Climate Repercussions in Yuna-Samana Bay Estuary, Sanchez, Dominican Republic: Case Study on Eastern Oyster (Crassostrea virginica) Exposure to Different Salinity Regimes
Received: 10-Sep-2012 / Accepted Date: 27-Nov-2012 / Published Date: 29-Nov-2012 DOI: 10.4172/2157-7617.S12-003
Abstract
The Eastern oyster (Crassostrea virginica), is densely populated in estuarine areas subjected to various abiotic and biotic factors. The density of C. virginica along the Atlantic coast of U.S. has declined to 1-3% of historic population level. Anthropogenic influences exacerbate stress on oyster populations by continuously modifying estuarine salinity gradients. One of the limiting environmental parameters is manipulating the osmotic conditions. Our objective in this study was to investigate Eastern oysters’ tolerance to a wide range of salinity levels. The varying salinity regimes include 36ppt, 32.5ppt, 25ppt, 18ppt, 11ppt (control) and 3.5ppt through in vivo experimentation by monitoring sensitivity on respiratory functions (gaping) and mortality during 7 days. The influence of various salinity concentrations on oysters was analyzed and compared through Fischer’s Pair wise test using Graph Pad software where significant P value was observed for 11ppt that differs from other treatments. Our study shows that oysters can tolerate both hyper-saline and a hypo-saline concentration for a temporary period but an optimal salinity level of 11ppt is required for their survival. The least amount of gaping was experienced under a hypo-saline condition (3.5ppt). This study shows that oysters’ respiratory function and survival rates are affected by the salinity changes which reflect the natural conditions where anthropogenic effects on water bodies decrease the oysters’ population that is a significant part of the ecosystem in the Yuna-Samana Bay Estuary. The timing and magnitude of deleterious effects of climate change on aquatic systems have yet to be determined for this system. However, precautions to mitigate repercussions must be undertaken now if we are to have any hope of protecting this valuable natural resource.
Keywords: Salinity; Gaping; Respiration; Mortality; Crassostrea virginica; Fischer’s pair wise test
10379Introduction
Oysters are ecological engineers that fabricate the infrastructure of the estuarine habitat; their depletion creates a profound domino effect on the ecosystem. They are considered a “keystone species” providing many benefits to estuarine environments (SCDNR 2010). Oysters stimulate diversity in an aquatic ecosystem by providing shelter and habitat to other biota, creating spawning substrate for a myriad of fish and concentrate prey species for high trophic fish. Further contributions include stabilizing bottom sediment for benthic organisms and flora, and manipulating a natural filtration system for clarification of the aquatic habitat. Restoring sustainable oyster populations is about much more than saving the marine mollusk themselves-it’s about ensuring a healthy balance in the aquatic ecosystem [1-7]. In response, government agencies are innovating strategies to restore and protect estuarine habitats for relief on oyster and shellfish populations. National Oceanic and Atmospheric Administration and the Army Corps of Engineers lead a revitalized effort to recover native oyster reefs and establish selfsustaining native oyster reefs sanctuaries in key tributaries by 2020 [8].
A large quantity of oyster biomass can depress die-offs caused by anthropogenically nourished eutrophication. Oysters are capable of filtering large quantities of particulate carbon and algae that magnify anoxic conditions [9]. Oysters as filter feeders remove sediments and algae from the water column to improve local water quality and clarity while facilitating the removal of nitrogen and phosphorous from eutrophic waters. Depressing eutrophication simultaneously decreases acidification, while aiding in the maintenance of desirable dissolved oxygen (DO) and pH levels [3,6,10]. Until recently, oysters have overcome adversity through exquisite adaptations to their environment. Oysters are a resilient species, equipped with physicochemical defense apparatus that provides a tolerance gradient against environmental modifications. Environmental forces, both natural and anthropogenic impinge upon the sensory organs and niche of individual oysters.
Anthropogenic influences, including pollution by toxic industrial chemicals, pesticide runoffs from agriculture practices, invasive fishing methods, and habitat degradation induce environmental fluctuations that exacerbate stress on oyster sustainability [2,3,11]. Specifically, anthropogenic influences affect the salinity gradient of estuaries by development and utilization of the shore line. An illustration of anthropogenic influences altering estuarine salinity composition is through the construction of dikes which produces hypo-saline environments, irrigation creates hyper-saline conditions. Dredging channels also increase transportation of salt water into the estuary [12].
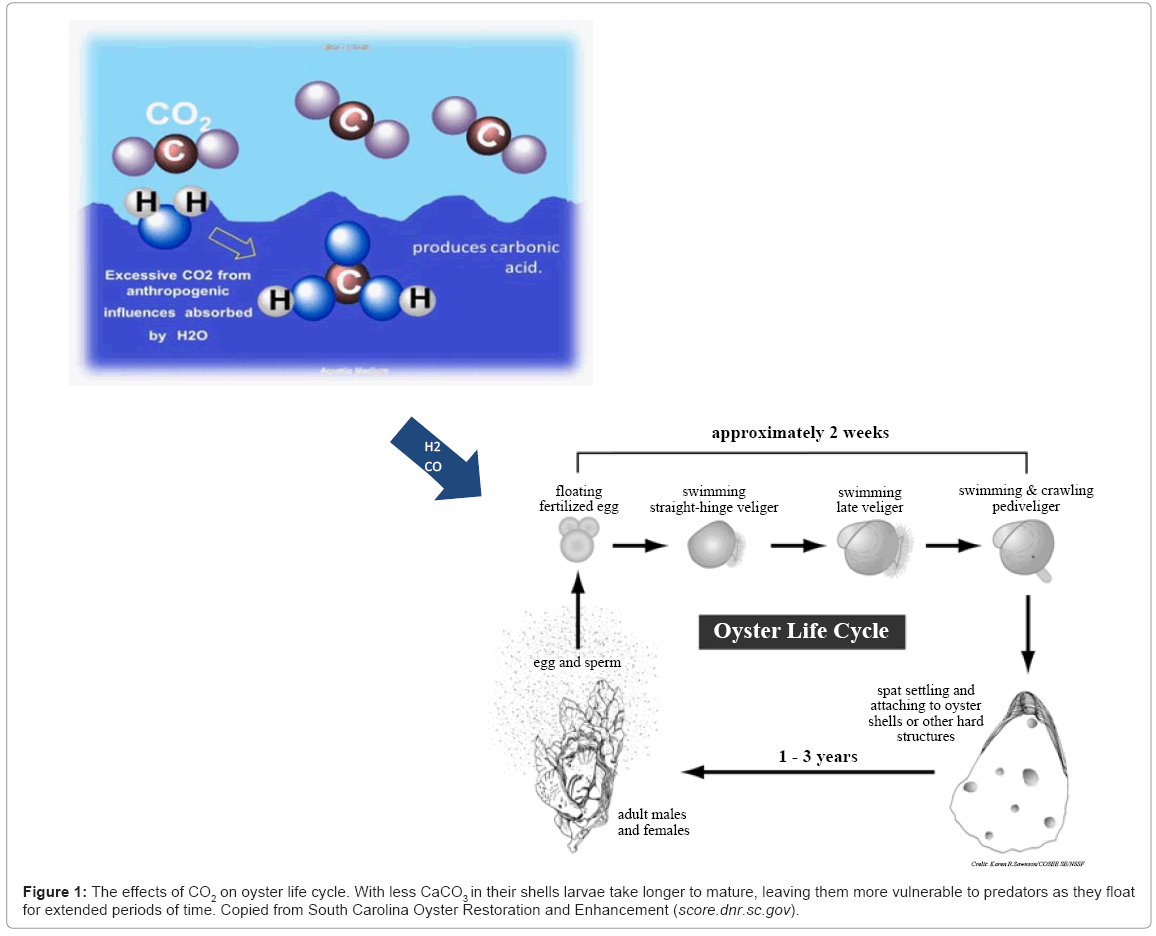
Over the past 200 hundred years, the increasing CO2 emissions from fossil fuel combustion has led to an exponential increase in the net amount of CO2 being dissolved into oceans [13]. Excessive CO2 in the aquatic system is dissolved to form CaCO3 (Figure 1). The conversion of CO2 into carbonate allows acidification to prevail. Oysters are becoming smaller and less robust because they cannot compensate for the amount of energy exerted to rid themselves of protons due to the conversion of carbon dioxide into carbonate [14]. Not only does this slow down the calcification rate, moreover, carbonic acid is corrosive to the oysters’ formation of a calcium carbonate shell; this corrosion is more pronounced on juveniles and larva.
Planktonic trochophore larva requires specific salinity concentrations to develop into fully shelled veliger larva; however, with less calcium in their shells, larva takes longer to mature. Unable to metamorph into spat, larva remains planktonic for longer durations, leaving them more vulnerable to predators [14]. Indirectly, acidification from carbon dioxide increase, affects shell formation through physiological impacts, such as respiration rate, which can impact energy budgets and thus alter the bivalves ability to produce shell material.
Oysters have an open circulatory system that is continuously exposed to physico-chemical modifications. Oysters’ haemocytes are able to synthesize osmotic shock proteins therefore protecting themselves from acute salinity variations [8]. Despite this advanced physiology, rising sea temperatures may directly inhibit the oysters’ haemocyte functions involved in their defense mechanism. This defense mechanism is critical for reducing oysters’ vulnerability to disease, such as Dermo and MSX, which proliferate in warm temperatures and high salinities [15]. Osmoregulation maintains specific concentrations of water and salt in the oysters’ body through retention and excretion. This balance is imperative for the oysters’ cell membrane to function. In a natural environment, oysters have an acute tolerance for a wide salinity regime. For optimum health and reproduction, oysters require a salinity range from 10 to 30ppt (FLDEP 2010).
The gills of oysters, by means of cilia, pump a stream of water from which food particles are filtered and passed along definite grooves to the labial palps, which convey them to the mouth [16-19]. It appears that salinity may be one of the important factors influencing the feeding of oysters [20]. Any significant change in salinity causes an immediate slowing or cessation in the rate of pumping [20].
The purpose of this study was to investigate an acute salinity gradient of Crassostrea virginica and to reveal the effects of anthropogenic activity on their salinity regime. This study observed the mortality and respiratory function (gaping response) of the gills as influenced by a salinity gradient in C. virginica retrieved from the Yuna-Samana Bay Estuary in Sanchez, Dominican Republic.
Material and Methods
Study site
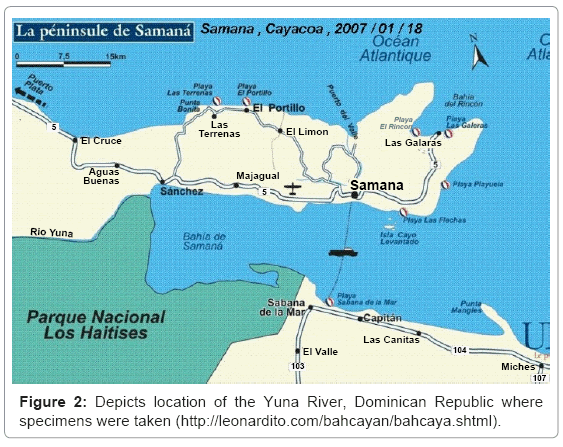
The Yuna-Samana Bay Estuary situated in the North-Central region of Dominican Republic and its many tributaries provide essential resources for the local populace; water depended sectors include: agriculture, fisheries, mining and tourism. The Yuna River (208 km in length) is the largest river in Dominican Republic in terms of fresh water output, carrying quantities (>10 to 100 m3/s) of fresh water year round [7]. The specimen for the study were taken from the downstream region (from Pimentel to the Bay of Samana), characterized by low plain and opened valleys with clayed soils (Figure 2). The nutrient rich water outflow and ideal temperatures (between 25-32°C), pH (between 7.7- 8.5), and salinity (0-25%) Tobey [7] has supported sustainable oyster population densities in the estuary. However, increased anthropogenic activity such as deforestation of mangroves, increased biological and chemical contaminations and cultivation with limited management contribute to amplify runoff impacts on degradation and sediment transport.
Experimental set-up
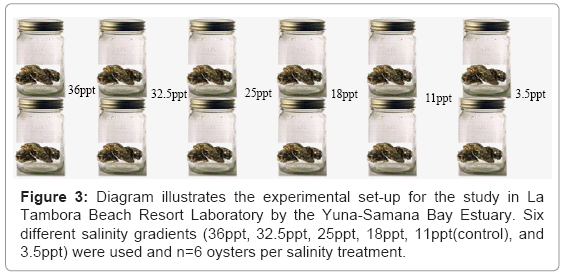
Eastern oysters (Crassostrea virginica) were retrieved from Yuna River. Before the experiment, the oysters were acclimated (using water from the Yuna-Samana Bay water source) in a bucket containing the estuarine water. In order to determine the short-term and acute response to salinity, the oysters were transferred directly from the acclimated condition into the experimental medium according to Shumway and Koehn [17]. Three adult oysters (>75 mm) were housed per a 2.5 gallon tank, providing a total of thirty-six oysters for observation (Figure 3). Experiments were conducted for seven days.
Water samples were collected at two different locations: Yuna River in Sanchez and Samana Bay at La Tambora Beach Resort. Both sample locations were in a place where the water was flowing. The sample containers were rinsed three times with the sampling water prior to collection. Samples were brought back to the lab station at La Tambora Beach Resort and diluted with distilled water, using one liter and twenty-five ml graduated cylinders, to make the appropriate test salinities. A hydrometer was used to measure the specific gravity of all the samples as percent salinity and then calculated to parts per thousand. The hydrometer was gently lowered into the sample containers and allowed to stabilize. The water sample was able to flow freely, and the reading was recorded when the surface of sample reached the stem of the hydrometer. This protocol was replicated for six different salinity gradients 3.5 ppt(10%); 11ppt(control)(30%); 18ppt(50%); 25ppt(70%); 32.5ppt(90%); and 36ppt(100%)). The salinity of the bay the oysters were collected in this study was recorded about 9-11ppt.
Statistical analysis
Oyster gaping was depicted as a cumulative gaping per day in a graph due to the labels’ coming off in the tanks during seven days experimental period. The mortality of oysters with respect to different salinity gradients was analyzed using Fisher’s exact test/pair wise test using Graph Pad software. P values were calculated using one tailed test. Our first alternate hypothesis (Ha1) was: oyster mortality would be high in lower salinity treatment. Our second alternate hypothesis (Ha2) was: oyster respiration (gaping) would be low in lower salinity treatment. We used 90% confidence interval where P
Results
Oysters were exposed to varying salinity gradient to monitor their tolerance levels and respiratory functions. As shown in Table 1, we found significant difference (P<0.10) between oysters survived in 11ppt (control) treatment vs. oysters in the rest of other treatments.
| Salinity Treatment | 36ppt | 32.5ppt | 25ppt | 18ppt | 11ppt (Control) |
|---|---|---|---|---|---|
| 32.5ppt | 1 | ||||
| 25ppt | 1 | 1 | |||
| 18ppt | 0.22 | 0.22 | 0.22 | ||
| 11ppt (Control) | 0.001S | 0.001S | 0.001S | 0.03S | |
| 3.5ppt | 0.22 | 0.22 | 0.22 | 0.72 | 0.03S |
Table 1: Fisher’s pair wise test results comparing oyster mortality at different salinities. P
Pairwise comparisons display a P value of 0.001 for 11ppt vs. 36ppt; 11ppt vs. 32.5ppt and 11ppt vs. 25ppt. These results are highly significant and it rejects our first alternate hypothesis. This means that 11ppt (control) salinity treatment is the optimal concentration for oysters in our study. For 11ppt vs. 18ppt the P value is 0.03 which also rejects our hypothesis that 11ppt salinity gradient is more optimal than 18ppt salinity treatment in our study. Mortality was absent in 11ppt whereas mortality was less in 18ppt when compared to other treatments. Specifically, no mortality was recorded during the first two days of experimentation for 3.5ppt, 11ppt and 32.5ppt salinity treatments, which means that oysters tolerated high and low salinity concentrations for a short period.
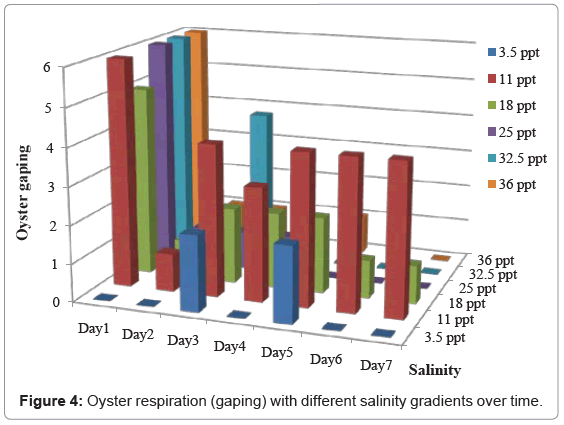
Gaping was prescribed as a measurement of respiratory functions. Figure 4 shows the oysters’ gaping response under different salinity gradients.
On day 1, oysters gaped in all salinity treatments except oysters in 3.5ppt treatment where our second alternate hypothesis was rejected. Gaping was seen in 3.5ppt treatment on day 3 and 5 but the number of oysters responded was very less and on days 6 and 7 gaping was absent in all oysters. For other treatments (11 to 36ppt), most of the oysters gaped on day 1 and no gaping was recorded in oysters in hyper-saline treatments resulting decrease respiration function and then mortality of those oysters. Gaping of oysters decreased gradually from day 2 for most of the treatments including control oysters. On day 2, gaping was absent only in 32.5 and 3.5ppt treatments. We need to count the fact that some of these oysters take more time to acclimatize to different salinity gradients resulting those oysters gaped later in our study such as oysters in 3.5ppt treatment. Although, dissolved oxygen is more readily available in lower salinity concentrations than in hyper-saline concentrations, oysters in 3.5ppt hypo-saline treatment stressed as much as hyper-saline treated oysters in our study.
Figure 4 show that oysters under hypo-saline (3.5ppt/10%) conditions did not experience significant respiratory or feeding functions throughout the entire study. Oysters held in (11ppt/30%) saline conditions revealed relatively constant respiratory and feeding functions and no mortality throughout the experiment (Table 1) suggesting how vulnerable those oysters to acute change in salinity from anthropogenic disturbance.
Discussion
Oyster hemocytes are able to synthesize osmotic shock proteins therefore protecting themselves from acute salinity variations [21]. Therefore, they are tolerant to acute salinity gradients. The purpose of this experiment was to check the tolerance gradient of Eastern oysters (Crassostrea virginica). When optimum saline concentration is accomplished, oysters respire and feed better by opening their valves. When oysters are exposed to hyper-saline concentrations for duration of time, they have tendency to shut down and mortality is expected from increased acute osmotic shock. Temperature range (25-32°C) under which this experiment was performed was optimum and any fluctuation in temperature would have added further complication and negative effect on the biological function of oysters such as gaping and filtration (Ozbay-pers. comm.).
Tolerance of oysters to salinity gradient is well depicted in our study because gaping was present in all salinity treatments in the first day except 3.5ppt salinity treatment. Gradually oyster gaping decreased in all hyper-saline treatments this may be due to this acute osmotic pressure. Our study also shows that 30% or 11ppt salinity concentration is optimum for oysters which corresponds with the literature (FLDEP 2010). At 10% or 3.5ppt salinity concentration, oyster mortality was recorded which explains why hypo-saline concentrations are lethal to oysters’ survival and respiration. Although the oysters were exposed to both acute hypo- and hyper-saline conditions in this experiment, all oysters survived and gaped in Day 1 supports their tolerance for the acute salinity changes for short duration as indicated by Tirard et al. [21].
Oyster mortalities may be contributed to anthropogenic disturbance as well as natural. Climate variability is of imminent concern because changes in average climate patterns could subject oysters to fluctuations and agitation from unfavorable environmental conditions. A combination of stressors, specifically rapidly changing salinity levels, may contribute to oyster mortalities as long as the conditions persist [14]. Additionally, intense storm surges and frequent hurricanes will increase freshwater inputs that recede into estuaries; causing prolonged periods of reduced salinity concentrations.
Climate variability and stochastic events have a profound influence on the Yuna River flow regime [22]. Given its geographic orientation, Dominican Republic is vulnerable to climate variability and natural hazards such as hurricanes, storm surges, and earthquakes. Anthropogenic activity and one or more natural phenomena can have a deleterious impact on the river’s physical parameters, including land use practices, water diversion, and construction of dams [22]. Operation of dams exerts some influence on the river’s flow dynamics, such as freshwater inputs; however, adequate research has not been conducted to examine the dynamic effects on the ecosystem. The Yuna River is the largest tributary that flows into Samana Bay-one of the largest estuaries in the Caribbean. The Samana Bay is of special interest because it supports winter populations of humpback whales in densities second only to those found on Silver and Navidad Banks [23]. Endangered species of sea turtles also call the estuary home.
Estuaries are kinetic ecosystems with natural environmental fluctuations associated to processes such as tidal flow and day-to-day temperature patterns. Superimposed upon these natural processes of fluctuating patterns are stochastic events such as weather related phenomena, which can create significant alterations in the estuarine environment. Salinity dynamics appear highly vulnerable to climatic pressures. Salinity can have a direct influence on the physical and biotic components of estuaries [24]. Numerous species of estuarine fauna, including oysters, cannot exist below particular salinity thresholds [25]. Salinity has an important association with estuarine equilibrium. Current climate change due to increases in atmospheric CO2 , may affect estuarine salinity equilibrium in a myriad of ways. Sea-level rise is speculated to have the most imminent impact because as sealevel rises, salt water intrudes the delicate estuarine water dynamics. It is apparent from these observations that further research is critical to fully understand the correlation between sea-level rise and estuary salinity dynamics. The timing and magnitude of deleterious effects of climate change on aquatic systems have yet to be determined. However, precautions to mitigate repercussions must be undertaken now if we are to have any hope of protecting this valuable natural resource.
Acknowledgements
We would like to acknowledge Marion McClary, Hector Ramirez, Anthony Stampul, Jennilee Jankowski, and Kenneth Hannum for their constant support and contribution. We would like to thank Dr. Karuna Chintapenta for her assistance in editing the manuscript. This project is partially funded by USDA-NIFA and USDAEvans- Allen Grant Program awarded to Dr. Gulnihal Ozbay.
References
- Erbland PJ, Ozbay G (2008) A comparison of the macrofaunal communities inhabiting a Crassostrea virginica oyster reef and oyster aquaculture gear in Indian River Bay, Delaware. J Shellfish Res 27: 757-768.
- Rotschild B, Ault J, Goulletquer P, Heral M (1994) Decline of the Chesapeake Bay oyster population : a century of habitat destruction and overfishing. Mar Ecol Prog Ser 111: 29-39.
- Kennedy VS (1996) The ecological role of the eastern oyster, Crassostrea virginica, with remarks on disease. J Shellfish Res 15: 177-183.
- Breitburg DL (1998) Are three-dimensional structure and healthy oyster populations the keys to an ecologically interesting and important fish community? Pages 239-250 in Luckenbach M, Mann R and Wesson JA (eds). Oyster reef habitat restoration: a synopsis and synthesis of approaches. VIMS Press, Gloucester Point, Virginia.
- Posey MH, Alphin TD, Powell CM, Townsend E (1998) Use of oyster reefs as habitat for epibenthic fish and decapods. In: Luckenbach MW, Mann R and Wesson JA, editors. Oyster reef habitat restoration: a synopsis and synthesis of approaches. VIMS Press, Gloucester Point, Virginia. 229-237.
- Newell RIE (2004) Ecosystem influences of natural and cultivated populations of suspension-feeding bivalve mollusks: a review. J Shellfish Res 23: 51-61.
- Tolley SG, Volety AK (2005) The role of oysters in habitat use of oyster reefs by resident fishes and decapod crustaceans. J Shellfish Res 24: 1007-1012.
- Loop T, Vickery H (2009) Agencies Release Draft Strategy for Chesapeake Bay Focused on Federal Action and Accountability.
- Cerco CF, Noel MR (2007) Can oyster restoration reverse cultural eutrophication in Chesapeake Bay? Estuaries and Coasts 30: 331-343.
- Nelson KA, Leonard LA, Posey MH, Alphin TD, Mallin MA (2004) Using transplanted oyster (Crassostrea virginica) beds to improve water quality in small tidal creeks: a pilot study. J Exp Mar Biol Ecol 298: 347-368.
- Brumbaugh RD, Sorabella LA, Garcia CO, Goldsborough WJ, Wesson JA (2000) Making a case for community-based oyster restoration: An example from Hampton Roads, Virginia, U.S.A. J Shellfish Res 19: 467-472.
- Wilber DH, Clarke DG (2001) Biological effects of suspended sediments: a review of suspended sediment impacts on fish and shellfish with relation to dredging activities in estuaries. N Am J Fish Manage 21: 855-875.
- Watson RT, Rodhe H, Oeschger H, Siegenthaler U (1990) Greenhouse gases and aerosols. Climate Change: The IPCC Scientific Assessment, Houghton JT, Jenkins G J,Ephraums JJ (eds). Cambridge University Press 1-40.
- Richmond CE, Woodwin SA (1996) Short-term fluctuations in salinity: effects on Planktonic invertebrate larvae. Mar Ecol Prog Ser 133: 167-177.
- Harvell CD, Kim K, Burkholder JM, Colwell RR, Epstein PR, et al. (1999) Emerging Marine Diseases--Climate Links and Anthropogenic Factors. Science 258: 1505-1510.
- Newell RIE, Jordan SJ (1983) Preferential ingestion of organic material by the American oyster Crassostrea virginica. Mar Ecol Prog Ser 13: 47-53.
- Shumway SE, Cucci TL, Newell RC, Yentsch CM (1985) Particle Selection, Ingestion, and Absorption in Filter-Feeding Bivalves. J Exp Mar Biol Ecol 91: 77-92.
- Nelson TC (1923) On the feeding habits of oysters. Proc Soc Exp Biol Med 21: 90-91.
- Nelson TC (1923) The mechanism of feeding in the oyster. Proc Soc Exp Biol Med 21: 166-168.
- Hopkins AE (1936) Adaptation of the Feeding Mechanism of the Oyster (Ostrea gigas) to Changes in Salinity. US Department of Commerce, Bureau of Fisheries, From Bulletin of the Bureau of Fisheries, Bulletin No. 21, Volume XLVIII: 344-363.
- Tirard CT, Grossfeld RM, Levine JF, Kennedy-Stoskopf S (1997) Effect of Osmotic Shock on Protein Synthesis of Oyster Hemocytes In Vitro. Comp Biochem Physiol Part A: Mol Integr Physiol 116: 43-49.
- Warner A (2005) Yuna River Hydrologic Characterization, Coastal Resources Center, University of Rhode Island, Narragansett, RI USA.
- Mattila DK, Clapham PJ, Katona SK, Stone GS (1989) Population composition of humpback whales, Megaptera novaeangliae, on Silver Bank, 1984. Can J Zool 67: 281-285.
- Gibson JK, Najjar RG (2000) The response of Chesapeake Bay salinity to climate-induced changes in stream flow. Limnol Oceanogr 45: 1764-1772.
- Jackson DK, Jesien R (1996) Chesapeake Bay low flow study. Susquehanna River Basin Commission.
Citation: Stampul PM, Ozbay G (2012) Climate Repercussions in Yuna-Samana Bay Estuary, Sanchez, Dominican Republic: Case Study on Eastern Oyster (Crassostrea virginica) Exposure to Different Salinity Regimes. J Earth Sci Climat Change S12: 003. DOI: 10.4172/2157-7617.S12-003
Copyright: ©2012 Stampul PM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 15348
- [From(publication date): 0-2013 - Apr 03, 2025]
- Breakdown by view type
- HTML page views: 10794
- PDF downloads: 4554