Data collection
A retrospective study was conducted in all 65 patients that underwent pancreatoduodenectomy at the department of Hepatobiliary, Pancreatic Surgery and Liver Transplantation between the periods of January 2008 and December 2015. All the records were extracted from the hospital database as well as from follow up clinics.
The pre-operative parameters that were considered for analysis included–patient demographics, co-morbidities, history of current illness, past illness, laboratory data and imaging data. The patients that underwent diagnostic computed tomography were only considered for the surgery.
Similarly, the evaluation of operability was done by endoscopic ultrasound as well as endoscopic retrograde Cholangiopancreatography in selected cases.
The surgery
The surgical procedure was performed by four PD specialists. The classical Whipple’s technique was employed for the anastomosis construction. Firstly, the proximal jejunum was brought from behind the colon to the right half of the middle colic vessels. Then, modified Cattell's pancreaticojejunostomy [16] was performed first and the most proximal part of the loop in an end to side manner in two layers.
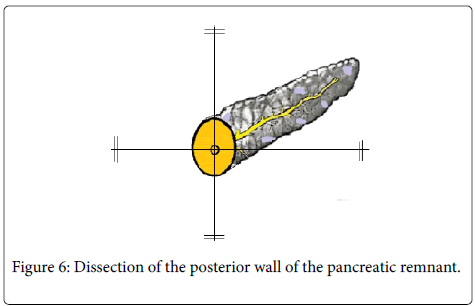
For these procedures, absorbable monofilament polydioxanone sutures (Johnson and Johnson: USA) with atraumatic needles were used. The procedure utilized is depicted in Figure 6 where the back wall of the pancreatic remnant was dissected off the splenic vein as well as from the retro peritoneum for a distance of around 2 cm from its cut edge.
This was followed by application of four 6/0 double armed interrupted sutures on the pancreatic duct at 3, 6, 9 and 12 o'clock position before starting the anastomosis procedure. These sutures were placed in an inside-out manner and if the pancreatic duct caliber was small then loupe magnification (2.5X) was performed.
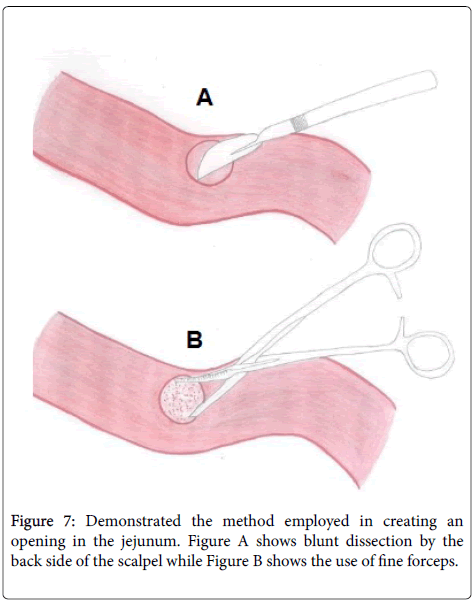
This was followed by seromyotomy of the back of the jejunum using the back side of the scalpel (Figure 7A) and the mucosa was removed off the seromucosal layer by the use of fine forceps (Figure 7B) for the necessary space that the pancreatic stump remnant would occupy in the anastomosis.
Finally, the outermost layers of the anastomosis were apposed by means of a running suture in an inside-out manner on the side of the pancreatic stump, taking enough pancreatic tissue from the pancreatic stump.
Anastomosis details
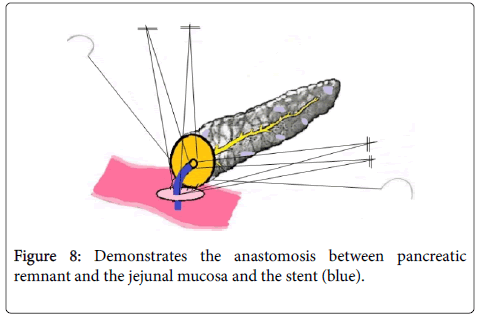
Firstly external anastomosis was created by connecting the posterior walls of the external layer of the divided pancreatic neck and the seromuscular layer of jejunum using a 4/0 running suture (Johnson and Johnson). It was made sure that the pancreatic duct was excluded from the suture line by placing a stent into the duct. The size of the stent (4-10 Fr) to be inserted into the pancreatic duct was same as the internal diameter of the pancreatic duct extent and accordingly an aperture (1-2 mm) was created into the jejunum. This was followed by duct to mucosa anastomosis between the main pancreatic duct and the jejunal mucosa with four interrupted 6-0 sutures over the inner transanastomotic stent.
The length of the stent used was about 8-10 cm and it was inserted half into the main pancreatic duct and half into the jejunum. The stent was supported by one of the sutures of the pancreatic duct and the sutures closed after all the sutures participating in the anastomosis were tied down. The knots were placed externally in relation to the anastomosis. This was followed by performing anastomosis between the anterior walls of the divided pancreatic neck and the jejunum with the help of abundant bites of 4/0 running sutures (Figure 8).
Care was always taken to avoid inadvertent pulling of the holding suture by the assisting surgeon so as to avoid laceration and cutting of the freshly sewn pancreatic remnant tissues. Following this procedure, two drains with negative suction were placed in the pancreatic anastomotic territory as well as nasogastric tube in the stomach. For the purpose of enteral feeding, a needle-catheter feeding jejunostomy was placed in all patients.
Post procedure, the patients received standard post-operative care. This comprised of continuation of antibiotics for 24 hours, proton pump inhibitors, and also subcutaneous octreotide 3 × 100 μg every day for 7 days and low molecular weight heparin until the patients were discharged. The NG tube was taken out the next day or later if the amount of drainage was less than 500 ml/day. The patients were allowed to drink water at day 6 of surgery while enteral feeding was continued upto the 7th.
Post-operative day with progressively introducing pancreatic (low fat) diet as tolerated. The abdominal drains were checked regularly and specifically for the amylase content during the 2nd and 5th postoperative day to detect formation of pancreatic fistula. The pancreatic fistula was diagnosed if the drain, however small the content be, contained amylase that was three times the value of plasma amylase levels after the third post-operative day [17,18].
Similarly, if the amylase content in the drain was less or if the drain had fluid volume less than 100 ml, then the drain was removed on the fourth postoperative day. Likewise, delayed gastric emptying was diagnosed if the stomach aspirate contained fluid volume of more than 500 ml/day for ≥ 10 days of surgery or if the patient could not take in normal food or persistent vomiting requiring NG tube reinsertion by 14th post-operative day [19,20].
The overall complications were evaluated by 5-grading scale proposed by De Oliveira et al. [21] that is depicted in Table 3. The mortality incorporated every single death that occurred within the 30 days of procedure including hospital deaths.
| Grade | Definition |
|---|---|
| I | Any deviation from the normal postoperative course without the need for pharmacological treatment or surgical, endoscopic, and radiological interventions. Allowed therapeutic regimen are: drugs as antiemetics, antipyretics, analgesics, diuretics, electrolytes, and physiotherapy. This grade also includes wound infections opened at the bedside. |
| II | Requiring pharmacological treatment with drugs other than such allowed for grade I complications. Blood transfusions and total parental nutrition are also included |
| III | Requiring surgical, endoscopic or radiological intervention |
| IIIA | Intervention not under general anesthesia |
| IIIB | Interventions under general anesthesia |
| IV | Life- threatening complication (including CNS complications)* requiring IC/ICU management |
| IVA | Single organ dysfunction |
| IVB | Multiorgan dysfunction |
| V | Death of patient |
| Suffix ‘d’ | If the patient suffers from a complication at the time of discharge, the suffix‘d’ (for disability) is added to the respective grade of complication. This label indicates the need for a follow up to fully evaluate the complication. |
| *Brain hemorrhage, ischemic stroke, subarachnoid bleeding, but excluding transient ischemic attacks. CNS, central nervous system, IC -intermediate care, ICU-intensive care unit. | |
Table 3: Shows the standard grading scale proposed and widely used by surgeons worldwide regarding post-operative grading of complications following PD procedure.
Statistical analysis
All the collected data’s were tabulated and were subjected to rigorous statistical analysis. The statistical method used was Yates correction and Fisher tests applying the SPSS (version-16, Chicago, USA) program.
The p-value < 0.05 was considered as statistical significant. The graphs and figures were constructed by graph pad prism and paint brush.
Results
A total of 65 patients underwent PD out of which 39 were male and 26 were female patients and the mean age of the patients were 60 (range 45-76) years and most were 60% (39/65) were men. The surgical indications considered for the procedure are outlined in Table 4. Preoperative endoscopic biliary stenting was doing in 24 patients.
| Age* (years) | 60.8 (45-76) |
| Male/Female | 39/26 |
| Malignant tumor | 55 |
| Histological diagnosis | |
| Pancreatic head cancer | 41 |
| Distal bile duct cancer | 2 |
| Ampullary cancer | 8 |
| Metastasis to the pancreas | 1 |
| Other | 3 |
| Benign tumor | 10 |
| Chronic pancreatitis | 7 |
| Cystadenoma | 1 |
| Ampullary adenoma | 2 |
| *data expressed as median (range) | |
Table 4: Patient characteristics.
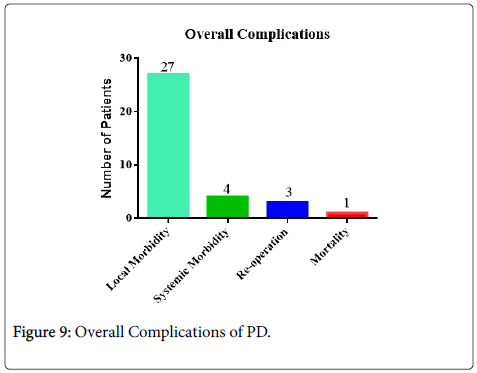
It was observed that the occurrence of post-operative complications was in 47.6% of patients (31/65) out of which 41.5% and 6.1% were local and systemic complications respectively (Table 2) also depicted in Figure 9. When the complications were categorized into standard grading system (Table 1), it was found that the patients had major complications in 5% (≥ grade 3) that required surgical treatment (n=2) or ICU management (n=1). Similarly, two patients (3%) had to undergo reoperation as one of them developed intra-abdominal hemorrhage while the other had mesenteric ischemia. The latter patient had to be admitted to the ICU too due to development of multi-organ failure due to development of pancreatitis of the remnant pancreas. The median length of hospital stay was 25 (territory 13-47) days.
The injury contaminations were brought about by Escherichia coli and Enterococcus faeciumand determined after run of the mill administration with anti-microbials and waste. Pancreatic anastomotic hole happened in standout patient (1.5%) with the nonappearance of any indications and the conclusion made on the third postoperative day by routine assessment of amylase substance in the seepage liquid. The patient was treated with support of outside seepage, all out enteral sustenance by means of the sustaining jejunostomy, intravenous antiinfection agents (piperacillin/tazobactam 4.5 gm 3 times every day) and proceeded with octreotide organization subcutaneously (0.1 mg 3 times day by day) until the spillage halted on the 21st postoperative day. The registration figured tomography examine did not uncover any irregularity and the patient was released home 24 days taking after surgery.
Local complication in the form of intra-abdominal hemorrhage was observed in 2 patients on day 2 and day 5. These patients were urgently taken to the OT and urgent re-laparotomy was performed followed by ligation of mesenteric roots that were the source of bleeding. Moreover, one of the patients developed biliary leakage after the procedure at day 6 and again after 1 day of performing re-laparotomy. This patient was placed with an abdominal drain until no fluid drained out that was for about 29 after which the drain was taken out. Unfortunately, the patient developed tight biliary stricture at the anastomosis site at 12 months and is still in continuous follow up and is being treated with percutaneous trans-hepatic biliary drainage procedure. He is well till now. He still has the trans-anastomotic biliary channel at 30 month of follow-up.
Among the local complications, wound infection was most common that occurred in 20% (13/65) of the cases. 41.7% of these patients (10/24) had endoscopically implanted biliary stent preceding surgery while 7.3% of the patients (3/41) did not have biliary stents before surgery (P< 0.0001). Pathological reports obtained from the laboratory revealed that wound infection was chiefly caused by bacterial infections with Escherichia coli and Enterococcus faecium which were successfully treated with antibiotics.
Pancreatic anastomotic leakage was observed in 1 patient (1.5%) that remained asymptomatic and the diagnosis was only revealed in 3rd post-operative day when the drain fluid revealed significant higher levels of amylase. The patient was thus treated with continuous external drainage of the fluid, enteral feeding via feeding jejunostomy, intravenous antibiotics (piperacillin/tazobactam 4.5 gm 3 times every day) and subcutaneous Octreotide (0.1 mg 3 times day by day). This treatment was followed until there was no fluid in the drain, which was seen at 21st post-operative day. The absence of leakage was confirmed by a CT scan and the patient was discharged at 24 days of the surgery.
Similarly as depicted in Tables 3 and 4 patients suffered from systemic complications, where pneumonia predominated and some patients also developed MI and stroke. All of these patients were managed with standard medical that recovered.
There was 1 case of in hospital death that occurred as a result of major post-surgical complication, the patient died because of serious stomach sepsis as a result of extensive intestinal necrosis that developed at day 6 of surgery. This patient was diagnosed as a case of pancreatic head carcinoma, thus the superior mesenteric artery was dissected as the tumor mass had encroached the artery and reaching the border of tumor was mandatory. This patient died at day 9 (Table 5).
| Post-operative complication | Total number of patients N=65 |
|---|---|
| Overall morbidity | 31 |
| Local morbidity | 27 |
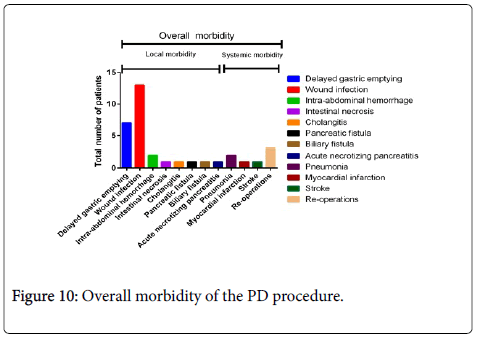
| Delayed gastric emptying | 7 |
| Wound infection | 13 |
| Intra-abdominal hemorrhage | 2 |
| Intestinal necrosis† | 1 |
| Cholangitis | 1 |
| Pancreatic fistula | 1 |
| Biliary fistula | 1 |
| Acute necrotizing pancreatitis | 1 |
| Systemic morbidity | 4 |
| Pneumonia | 2 |
| Myocardial infection | 1 |
| Stroke | 1 |
| Re-operations | 3 |
| Mortality | 1 |
| Hospital stay(days)* | 25(13-47) |
Table 5: Post-operative complications.
Discussion
Among commonly performed surgical procedures, PD is considered to be one of the most challenging procedures as it is associated with significant mortality and morbidity. It has been reported that even in specialized units, the occurrence of morbidity and mortality with this procedure is observed to be 40-60% and 0-5% respectively [3,22-25]. The occurrence of pancreatic fistula as the major complication carries the major bulk of complication and significant amount of in-hospital mortality while 20-40% postoperative deaths [3,9-12,26-28]. Are contributed by other procedural complications like sepsis, peritonitis, abscess formation or intra-abdominal hemorrhage. Although there are many causes of pancreatic fistula formation, surgical causes or iatrogenic causes chiefly revolves around per procedural causes. The diseased pancreatic tissue is very fragile with soft texture and excising the tissue as well as suturing the tissue becomes difficult. Tying the knot around the pancreatic remnant primarily determines whether there will be any leakage or fistula in the future or not.
The stability and viability of the knot still depends on the amount of texture and fatty infiltration on the gland which are the main risk factors for knot stability and development of pancreatic fistula [25-30]. Besides, the delicate diseased pancreas may produce large amount of pancreatic juice compared to fibrosed pancreas. This has been validated by studies that have demonstrated lower occurrence of anastomosis based complications in fibrosed pancreatic tissue (chronic pancreatitis) compared to friable soft textured pancreatic tissue [31]. Thus numerous researches have aimed at finding a suitable agent or a suitable method to address this issue of anastomotic leakage and recently trans-pancreatic U-sutures have also been suggested so as to avoid or minimize tangential sheer forces during knot tying so as to avoid the extra stress in the tissue by the knot [28,32].
In this study, we observed that the occurrence of pancreatic anastomotic leakage after PD was significantly lower (1.5%) with modified duct to-mucosa pancreaticojejunostomy procedure. This finding of ours was in lieu with previous findings of some studies conducted earlier [3,33,34]. However, this finding was also contradictory with other studies that have found the reverse [35-37]. These lower rates of pancreatic anastomosis leaks have also been documented in both series that do and do not use pancreatic stents [3,28,37]. A noteworthy technique that they have deployed that differs from ours was that they constructed an external layer of the anastomosis with interrupted sutures and did not use pancreatic duct stent for duct to-mucosa anastomosis. Furthermore they also provided a buttress to the pancreatic stump by providing some horizontal mattress U-sutures in cases of soft and intermediate textured pancreas. The goal of these procedures was similar to our technique of incorporating the thick bites of thick pancreatic tissue so as to achieve a firm and tight anastomosis. Similarly the internal layer of duct to mucosa should be perfectly apposed to the wall so as to ensure undisturbed flow of pancreatic juice into the jejunum. The jejunum too be vigilantly so as to prevent cracks and crevices from where the pancreatic juice can leak out (Figure 2). The use of an internal transanastomotic stent and loupe amplification can be particularly important in securing a tight anastomosis too.
Whether to use the pancreatic stent or not has received conflicting opinions but it is generally agreed in some studies that its use especially in cases of normal pancreatic duct and with normal textured pancreas, the use of stent is beneficial [38-40]. Yet, as stated earlier, there are studies that have opined in contradiction to the use of pancreatic stent [41-43], making generalized opinion difficult. We in our study used the inner trans-anastomotic stent routinely in every one of our patients regardless of the status of the pancreatic duct or the pancreatic texture.
The in hospital mortality rate was 1.5% in our study, which is low as compared to other studies. Our study also revealed that the rate of post-operative wound infections were relatively higher (20%). This was specially observed in patients that had undergone biliary stenting prior to the procedure. This observations of ours has also been acknowledged in previous studies by different authors [44,45]. This has led us to identify biliary stent in-situ as an important risk factor in the development of wound infection that would require further studies in the future.
Conclusion
Based on our studies we can say that the utilization of two-layered duct to-mucosa pancreaticojejunostomy along with the transanastomotic stent as a connecting channel between the pancreas and jejunum is the optimal method to avoid trans-anastomotic leakage, pancreatic fistula formation and reduce the in-hospital complication rates. This process is safe and is associated with lower mortality. The practice of electively putting in biliary stent and PD performed with an intrinsic biliary stent is associated with higher chances of postoperative wound infection. Given the overall rarity of complications that require surgery familiarity with the published data and sound clinical judgment are key to successful patient outcomes.
References
- Horstmann O, Markus PM, Ghadimi MB, Becker H (2004) Pylorus preservation has no impact on delayed gastric emptying after pancreatic head resection. Pancreas28: 69-74.
- Tajima Y, Kuroki T, Tsuneoka N, Adachi T, Kosaka T, et al. (2009) Anatomy-specific pancreatic stump management to reduce the risk of pancreatic fistula after pancreatic head resection. World J Surg 33: 2166-2176.
- Büchler MW, Friess H, Wagner M, Kulli C, Wagener V, et al. (2000) Pancreatic fistula after pancreatic head resection. Br J Surg87:883-889.
- Sato N, Yamaguchi K, Chijiiwa K, Tanaka M (1998) Risk analysis of pancreatic fistula after pancreatic head resection. Arch Surg 133: 1094-1098.
- Hashimoto N, Yasuda C, Ohyanagi H (2003) Pancreatic fistula after pancreatic head resection; incidence, significance and management. Hepatogastroenterology50:1658-1660.
- Nazarian SM, Yenokyan G, Thompson RE, Griswold ME, Chang DC, et al. (2008). Statistical modeling of the volume-outcome effect for carotid endarterectomy for 10 years of a statewide database. J VascSurg48: 343-350
- Schmidt CM, Turrini O, Parikh P, House MG, Zyromski NJ, et al. (2010) Effect of hospital volume, surgeon experience, and surgeon volume on patient outcomes after pancreaticoduodenectomy: a single-institution experience. Arch Surg 145: 634-640.
- Khithani A, Christian D, Lowe K, Saad AJ, Linder JD, et al. (2011) Feasibility of pancreaticoduodenectomy in a non-university tertiary care center: what are the key elements of success? Am Surg 77:545-551.
- Chew DK, Attiyeh FF (1997) Experience with the Whipple procedure (pancreaticoduodenectomy) in a university-affiliated community hospital. Am J Surg 174: 312-315.
- Cameron JL, Riall TS, Coleman J, Belcher KA (2006) One thousand consecutive pancreaticoduodenectomies. Ann Surg 244:10-15
- Cameron JL, He J (2015) Two thousand consecutive pancreaticoduodenectomies. J Am CollSurg; 220: 530-536.
- Yeo CJ, Cameron JL, Sohn TA, Lillemoe KD, Pitt HA, et al. (1997) Six hundred fifty consecutivepancreaticoduodenectomies in the 1990s: pathology, complications, and outcomes. Ann Surg 226:248-260.
- Kleespies A, Albertsmeier M, Obeidat F, Seeliger H, Jauch KW, et al. (2008) The challenge of pancreatic anastomosis. Langenbecks Arch Surg. 2008; 393: 459-471.
- Stojadinovic A, Brooks A, Hoos A, Jaques DP, Conlon KC, et al. (2003) An evidence-based approach to the surgical management of resectablepancreatic adenocarcinoma. J Am CollSurg196:954-964.
- Lai EC, Lau SH, Lau WY (2009) Measures to preventpancreaticfistula after pancreatoduodenectomy. A comprehensive review. Arch Surg 144:1074-1080.
- Varco RL (1945) A method of implanting the pancreatic duct into the jejunum in the Whipple operation for carcinoma of the pancreas. Surgery 32:569-573.
- You D, Jung K, Lee H, Heo J, Choi S, et al. (2009) Comparison of different pancreatic anastomosis techniques using the definitions of the International Study Group of Pancreatic Surgery: a single surgeon's experience. Pancreas 38:896-902.
- Butturini G, Daskalaki D, Molinari E, Scopelliti F, Casarotto A, et al. (2008) Pancreatic fistula: definition and current problems. J HepatobiliaryPancreatSurg15:247-251.
- Park JS, Hwang HK, Kim JK, Cho SI, Yoon DS, et al. (2009) Clinical validation and risk factors for delayed gastric emptying based on the International Study Group of Pancreatic Surgery (ISGPS) Classification. Surgery 146:882-887.
- Welsch T, Borm M, Degrate L, Hinz U, Büchler MW, et al. (2010) Evaluation of the International Study Group of Pancreatic Surgery definition of delayed gastric emptying after pancreatoduodenectomy in a high-volume centre. Br J Surg 97:1043-1050.
- DeOliveira ML, Winter JM, Schafer M, Cunningham SC, Cameron JL, et al. (2006) Assessmentofcomplications after pancreatic surgery. A novel grading systemapplied to 633 patients undergoing pancreaticoduodenectomy.AnnSurg 2006; 244:931-937.
- Schmidt CM, Powell ES, Yiannoutsos CT,Howard TJ, Wiebke EA, et al. (2004) Pancreaticoduodenectomy: a 20-year experience in 516 patients. Arch Surg 139: 718-727.
- Gouma DJ, Van Geenen RC, van Gulik TM, de Haan RJ, de Wit LT, et al. (2000) Rates of complications and death after pancreaticoduodenectomy: risk factors and the impact of hospital volume. Ann Surg 232:786-795.
- Hilal MA, Malik HZ, Hamilton-Burke W, Verbeke C, MenonKV (2009) Modified Cattell’spancreaticojejunostomy, buttressingfor soft pancreas and an isolated biliopancreatic loop aresafety measurements that improve outcome after pancreaticoduodenectomy:a pilot study. HPB (Oxford) 11: 154-160.
- Winter JM, Cameron JL, Campbell KA, Chang DC, Riall TS, et al. (2006) Does pancreatic duct stenting decrease the rate of pancreatic fistula following pancreaticoduodenectomy? Results of a prospective randomized trial. J GastrointestSurg 10:1280-1290.
- Muscari F, Suc B, Kirzin S, Hay JM, Fourtanier G, et al. (2006) Risk factors for mortality and intra-abdominal complications after pancreatoduodenectomy: multivariate analysis in 300 patients. Surgery 139:591-598.
- Duffas JP, Suc B, Msika S, Fourtanier G, Muscari F, et al. (2005) A controlled randomized multicenter trial of pancreatogastrostomy or pancreatojejunostomy after pancreatoduodenectomy. Am J Surg 189:720-729.
- Berger AC, Howard TJ, Kennedy EP, Sauter PK, Bower-Cherry M, et al. (2009) Does type of pancreaticojejunostomy after pancreaticoduodenectomy decrease rate of pancreatic fistula? A randomized, prospective, dual-institution trial. J Am CollSurg 208: 738-747.
- Su AP, Zhang Y, Ke NW, Lu HM, Tian BL, et al. (2013) Triple-layer duct-to-mucosa pancreaticojejunostomy with resection of jejunal serosa decreased pancreatic fistula after pancreaticoduodenectomy. J Surg Res 186:184-191.
- Gaujoux S, Cortes A, Couvelard A, Noullet S, Clavel L, et al. (2010) Fatty pancreas and increased body mass index are risk factors of pancreatic fistula after pancreaticoduodenectomy. Surgery 148:15-23.
- Friess H, Malfertheiner P, Isenmann R, Kühne H, Beger HG, et al. (1996) The risk of pancreatic intestinal anastomosis can be predicted preoperatively. Pancreas 13:202-208.
- Grobmyer SR, Kooby DA, Hochwald SN, Blumgart LH (2009) Blumgart anastomosis for pancreaticojejunostomy minimizes severe complications after pancreatic head resection Br J Surg 96: 741-750.
- Strasberg SM, Drebin JA, Mokadam NA,Green DW, Jones KL, et al. (2002) Prospectivetrial of a blood supply-based technique of pancreaticojejunostomy:effect on anastomotic failure in the Whipple procedure.J Am CollSurg194:746-758.
- Strasberg SM, McNevin MS ()Results of a technique of pancreaticojejunostomy that optimizes blood supply to the pancreas. J Am CollSurg187:591-596.
- Ota T, Araida T, Yamamoto M, Takasaki K (2007)Operative outcome and problems of right hepatic lobectomy with pancreatoduodenectomy for advanced carcinoma of the biliary tract. J HepatobiliaryPancreatSurg14:155-158.
- Sakorafas GH, Friess H, Balsiger BM, Büchler MW, SarrMG (2001) Problems of reconstruction duringpancreatoduodenectomy.Dig Surg18: 363-369.
- Fragulidis GP, Arkadopoulos N, Vassiliou I, Marinis A, Theodosopoulos T, et al. (2009) Pancreatic leakage after pancreaticoduodenectomy: the impact of the isolated jejunal loop length and anastomotic technique of the pancreatic stump. Pancreas 38: 177-182.
- Yang YM, Tian XD, Zhuang Y, Wang WM, Wan YL, et al. (2005) Risk factors of pancreatic leakage after pancreaticoduodenectomy. World J Gastroenterol11:2456-2461.
- Tajima Y, Kuroki T, Tsutsumi R, Fukuda K, Kitasato A, et al. (2006) Risk factors for pancreatic anastomotic leakage: the significance of preoperative dynamic magnetic resonance imaging of the pancreas as a predictor of leakage. J Am CollSurg202:723-731.
- Howard JM (1997) Pancreaticojejunostomy: leakage is a preventablecomplication of the Whipple resection. J Am CollSurg184: 454-457.
- Smyrniotis V, Arkadopoulos N, Kyriazi MA (2010) Does internal stenting of pancreaticojejunostomy improve outcomes after pancreatoduodenectomy? A prospective study.Does internal stenting of pancreaticojejunostomy improve outcomes after pancreatoduodenectomy? A prospective study. Langenbecks Arch Surg395:195-200.
- Satoi S, Toyokawa H, Yanagimoto H, Yamamoto T, Hirooka S, et al. (2010) Is a nonstented duct-to-mucosa anastomosis using the modified Kakita method a safe procedure? Pancreas 39:165-170.
- Ohwada S, Tanahashi Y, Ogawa T, Kawate S, Hamada K, et al. (2002) In situ vs ex situ pancreatic duct stents of duct-to-mucosa pancreaticojejunostomy after pancreaticoduodenectomy with billroth I-type reconstruction. Arch Surg 2002;137:1289-1293
- Sohn TA, Yeo CJ, Cameron JL, Pitt HA, Lillemoe KD (2000) Do preoperativebiliary stents increase post-pancreaticoduodenectomycomplications? J GastrointestSurg4:258-267.
- Sewnath ME, Birjmohun RS, Rauws EA, Huibregtse K, Obertop H, et al. (2001) The effect of preoperative biliary drainage on postoperative complications after pancreaticoduodenectomy. J Am CollSurg192:726-734.
Relevant Topics
- Constipation
- Digestive Enzymes
- Endoscopy
- Epigastric Pain
- Gall Bladder
- Gastric Cancer
- Gastrointestinal Bleeding
- Gastrointestinal Hormones
- Gastrointestinal Infections
- Gastrointestinal Inflammation
- Gastrointestinal Pathology
- Gastrointestinal Pharmacology
- Gastrointestinal Radiology
- Gastrointestinal Surgery
- Gastrointestinal Tuberculosis
- GIST Sarcoma
- Intestinal Blockage
- Pancreas
- Salivary Glands
- Stomach Bloating
- Stomach Cramps
- Stomach Disorders
- Stomach Ulcer
Recommended Journals
Article Tools
Article Usage
- Total views: 5926
- [From(publication date):
December-2016 - Apr 18, 2025] - Breakdown by view type
- HTML page views : 5008
- PDF downloads : 918