Research Article Open Access
Climatic Consequences of Long-term Global Salination of Ocean
Gaspar Banfalvi*
Department of Biotechnology and Microbiology, University of Debrecen, Hungary
- Corresponding Author:
- Gaspar Banfalvi
University of Debrecen
Department of Biotechnology and Microbiology
Life Sciences Building 1.1021
Egyetem Square, Debrecen 4010, Hungary
Tel: 36 52 512 900
Fax: 36 52 512 925
E-mail: bgaspar@unideb.hu
Received Date: May 13, 2016; Accepted Date: June 03, 2016; Published Date: June 06, 2016
Citation: Banfalvi G (2016) Climatic Consequences of Long-term Global Salination of Ocean. J Marine Sci Res Dev 6:196. doi:10.4172/2155-9910.1000196
Copyright: © 2016 Banfalvi G. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Marine Science: Research & Development
Abstract
The long-term salination of two major osmolyte systems, the ocean and the inner environment of vertebrates has been compared. The average osmolality of today’s sea (1.09 Osm) is more than three times higher than that of the blood of land vertebrates (~0.3 Osm). Of the two major strategies for ionic adaptation, in the first pattern the osmolarity of organisms (unicells, invertebrates, primitive vertebrates) equals that of the surrounding water, but the qualitative composition of body or cells fluid differ from those of the environment. In the second pattern of ionic adaptation advanced invertebrates and vertebrates maintain their energy consuming osmotic content and ionic composition of their extracellular environment. In spite of geological changes, terrestrial vertebrates maintained their physiological ionic concentration that corresponded to the sea at the time of their emergence in the Devonian era, rather than the osmolality of the ocean maintained its constancy. Paradoxically, the recent global melting of ice and snow with its temporary dilution effect is opposed by the long-term salination of the ocean. To resolve the contradiction between the salination process and its short term oscillations supported by Raoult’s law of dilute solutions, glacial periods favoring salination and interglacial periods of dilutions are: a) characterized as salinity fluctuations over geological ages, b) representing a dynamic osmolyte system against a general geochemical balance, c) directed towards a general salination process of oceans interrupted by glacial and interglacial oscillations. The gradually increasing salinity of ocean poses a long-term threat to the biodiversity and global life. It is assumed that unless mitigative measures of global proportion will be implemented, biodiversity of life on Earth will be endangered by the salinity of oceans and the shrinkage of the fresh water resources.
Keywords
Osmolytes; Salination; Glacial concentration; Interglacial dilution; Biodiversity
Introduction
Climate change is a global phenomenon affecting both terrestrial and aquatic life. Global warming and the melting of polar and Greenland ice have a diluting effect on oceans. The sea level rise at low sea temperature is hardly affected by the thermal expansion of oceans, but seriously impacted by the huge amount of water released by the melting snow and ice of the arctic ice caps and the Greenland ice sheet.
Another threat of global importance that has not been dealt with is known as salinity oscillation discussed here in terms of global vapor pressure changes governed by Rault’s law of dilute solutions. Thermosteric changes have received considerable attention, but salinity-driven halosteric patterns of long-term sea level change have not been throughly investigated [1]. Only regional halosteric anomalies have been considered as important drivers of the sea level variability [2-7]. Medium-term, multidecadal changes have been ignored because the halosteric fluctuations could not be detected or were close to zero [8-11], as well as historical salinity measurements [12,13] were not sufficient to come to any conclusion and primarily thermosteric effects have been taken into consideration in climatic changes [14,15].
This review focuses primarily on the second aspect of global threat related to the long-term halosteric changes of ocean starting with the evolution of dilute solutions, followed by sea level rises and falls, ocean as a dilute solution, global water circulation, salination and dilution processes, long-term salination versus temporary dilutions and future aspects of salination affecting life on Earth.
Evolution of Dilute Solutions
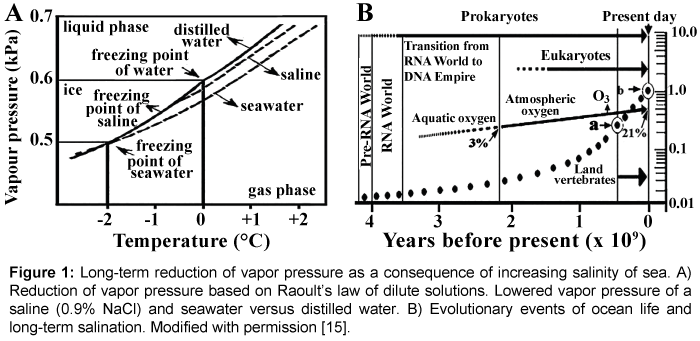
The thermodynamic law of dilute solutions established by François- Marie Raoult is summarized in Figure 1A showing the equilibrium of various phase-transitions of water around the freezing point. The freezing point of distilled water at the sea level is 0°C. By multiplying the osmolarity of seawater (1.09 Osm) with the molar freezing point depression (-1.86°C) the freezing point for saline (0.3 Osm × -1.86°C) is -0.56°C and for seawater (-1.86 × 1.09) -2°C. The vapor pressure of dilute solutions including the ocean is a fundamental property of icevapor- liquid equilibrium system of Earth known as biosphere. This system implies that at all temperatures the vapor pressure of dilute solutions (e.g. saline solution, seawater) is lower than that of the pure solvent (distilled water) and indicates that the freezing point of a solution depends strongly on its vapor pressure. In dilute solutions, the vapor pressure will be higher at each temperature than that of the pure solvent, and the freezing-point will be lower than 0°C. Similarly the boiling point elevation and osmolality of a dilute solution is dependent on its concentration, the higher the concentration of the solution, the higher its boiling point elevation and osmotic concentration will be. The graph also shows that below a certain vapor pressure (0.6 kPa) water is not in liquid form but e×ists as ice. Important to mention that the higher the temperature, the larger the difference in vapor pressure will be between between distilled water and solutions. For more information on the thermodynamics of water see [16].
Major evolutionary events on Earth including the evolutionary aspects of ocean life and the progress of increasing salt content of ocean are summarized in Figure 1B. Prebiotic life could have started 4.2 billion years ago. The ”RNA World” could have lasted for an estimated 400 million years, and replaced by DNA-based cells. An estimated 3.6 billion years ago photosynthetic stromatolytes and cyanobacteria started to hydrolyze water leading to the appearance of atmospheric oxygen ~2.2 billion years ago. Eukaryotic cells came into being ~2-1.5 billion, plants appeared between 1.2-1.5 billion, land vertebrates some 450 million years ago. The hypothetical salination of ocean is indicated by the dotted curve. Unfortunately the initial salt concentration after the World Ocean cooled down to the temperature tolerable for primitive life is unknown, but was probably much lower than the recent salinity value. Attention is called to a and b salt concentrations (circled black dots) in Figure 1: a) Devonian salt concentration of ocean is believed to have corresponded to the concentration of blood electrolytes (0.3 Osm) at the time when land vertebrates left the sea [17-20]. b) This dot in Figure 2 corresponds to present day’s sea osmolality (1.09 Osm) after 4.55 billion years of global history.
Figure 1: Long-term reduction of vapor pressure as a consequence of increasing salinity of sea. A) Reduction of vapor pressure based on Raoult’s law of dilute solutions. Lowered vapor pressure of a saline (0.9% NaCl) and seawater versus distilled water. B) Evolutionary events of ocean life and long-term salination. Modified with permission [15].
Sea Level Rise above its Present Level
The global temperature increase and recent sea level rise raise the question: How far the recent sea level increase could go taking into account the available fresh water reservoirs? These reservoires include the arctic ice, glaciers and the permanent snow. To answer the question, fundamental data are given in Table 1.
| Earth | Mean Radius | Volume | Reference |
|---|---|---|---|
| m | m3 | ||
| Earth radius | 6,367,450 | 1.0814 × 1021 | [21,22,26] |
| Sea volume | 1.33 ×1018 | [23] | |
| Sea volume | 6,363,767.80 | 1.332 × 1018 | [24] |
| Hypothetical radius | |||
| after 100 m sea level rise | 6,367,550 | 1.08145 × 1021 | Estimated |
| after 50 m sea level rise | 6,367,500 | 1.08142 × 1021 | Estimated |
| Volume needed for 100m sea level rise | ~5 × 1016 | Estimated | |
| 50 m sea level rise Estimated | ~2.0 × 1016 | ||
| Fresh water reserv (ice, galciers, permanent snow) | ~2.4 × 1016 | [25] |
Table 1: Highest potential sea level rise.
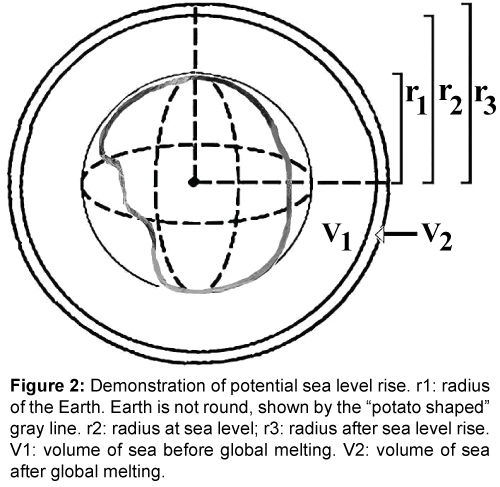
These data include
r1 = Average radius of Earth, the distance from Earth’s center to its solid surface without sea (6,368 km),
r2 = The radius of Earth at sea level (6,371 km) [21,22],
r3 = Earth radius after a hypothetical 100 m sea level rise (6,371.1 km)
V1 = Volume of Earth + sea = 1.08321 × 1012 km3
Volume of sea 1.332 × 109 km3 [23,24].
Volume of Earth + hypothetical sea volume after 100 m sea level rise: 1.08326 × 1012 km3
Volume of hypothetical sea level rise: 5 ×109 km3.
Volume of fresh water reserve (ice, galciers, permanent snow) ~2.4 × 109 km3 [25,26].
Data of fresh water supply are given in Table 1. These data have been used to estimate the potential sea level rise as a consequence of global warming and melting.
The potential sea level rise is demonstrated in Figure 2. From the data of Table 1 one can estimate that the available fresh water reservoir would not be sufficient to supply a 100 m sea level rise. The volume of the available fresh water reserve (2.4 × 109 km3) would cause less than 60 m elevation of sea water. Taking into account that the continental shelf narrows upwards increasingly more water will be necessary as the sea level rises. Based on above estimations and to answer the question how far the recent sea level increase could go, the maximum of sea level rise would be closer to 50 than to 60 m. One can also expect that the thaw of fresh water reserve will never be complete, which further reduces the possible sea level rise. One can also predict from the available small buffer capacity of the fresh water reservoirs, that future interglacial oscillations of sea level rises will take place with higher frequencies but decreasing amplitudes, in long-term not be able to compensate the progression of salination process of the ocean.
A further danger concerns the constancy of water volume on Earth, challenged by those who suspected that Earth’s air gradually leaks back into space and Earth could become dry like Venus [27]. The quote of Benjamin Franklin that “a small leak can sink a great ship” could be perplexing at global proportion by the observation that in the highest reaches the atmosphere is shrinking [28-30]. Air leakage on Earth would mean fresh water loss in the form of water vapor. Earlier changes leading to the recent climate of Mars reflect what Earth could also become, such as the Martian polar ice caps, characteristic seasonal changes and weather patterns with very little atmosphere. The interaction of the ionosphere of Mars with solar wind gradually stripping away atoms from the upper atmosphere [31] could be another similarity between these sister planets.
Ocean as a Dilute Solution
By viewing the ocean as the oldest dilute osmolyte system, the laws of dilute solutions should apply to the ocean.
Arguments that supply this notion
In dilute aqueous solutions the molar excess of solvent over solute is more than one hundred. The molar concentration of water is 55.5 M (1 M = 18 g/l, 55.5 M = 1000 g/l) consequently the border between a dilute and concentrated solutions can be drawn at 0.555 Osm (3.34 × 1023 particles/kg). The osmotic concentration calculated from the salinity and from the freezing-point depression of seawater (-2°C) corresponds to 1.09 molar non-dissociable solute concentration. In dilute solutions there are practically no interactions between the molecules of the solute. Although, the osmolality of the present day ocean (1.09 Osm) is almost two times higher than in ideal dilute solutions (<0.555 Osm) the solute-solvent interactions are still negligible.
The solutes are non-volatile. The sea contains primarily nonvolatile salts. One notable exception is iodine, but present in very low concentration (<0.001 ppm = 10-7%). Due to its volatility, the iodine content of rain inside the continents diminishes with the distance from the coast.
The solvent evaporates. More than 80% of the evaporated water close to distilled water quality comes from the sea surface, less from the surface of the land and from fresh water reservoirs, lakes and rivers (Figure 3C). As far as evaporation is concerned beside the concentration, the surface temperature is an important factor.
More water molecules break away from the surface at higher temperature, resulting generally in higher vapor pressure over the sea and more precipitate formation. The so called Southern Oscillation causes variations in the temperature of the surface with warming accompanied by high air surface pressure of the Tropical Eastern Pacific (El Niño) and cooling with low pressure (La Niña) in the Tropical Western Pacific. The Tropical Western Pacific area (Manus, Papua New Guinea, Naru, Northern Australia) with its warmest sea-surface temperature is the ”warm pool” of the Pacific ocean causing a highaltitude cirrus cloud system affecting both the solar energy absorption and the suspected escape of heat energy into the space. The Western Pacific system demonstrates that the biosphere is a semi-closed system the permeability of which is vulnerable.
Evaporation takes place in this semi-closed aquatic biome, which consists of marine and freshwater. In this system the salinity of the fresh water carried to the ocean constantly increases, while the total mass of water plus water vapor of the atmosphere remains relatively constant. This constancy has been challenged by the assumption that the atmosphere could be leaking back to the space [27,29,30]. Leakage of the thermosphere questions the Earth as a closed system as the equilibrium of the water cycle would be shifted, with gradual loss of upper atmosphere including fresh water as water vapor. Leakage in the water cycle would also contribute to the long-term salination process.
Balance between evaporation and precipitation in a closed system is reached when the numbers of water molecules leaving the surface of the sea are equal to the number of molecules returning to it. Evaporation and precipitation near the sea level are closer to equilibrium than at higher altitudes particularly at distant locations above the continents. Although, global balance could never be reached, local saturation occurs frequently, with the vertical profile of temperature determining the form of precipitation (rain, snow, sleet, etc.). The lack of saturation, when much less water returns to the surface than evaporated is generating and spreading deserts at an alarming rate. Cutting and burning plants in forests to create fields, poor conservation, overtaxed water supply, soaring population and recently global warming are blamed without mentioning the global effect of salinity increase and as a consequence the global reduction of vapor pressure in air.
The extent of cloud formation on Earth is influenced by Raoult’s law of dilute solutions. The higher the osmolality of the sea, the less the vapor pressure, evaporation and precipitation will be at global scale. To the contrary, the more dilute and higher the temperature of the sea, the more water molecules will evaporate increasing the vapor tension in air resulting in more cloud and precipitate formation. That the dilution effect at global scale is not uniform is seen at three major areas including the intensive melting in the two polar regions and near the Greenland Ice sheet.
That Charles’ law also prevails is evident from the fact that increasing temperature expands the volume of the ocean.
Global Water Circulation
The three physical variables in the ocean are temperature, salinity and density. The physical properties of seawater include both ‘thermodynamic properties’ like density and freezing point, as well as ‘transport properties’ like the electrical conductivity and viscosity. Density is an important property in ocean science because small spatial changes in density result in spatial variations in pressure at a given depth, which in turn drive the ocean circulation [32].
The Arctic ice contains at least 15% sea ice and its fresh water stream is carried by ocean currents. The driving mechanism of global circulation is known as thermohaline circulation that depends on the complex interplay of sea water temperature and salinity across the globe. The seawater is circulated by ocean currents of warm or cold water generated and driven by solar warming, volcanic heat, hydrothermal circulation of hot Earth’s crust, arctic cooling, Earth’s rotation, gravity, gravitation of the moon, winds and temperature, all influencing the climates of the continents. Nevertheless, it is the salinity at the ocean surface that is the fundamental factor in the large-scale ocean climate, affected to a large extent by air-sea boundary processes such as evaporation and precipitation, and to a lesser extent by the horizontal and vertical advection of adjacent water. Consequently, the changes in surface-layer salinity over a certain period allow the estimation of the corresponding changes in evaporation and precipitation [33].
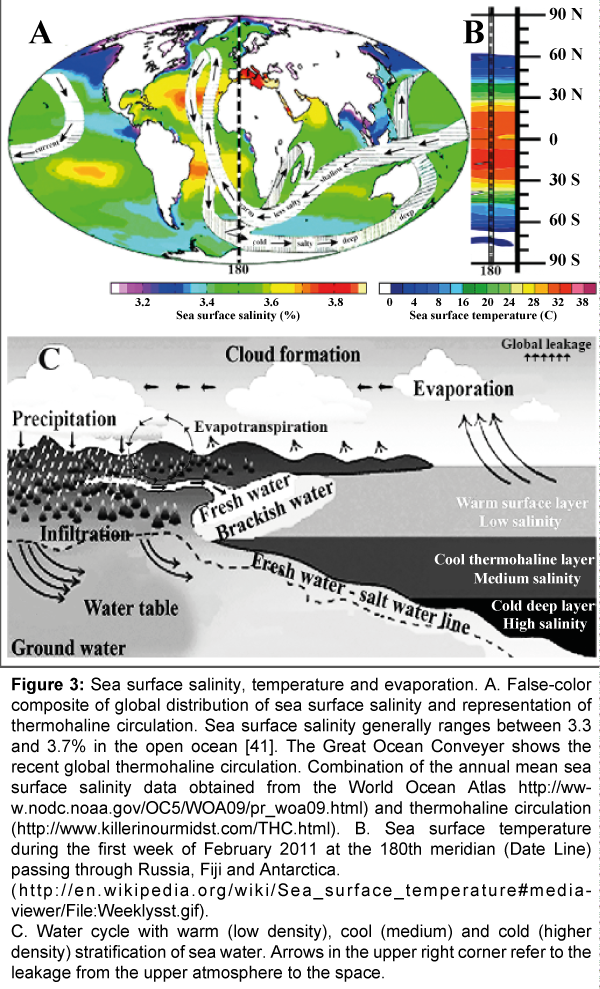
The Great Ocean Conveyor belt drives the global thermohaline circulation including the Gulf Stream – North Atlantic – Norway Current in the Atlantic Ocean, the Humboldt Current off South America, and the West Australian Current (Figure 3). Reduced circulation is causing severe meteorological activities near the circulating currents. As a consequence of local dilution in the north Atlantic region the storm intensity and frequency could be mentioned that have been doubled in the past 30 years. Due to the increased evaporation from diluted and warmer seawater, cloud formation, storm frequency and severity have been intensified. These events are not restricted to the North Atlantic region; more hurricanes hit the US South mainland, especially Texas than the average had been per decade in the previous 40 years.
Figure 3: Sea surface salinity, temperature and evaporation. A. False-color composite of global distribution of sea surface salinity and representation of thermohaline circulation. Sea surface salinity generally ranges between 3.3 and 3.7% in the open ocean [41]. The Great Ocean Conveyer shows the recent global thermohaline circulation. Combination of the annual mean sea surface salinity data obtained from the World Ocean Atlas http://www.nodc.noaa.gov/OC5/WOA09/pr_woa09.html) and thermohaline circulation (http://www.killerinourmidst.com/THC.html). B. Sea surface temperature during the first week of February 2011 at the 180th meridian (Date Line) passing through Russia, Fiji and Antarctica.
(http://en.wikipedia.org/wiki/Sea_surface_temperature#mediaviewer/File:Weeklysst.gif).
C. Water cycle with warm (low density), cool (medium) and cold (higher density) stratification of sea water. Arrows in the upper right corner refer to the leakage from the upper atmosphere to the space.
The sea surface temperature is measured near the surface layer confined to the top portion of the ocean from 1 mm to 20 m below the surface. The top 2-3 m layer has a heat capacity resembling the entire atmosphere above [34]. The sea surface temperature map created by satellite temperature measurements shows the regional variations across the globe in the vicinity of the 180th meridian. The sea surface temperature is high near the equator (27-30ºC) and below the freezing point (- 2ºC) within the polar region. Due to the horizontal movement of the conveyor belt, the eastern tropical regions are cooler than the western tropical regions subjected to more vertical upwelling and sinking. As temperature and salinity are related inversely, cold water is denser and warm water has lower density (Figure 3) (lower right corner).
Global Aspects of Salination
Detailed spatial salinity distributions were missing due to the lack of observational data. Satellite-based, ship-based and Argo-float measurements started at the beginning of the 21st century. It was the Argo Project that allowed to investigate medium-term, multidecadal salinity changes and continues to provide an immense amount of surface salinity data of the World Ocean [35,36]. Estimates of global and basin zonal-mean near-surface salinity changes date back to the 1950 - 2000 period showing moderately increasing values of local freshening and salination areas of the oceans. There is a general agreement regarding the dilution of the Pacific ocean between the 0 and 60 N latitudes, salinity increase in the Northern latitudes of the Atlantic and Indian oceans [33,37,38].
As far as the long-term constancy of salinity of the oceans is concerned we do not have good records. The salinity of the ancient oceans could not be measured directly. However, the glacial and interglacial ages reflect dynamic changes known as concentration and dilution periods with lowering and rising sea levels. Both experimental and theoretical studies have dealt mostly with the long-term mean of salt concentration. The idea of a general geochemical balance provided a model to make such constancy plausible [39,40]. However, by viewing the oceans as a dynamic osmolyte system rather than a steady-state one, long-term salinity changes cannot be disregarded.
There is a general consensus that extracellular and intracellular salt concentrations of living organisms were identical and vertebrates remained in osmotic equilibrium with the sea before the migration to land in the early Devonian Era some 450 million years ago [17-20]. This view needs to be reconciled with the fact that the average osmotic concentration of seawater is now 1.09 Osm (35 ppt). Incomplete mixing is indicated by some variation in salinity; in open seas, but this stratification ranges only between 33 and 37 ppt [41-45]. Incomplete mixing does not explain why the salinity of the sea became more than three times higher than the isotonic concentration of the blood of land vertebrates (~0.3 Osm) (Table 2). What distinguished the long-term changes at the origin of tetrapods of all land-living vertebrates were the many selective factors associated with the shift in environment from sea to fresh water and onto land. These changes included locomotion, skeletal support, feeding, respiration, sensory apparatus and reproduction [30]. All changed in the long run, but in spite of the diversification the salinity of their body fluids remained constant. The uniform osmolality of blood in land vertebrates (300 ± 15 mOsm) with the notable exception of amphibians (160-240 mOsm) is a reflection of osmolality of seawater at the time of their evolutionary emergence from the marine environment (Table 2). Although, water vapor of the ocean greatly impacts the humidity and precipitation formation not only over the sea, but also over the land, but in this respect not only long-term, but also short-term humidity and precipitation data are missing.
| Osmotic values of vertebrates mOsm | |
| Terrestrial vertebrates [42] | |
| Mammals | 295 |
| Birds | 317 |
| Reptiles | 286 |
| Lizards | 307 |
| Snakes | 300 |
| Turtles | 287 |
| Amphibians | 160-240 |
| Marine vertebrates | |
| Fishes | 60-80 |
| Teleosts (bonny fish) | |
| African lungfish (P. aethiopicus) [43] | |
| Preaestivation | 234 (urea: 4.2 mM) |
| Aestivation (13 mo) | 650 (urea: 203 mM) |
| Cartlilagenous | |
| Shark (Bonnethead) | 1094[44] |
| Seawater | 1094[42] |
Table 2: Characteristic data of the two major osmolyte systems
1 Osm = 6×1023 dissolved particles/kg water.
Convergent evolution, an extremely important topic within biodiversity, refers to the independent development of similar features in the descendants of different ancestral groups including land vertebrates. In spite of their convergent evolution land vertebrates maintained their uniform osmolality. It has been reasoned that at higher than 0.4 Osm the function of informational macromolecules (DNA, RNA, protein) would be threatened. Because of the strong concentration dependence of salt effect it would be difficult to design a protein that could maintain its optimal functional abilities over a wide range of salt concentration [46]. This explanation is in conformity with the existence of many isoenzymes that compensate small microenvironmental changes in different tissues and organs. Isozymes consist of slightly different amino acid sequences, but catalyze the same biochemical reaction.
Marine organisms are highly exposed to salinity changes in a process known as osmosis. As the dissolved salt generally does not easily cross biological membranes, it is the water that flows in or out to create equilibrium. Poikilosmotic conformers are marine organisms that maintain the salinity of the sea in their ionic makeup. These osmoconformers did not develop mechanisms to control osmosis and their cells have the same or similar salt content as the sea. Osmotic regulators (homeosmotic organisms) developed a variety of mechanisms to maintain a relatively constant bodily osmotic pressure independent of the osmotic pressure of the external environment.
Beside marine osmotic regulators, land vertebrates also encountered changes in salinity. The stability of the inner environment of land vertebrates versus the salination taking place in the sea dwelling animals indicates that salination in sea was followed mainly by osmoconformers rather than prevented by osmoregulation [42]. Major processes that could have caused the salination of ocean will be detailed.
As already mentioned global loss of fresh water reservoirs is likely to lead to diminishing oscillations in sea level rises and falls. The chronology of the Paleozoic sea-level changes seems to support this notion, with a gradual sea level rise through the Cambrian, reaching an ~200 m zenith in the Late Ordovician [47]. Sea-level data also reflect that even at the highest sea level rise the dilution effect was less than 10% taking into account the average radius of the Earth and the average depth of oceans across the planet (3682 m) [23]. These estimations also show that salinity oscillations could not have caused the >3-fold salinity increase of ocean (1.09 Osm) relative to the osmotic concentration of land vertebrates (0.3 Osm). The current Phanerozoic eon [48,49] dating back to 500 million years is crucial from the point of view of terrestrial evolution, constituting the development of terrestrial plants, multicellular animals, emergence of terrestrial animals and the development of modern faunas. Within the Phanerozoic the recent wet, warm interglacial period with an average 3.4% salinty is more in favor of specietion than the earlier somewhat saltier sea containing less dissolved oxygen. It is expected that the recent interglacial period is likely to be extended in the future [50,51]. By the end of the latest ice age the volume of the ice was ~5 × 107 km3. An ~ 4 × 107 km3 loss of all polar and glacial ice has been predicted, corresponding to 80% loss of our fresh water reservoir [52]. However, others reported that the recent fresh water reserve (ice, glaciers, permanent snow) is only 2.4 ×107 km3 [25]. An 80% loss (1.9 × 107 km3) of this fresh water would cause not more than an estimated 50 m sea level rise by ignoring the thermal expansion of water. The attenuation of oscillations of wet and dry climatic periods provides convincing evidence of eustatic changes due to salinity variations and thermal expansions over geologic ages and contradicts the general geochemical balance and long-term constancy of ocean salinity.
Salination Processes
Ice formation
In Polar Regions and in mountains snow and ice formation cause solvent deficiency and salination of oceans. During the advances of ice ages the sea level decreased significantly which in turn increased its salinity. Based on Charles’ law the cooling effect of ice ages lowered the temperature of sea, decreased its volume and increased salt concentration. Lowered water temperature increased the solubility of oxygen, which in turn contributed to the diversification of aqueous life.
Continental drift and outpouring lava
The constant reshaping of seafloor occurs in the oceanic lithosphere (Earth’s crust plus upper solid mantle) where the oceanic plates are colliding, moving apart and moving one under or above the other causing an immense pollution and small but constant increase in the salt content of the sea. The upper part of the crust is formed from episodic extrusions and intrusions of basaltic melt. The lower crust is formed by rapid hydrothermal alterations of the mantle [53]. The volcanic gases (CO2, H2S, HCl, HCN, SO2) and the soluble salts of the new crustal material of the erupting lava are dissolved pollutants of the ocean.
Weathering and denudation
These processes carry away the surface waste of land, especially if not protected by forests. Endogenic processes (volcanoes, earthquakes, plate tectonic uplifts) also expose the continental crust to exogenic denudation processes of weathering and erosion. Mass wasting is accelerated by man. Most of the salt of the eroded surface of the soil and sand especially that of spreading deserts is deposited in the ocean as sedimentary rocks, the salt content of which increases the salinity of the sea. From osmotic point of view only the number of dissolved particles matters irrespective of the quality of the salinating material.
Hydrological cycle
This cycle also known as water cycle circulates from the land to the sky and back to the sea. It consists of evaporation from sea, evapotranspiration from soil and vegetation and precipitation. The flow back returns the fresh water bodies to sea carrying diluted dissolved salt (<0.1%). The ”salt clock” method that served to determine the age of Earth was based on the continuous salinity intake of ocean by the water cycle [39]. Although, the ”salt clock” did not work, but led to the recognition that salination is a long lasting process [54-56]. The salination effect of hydrological cycle is strengthened by the chemical pollution of rivers that has already been noticed e.g. in Amazonia due to the mining on industrial scale, forest burning and uncontrolled use of pesticides [57].
Sea-level regression
After the Cretaceous high from 304 to 119 million years ago [58], the sea level was lower in the final stage of the Cretaceous by more than at any other time in the Mesozoic Era, but there is no direct evidence for the cause of this regression. According to the currently accepted explanation, the mid-ocean ridges could have sunk under their own weight [59]. Such a severe regression could have resulted in a virtual loss of sea. The higher salt content of the ridges could have increased the salinity of sea. Other disturbances responsible for sea to sea variations in chemical composition, even if small, may have caused significant global climatic changes, the consequences of which may not all be known.
Seawater reverse osmosis
One would expect that desalination of ocean to obtain fresh water without removing salt would increase the salinity of sea. Reverse osmosis is a desalination membrane process based on semi-permeable membranes that has been commercially used since the 1970s [60]. The volume of desalinated water is 109-times less than the volume of the ocean and does not represent a real threat to affect ocean salinity. In fact the most important waste management programs include the use of nuclear energy, solar energy, tidal power, wave power of ocean surface waves and wind power could serve not only the generation of electricity, but also the removal of salt from sea by desalination. Desalination of seawater by reverse osmosis in huge quantities would have an opposite effect and could pump fresh water into fresh water reservoirs.
River pollution and dumping of contaminated wastes
The danger of river pollution and ocean dumping has been recognized and banned worldwide, but its prohibition has not been implemented. As already referred to it, irrespective of the origin of waste, salination increases osmosis, lowers the rate of evaporation and precipitation formation.
Local pollution: Convincing evidence was provided that tropical waters became saltier over the past 40 years, while polar seas that injected immense amount of fresh water into the ocean, lowered its salinity [61,62]. In spite of local chemical pollutions the mean ocean salinity has not been changed. For this illusorical constancy the mass action law gives a reasonable explanation. The mass of the ocean exceeds about 270-times the mass of the atmosphere (1.38 × 1021 kg/5.1 × 1018 kg = 270). It will probably take more time to trace the global metahaline salinity increase than air pollution, which became measurable within one century.
Themohaline circulation
Another threat of large and rapid salinity changes may be the disruption of the thermohaline circulation, a possibility to be considered by the sea surface salinity (SSS) programs. These programs are being executed by the European Space Administration (ESA), by the Aquarius/SAC-D mission of NASA and by the Space Agency of Argentina (Comisión Nacional de Actividades Espaciales, CONAE). Refining data of local changes in the open ocean by these measurements are expected to clarify several phenomena of the water cycle and ocean circulation, representing the two major components of the climate system of Earth. Estimating global ocean heat content and global steric sea surface level with temperature/salinity data from the Argo network revealed increasing values during the years from 2005 to 2012 [63].
Dilution Processes
Salt extraction
Beside the several processes of salination we have also to consider global dilution processes. Rising sea beds cause obstruction, limited flow, loss of contact of inner shallow seas to the ocean. Evaporate deposits could have temporarily decreased ocean salinity [48,64,65]. Others thought that salt extraction did not decrease significantly the salinity of sea [66].
Interglacial periods
These sea level rises are part of sea level oscillations and provide only temporary dilutions followed by temporary by glacial salination periods. The high sea level rises of the the Paleozoic sea-levels (~ 460- 470 million years ago) were followed by other smaller rises. During the latest glacial, the buildup of ice over the continents and polar regions lowered the global sea level by an estimated 130 m compared to its present level [67] increasing its salinity [68-70]. The predicted 80% loss of all polar and glacial ice being under way could be extended by the greenhouse effect that is expected to lengthen the recent interglacial period [50,61,62]. Wet and dry climatic periods provide convincing evidence of eustatic and euhaline changes due to thermal expansion and salinity variations over geologic time periods contradicting the general geochemical balance and constancy of ocean salinity.
Effect of thermal expansion on volume
Water expands at higher temperature but contracts as it is warmed up from 0 to 4ºC. The average deep sea temperature is 2ºC, the average sea surface temperature 17ºC. Assuming a 2ºC increase (from 2 to 4ºC) in global sea temperature the volumetric contraction would still be negligible if any and similarly the expansion would also be within a few %. By eliminating the negligible changes of thermal expansion and contraction of seawater, the global melting remains to be restricted to the availability of the fresh water reserve with its modest (2%) dilution effect on World Ocean.
Considering the many glacial advances and retreats that occurred in the past, it is assumed that the concentration of the ocean was not simply fluctuating during these wet and dry climatic periods. The glacialinterglacial cycles provided evidence that the ocean concentration was changing over geologic periods, and Halley’s ”salt clock” method led to the recognition of a long-lasting salination process [54,55,71]. The diminishing amplitudes of interglacial sea level rises over geological time support the notion of long-term salination and gradual shrinkage of fresh water reservoirs. The acceptance of salinity changes implies that a long-lasting geochemical balance that would provide constancy to the ocean does not exist.
Long-term Salination vs Temporary Fluctuations
The dilution and salination of ocean can be summarized as
1. Assuming a gradual change, at least a 1 million year period would be necessary to measure the long-term salination effect. We have been able to trace only local salinity changes and the short-term dilution effect of global warming [72]. The global surface air temperature increased by approximately 0.85°C since 1880 [73]. This temperature elevation is attributed predominantly to the increasing atmospheric concentration of greenhouse gases, which in turn have a cooling effect in the upper atmosphere known as “greenhouse cooling” [74].
2. Water with less salt warms up faster and generates higher vapor pressure. Even a small decrease in salinity may significantly contribute to cloud and precipitation formation. Local slow dilution of ocean generates increasing amount of precipitation, fast dilution causes storm intensification.
3. The recent slight decrease in ocean salinity [48] is likely to mask the small global effect of increasing chemical pollution. Nevertheless, local pollution is already taking its toll (e.g. Aral Sea, estuaries), and exerts its devastating effect on life in these areas.
4. Salinity imbalances with climatic consequences are noticed at locations where:
a) Upwelling returns deep water to surface (e.g. Indian, Pacific Ocean), explaining the high frequency of floods in Eastern Australia.
b) Radiation emitted by the sun is heating up the diluted surface water, generating saturation vapor pressure, tropical and subtropical storms (e.g. North Atlantic tropical cyclones). An estimated >90% of the excess energy of global warming is absorbed by the ocean. The rest of heat energy is melting sea and land ice, heating the land surface and atmosphere [75-77].
c) The input of fresh water by glacier melting and runoff into the North Atlantic could weaken the conveyor belt.
d) The estimated fresh water reservoir is about 2.4 × 106 Km3 [25] limiting the potential sea level rise to 50 m. Earlier much higher sea level rises (>200 m) indicate that a significant portion of the fresh water reservoir could have been consumed during the salination process. The reduction of the total amount of sea water could be the consequence of water leakage.
Assuming the continuing tendency of the natural long-term salination process alone or in combination with global atmospheric leakage and antropogenic pollution, the osmolality increase of sea is likely to speed up in the future. By extrapolating the recent speed the salination could be as high as ~5 Osm in 500 million years unless the pollution would reach a saturation level, slowed down and follow a sigmoidal curve with an extended period of life on Earth beyond 500 million years. Although, this conjecture for which the only evidence is the diminishing fresh water supply remains speculative, but the salination process cannot be ignored and mitigative measures are needed to slow down the process
Global temperature and salinity rise follow their own pattern in accord to the changing hydrological parameters. The absorption and radiation of heat by the atmosphere known as the natural greenhouseeffect has been contributed by man that has increased the average global surface temperature between 1884 and 2015 by 0.87°C [78]. The 6th annual Sustainable Innovation Forum (SIF15) at Stade de France with the participation of more than 190 countries was the largest event held during the annual Conference of Parties (COP21). The most important mitigative measure of SIF15 against the “dangerous anthropogenic interference” was the decision to keep the global mean surface temperature increase below 2°C relative to the pre-industrial times. Similar global decision would be necessary to prohibit dumping of wastes into water bodies and keep the fresh water of rivers and lakes under a determined osmolarity minimum to prevent the anthropogenic salinity increase of oceans.
Conclusion
To summarize the euhaline and eustatic changes of ocean, it is predicted that salination is becoming a serious concern of global life. Inversely correlated intermittent fluctuations of ocean volume and fresh water reservoires are restricted to glacial and interglacial periods. The long-term salination process leads to the conclusion that the glacial-interglacial cycles will take place at increasingly higher salt concentrations of oceans resulting in gradually shortened and consequently more frequently occurring ice ages with gradually diminishing fresh water reservoirs. The widening gap between the increasing concentration of oceans, containing less dissolved oxygen versus the diminishing fresh water, the declining rate of evaporation and the spread of deserts are warning signals. The global survival strategy to extend life on Earth needs to turn to the preservation of fresh water and slow down the salination process.
Competing Interest
The author declares to have no competing interest.
Acknowledgement
This work was supported by a grant of the Hungarian National Science and Research Foundation to GB (OTKA T42762 grant. The critical reading of the manuscript by Prof Henry Paulus is gratefully acknowledged.
References
- Paul J, Durack PJ, Susan E, Wijffels SE, Peter J, et al. (2014) Long-term sea-level change revisited: the role of salinity. Environ Res Letts 9: 114017.
- Pattullo J W, Munk R, Revelle R, Strong E (1955) The seasonal oscillation in sea level. J Mar Res 14: 88-156.
- Tabata S, Thomas B, Ramsen D (1986) Annual and interannual variability of steric sea level along line P in the Northeast Pacific Ocean. J PhysOceanogr 16: 1378-1398.
- Maes C (1998) Estimating the influence of salinity on sea level anomaly in the ocean Geophy. Res Lett 25: 3551-3554.
- Sato OT, Polito PS, Liu WT (2000) Importance of salinity measurements in the heat storage estimation from TOPEX/POSEIDON. Geophys Res Lettpp: 27549-27551.
- Wunsch C, Ponte RM, Heimbach P (2007) Decadal trends in sea level patterns: 1993–2004. J Clim 20: 5889-5911.
- Suzuki T, Ishii M (2011) Long-term regional sea level changes due to variations in water mass density during the period 1981-2007. Geophys Res Lett 38: L21604.
- Antonov JI, Levitus S, Boyer TP (2005) Thermosteric sea level rise, 1955–2003 Geophys Res Lett 32: L12602.
- Munk W (2003) Ocean freshening, sea level rising. Science 300: 2041-2043.
- Ishii M, Kimoto M, Sakamoto K, Iwasaki SI (2006) Steric sea level changes estimated from historical ocean subsurface temperature and salinity analyses. J Oceanogr 62: 155-170.
- Gregory JM, Banks H T, Stott P A, Lowe J A, Palmer MD (2004) Simulated and observed decadal variability in ocean heat content. Geophys Res Lett 31: L15312.
- Boyer T P, Antonov JI, Baranova OK, Coleman C, Garcia HE, et al. (2013) World Ocean Database 2013 edSLevitus and A Mishonov 209 (Technical ed). National Oceanographoc Data Center, Silver Spring, MD.
- Durack PJ, Wijffels SE, Boyer TP (2013) Long-term salinity changes and implications for the global water cycle Ocean Circulation and Climate, A 21st Century Perspective 2nd edn. Eds. G Siedler, SM Griffies, J Gould, JA Church Oxford: International Geophysics, Academic, Elsevier, vol 103 chapter 28: 727-757.
- Church JA, Clark PU, Casenave JM, Gregory JM, Jevrejeva S, et al. (2013) The Physical Science Basis (eds TF Stocker, D Qin, G-K Plattner, M Tignor, SK Allen, J Boschung, A Nauels, Y Xia, V Bex and P M Midgley. IPCC, Univ. Press, Cambridge, UK pp: 1137-1216.
- Banfalvi G (2009) DNA Empire. In: Banfalvi G (ed) Apoptotic chromatin changes. Springer, Dordrecht p: 10.
- Iribarne JV, Godson WL (1973) Atmospheric Thermodynamics. Geophysics and Astrophysics Monographs, D. ReidelPubl, Dordrecht Holland p: 222.
- Gradstein FM, Ogg JG, Smith AG (2004) Construction and summary of a geological time scale. In. A geologic time scale. Gradstein FM, Ogg JG, Smith AG (eds) Cambridge University Press, Cambridge pp: 455-462.
- McFarlan WN, Heiser JB (1979) Life in water: Its influence on basic vertebrate functions. In Vertebrate Life, Macmillan, New York pp: 221-284.
- Smith HW (1943) Lectures on the Kidney', Porter Lectures IX, University of Kansas Press, Lawrence.
- Smith HW (1953) From fish to filosopher', Little, Brown and Co, Boston.
- Selected Astronomical Constants (2011) The Astronomical Almanac. Retrieved 2011-02-25 World Geodetic System (WGS-84) (1984) Available online from National Geospatial-Intelligence Agency.
- Cazenave A (1995) Geoid, topography and distribution of landforms. In Ahrens TJ (ed). Global earth physics a handbook of physical constants. Washington, DC: American Geophysical Union.
- Charette MA, Smith W (2010) The volume of Earth’s Ocean. Oceanography 23: 112-114.
- Ocean's Depth and Volume Revealed.
- Shiklomanov IA (1995) World fresh water resources. In: Gleick PH (ed) Water in crisis: A guide to the world’s fresh water resources. Oxford University Press, New York p: 13-24.
- World Geodetic System (1984).
- Catling DC, Zahnle KI (2009) The planetary air leak. Scientific American 300: 36-43.
- Lastovicka J, Akmaev RA, Beig G, Bremer J, Emmert JT (2006) Global change in the upper atmosphere. Science 314: 1253-1254.
- Emmert JT, Picone JM, Meier RR (2008) Thermospheric global average density trends, 1967-2007, derived from orbits of 5000 near-Earth objects. Geophys Res Lett 35: L05101.
- Englert CR, Harlander JM, Emmert JT, Babcock DD, Roesler FL (2010) Initial ground-based thermospheric wind measurements using Doppler asymmetric spatial heterodyne spectroscopy (DASH). Opt Express 18: 27416-27430.
- The Solar Wind at Mars.
- Pawlowicz R (2013) Key Physical Variables in the Ocean: Temperature, Salinity, andDensity. Nature Education Knowledge 4: 13.
- Hosoda S, Suga T, Shikama N, Mizunol K (2009) Global surface layer salinity change detected by Argo and its implication for hydrological cycle Intensification. J Oceanography 65: 579-586.
- Soloviev A, Lukas R (2006) The near-surface layer of the ocean: structure, dynamics and applications. Athmospheric and Oceanographic Sciences Library, Springer, Dordrecht, The Netherlands pp: 1-79.
- Argo Science Team (2001) Report of the Argo Science Team Second Meeting. Argo Science Team Second Meeting, Southampton Oceanogr. Cent. Southampton, UK.
- Argo Steering Team (2007) Eighth meeting of the International Argo Steering Team. Argo Steering Team Eighth Meeting, Paris, France.
- Boyer TP, Levitus S, Antonov JI, Locarnini RA, Garcia HE (2005) Linear trends in salinity for the World Ocean, 1955-1998. Geophys Res Letts 32: L01604.
- Durack PJ, Wijffels SE (2010) Fifty-year trends in global ocean salinities and their relationship to broad-scale warming. J Climate 23: 4342-4362.
- Rubey WW (1951) Geologic history of sea water. GeolSoc Am Bull 62:1111-1147.
- Railsback LB, Anderson TF, Ackerly SC, Cisne JL (1989) Paleoceanographic modeling of temperature-salinity profiles from stable isotopic data. Paleoceanography 4: 585-591.
- Worthington LV (1981) The water masses of the World Ocean: Some results of a finescale census. In Evolution of Physical Oceanography. Warren B, Wunsch C (eds) MIT Press, Cambridge, MApp: 43-69.
- Banfalvi G (1991) Evolution of osmolyte systems. Biochem Education 19: 136-139.
- Delaney RG, Lahiri S, Hamilton R, Fishman AP (1977) Acid-base balance and plasma composition in the aestivating lungfish (Protopterus). Am J Physiol 232: R10-R17.
- Harms C, Ross T, SegarsA (2002) Plasma biochemistry reference values of wild bonnethead sharks, Sphyrnatiburo. Vet ClinPathol 31: 1-5.
- Carrol RL (2001) The origin and early radiation of terrestrial vertebrates. J Paleont 75: 1202-1213.
- Yancey PH, Clark ME, Hand SC, Bowlus RD, Somero GN (1982) Living with water stress: evolution of osmolyte systems. Science 217: 1214-1222.
- Haq BU, Schutter SR (2008) A chronology of Paleozoic sea-level changes. Science 322: 64-68.
- Hay WW, Migdisov A, Balukhovsky AN, Wold CN, Flögel S, et al. (2006) Evaporites and the salinity of the ocean during the Phanerozoic: Implications for climate, ocean circulation and life. Palaeogeography, Palaeoclimatology, Palaeoecology 240: 3-46.
- Holser WT (1984) Gradual and abrupt shifts in ocean chemistry during Phanerozoic time. In: H.D. Holland and AF Trendall (Editors), Patterns of Change in Earth Evolution. Springer-Verlag, Berlinpp: 123-144.
- Berger A, Loutre MF (2002) An exceptionally long interglacial ahead? Science 297: 1287-1288.
- Augustin L, Barbante C, Barnes PR, Barnola JM, Bigler M, et al. (2004) Eight glacial cycles from an Antarctic ice core. Nature 429: 623-628.
- Berger A, Loutre MF (1996) Modelling the climate response to astronomical and CO2 forcings. Ext Geophys Climate Environ C. R. Acad. $ci. Paris 323: 1-16.
- Lewis BTR (1983) The process of formation of ocean crust. Science 220: 151-157.
- Joly J (1899) An estimate the geological age of the Earth. Trans Royal Soc Dublin 2:23-66.
- Burchfield JD (1998) The age of the Earth and the invention of geological time. Geological Society, London, Special Publications 143: 137-143.
- Dalrymple GB (2001) The age of the Earth in the twentieth century: a problem (mostly) solved. Special Publications, Geological Society of London 190: 205-221.
- Dolbeck J, Mergler D, Larribe F, Roulet M, Lebel J, et al. (2001) Mercury contamination and fish diet of an Amazonian population (Brazil). Sci Total Environ 271: 87-97.
- Miller KG, Kominz MA, Browning JV, Wright JD, Mountain GS, et al. (2005) The Phanerozoic record of global sea-level change. Science 310: 1293-1298.
- Liangquan L, Keller G (1998) Abrupt deep-sea warming at the end of the Cretaceous. Geology 26: 995-998.
- Loeb S, Van Hessen F, Shahaf D (1976) Production of energy from concentrated brines by pressure-retarded osmosis: II. Experimental results and projected energy costs. J Membrane Sci 1: 249-269.
- Dickson B, Yashayaev I, Meincke J, Turell B, Dye S, et al. (2002) Rapid freshening of deep North Atlantic Ocean over the past four decades. Nature 416: 832-836.
- Curry R, Mauritzen C (2005) Dilution of the Northern North Atlantic Ocean in recent decades. Science 308: 1772-1774.
- Von Schuckmann K, Sallée JB, Chambers D, Le Traon PY, Cabanes C, et al. (2014) Consistency of the current global ocean observing systems from an Argo perspective. Ocean Sci 10: 547-557.
- Southam JR, Hay WW (1981) Global sedimentary mass balance and sea level changes. In: The Sea, Wiley-Interscience, New York 7:1617-1684.
- Zharkov MA (1981) History of Paleozoic salt accumulation, Springer-Verlag, Berlin.
- Holland HD, Lazar B, McCaffrey M (1986) Evolution of the atmosphare and oceans. Nature 320: 27-33.
- Lambeck K, Chappel J (2001) Sea level change through the last glacial cycle. Science 292: 679-686.
- Shackleton NJ (1987) Oxygen isotopes, ice volume and sea level. QuatSci Rev 6: 183-190.
- Duplessy JC, Bard E, Labeyrie L, Duprat J, Moyes J (1993) Oxygen isotope records and salinity changes in the northeastern Atlantic Ocean during the last 18,000 years. Paleoceanography 8: 341-350.
- Zachos JC, Stott LD, Lohmann KC (1994) Evolution of early Cenozoic marine temperatures. Paleoceanography 9: 353-387.
- Dalrymple GB (1991) The Age of the Earth. Stanford California: Stanford University Press.
- Antonov JI, Locarnini RA, Boyer TP, Mishonov AV, Garcia HE (2006) World Ocean Atlas 2005: Salinity S Levitus S (ed) (2006). NOAA Atlas NESDIS 62, US Government Printing Office, Washington, DC p: 182.
- Intergovernmental Panel on Climate Change (IPCC) (2013) The Physical Science Basis.
- Cicerone RJ (1990) Greenhouse cooling up high. Nature 344: 104-105.
- Hansen J, Sato M, Kharecha P, von Schuckmann K (2011) Earth’s energy imbalance and implications. AtmosChemPhys 11: 13421-13449.
- Church JA, White NJ, Konikow LF, Domingues CM, Cogley JG, et al. (2011) Revisiting the Earth’s sea-level and energy budgets from 1961 to 2008. Geophys Res Lett 38: L18601.
- Cazenave A, Llovel W (2010) Contemporary sea level rise, Ann Rev Marine Sci 2: 145-173.
- http://climate.nasa.gov/vital-signs/global-temperature.
Relevant Topics
- Algal Blooms
- Blue Carbon Sequestration
- Brackish Water
- Catfish
- Coral Bleaching
- Coral Reefs
- Deep Sea Fish
- Deep Sea Mining
- Ichthyoplankton
- Mangrove Ecosystem
- Marine Engineering
- Marine Fisheries
- Marine Mammal Research
- Marine Microbiome Analysis
- Marine Pollution
- Marine Reptiles
- Marine Science
- Ocean Currents
- Photoendosymbiosis
- Reef Biology
- Sea Food
- Sea Grass
- Sea Transportation
- Seaweed
Recommended Journals
Article Tools
Article Usage
- Total views: 11794
- [From(publication date):
June-2016 - Mar 31, 2025] - Breakdown by view type
- HTML page views : 10904
- PDF downloads : 890