Clear Cell Odontogenic Carcinoma (CCOC): Mini-Review of Literature and Case Report of Mandibular Radiolucency in 17-year Girl
Received: 27-Oct-2016 / Accepted Date: 23-Nov-2016 / Published Date: 29-Nov-2016 DOI: 10.4172/2476-2024.1000120
Abstract
A 17-year girl reported with painful right lower posterior teeth. Orthopantomogram showed unilocular radiolucency with scalloped non-sclerotic border at apical area of non-carious right mandibular molars and premolar. A provisional radiological diagnosis of ameloblastoma or odontogenic keratocyst was given. Histopathological examination revealed follicular areas of peripheral palisaded hyperchromatic basaloid cells and central round-polygonal clear cells. A diagnosis of clear cell odontogenic carcinoma (CCOC)-ameloblastomatous variant was made after assessing the provisional diagnoses. A nosological dilemma arose as many authors opined that the terms ‘clear cell ameloblastoma’ and ‘clear cell odontogenic tumor’ should be invalidated and CCOC should be the preferred diagnosis because of the reported aggressive nature of clear cell odontogenic neoplasms. The scientific literature gave variable biological behavior and prognosis with diverse therapeutic approaches leading to therapeutic dilemma in management of the case. The authors have attempted to resolve the diagnostic and therapeutic challenges by presenting the clinical, radiological and histological aspects of the case and discussing the differential diagnoses of clear cell lesions involving the maxillofacial region along with the therapeutic approaches and prognosis of CCOC.
Keywords: Clear cell; Odontogenic; Carcinoma; Tumor; Ameloblastoma; Metastatic; Salivary gland; CCOC; CCOT
7319Introduction
Clear cells are tumor cells that have vacuolated or ‘clear’ cytoplasm in routine hematoxylin and eosin stain as the intercellular components (glycogen, mucin, lipid) are lost when the tissue sections are subjected to organic solvents like xylene during tissue processing; or due to paucity in cell organelles [1]. In the head and neck region clear cells can originate from various sources and are more commonly seen in odontogenic tumors, salivary gland tumors, metastatic carcinomas, and melanotic tumors [1,2]. Tumors with clear cells can impose a diagnostic dilemma for the surgical pathologist with respect to the differential diagnoses and nosology, which extends as a therapeutic dilemma for the managing surgeons. Clear cell odontogenic carcinoma (CCOC), formerly known as clear cell odontogenic tumor (CCOT) was originally described by Hansen LS, et al. in 1985 as a benign locally aggressive tumour [3]. The World Health Organization (WHO) reclassified CCOT in 2005 as a malignant carcinoma of odontogenic origin owing to its infiltrative behavior, tendency for recurrence, metastasis to the regional lymph node, and rare distant metastasis to the lungs [4]. Complete understanding of the biological and prognostic behavior of CCOC remains elusive because of the paucity of cases described since 1985, and hence the treatment strategies described remain diverse. The authors hope this additional case shall further in improving our understanding of this odontogenic carcinoma and help elucidate the tumor biology. The article discusses the differential diagnoses of clear cell lesions involving the maxillofacial region, and the diagnostic and therapeutic approaches to CCOC.
Case
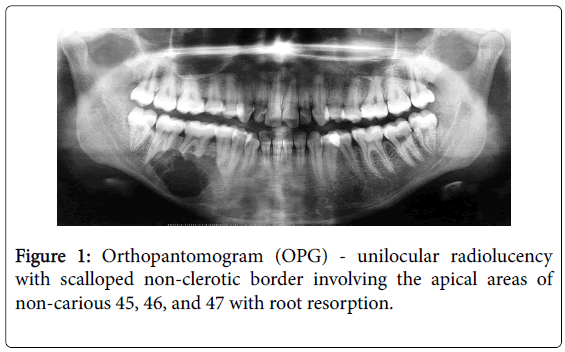
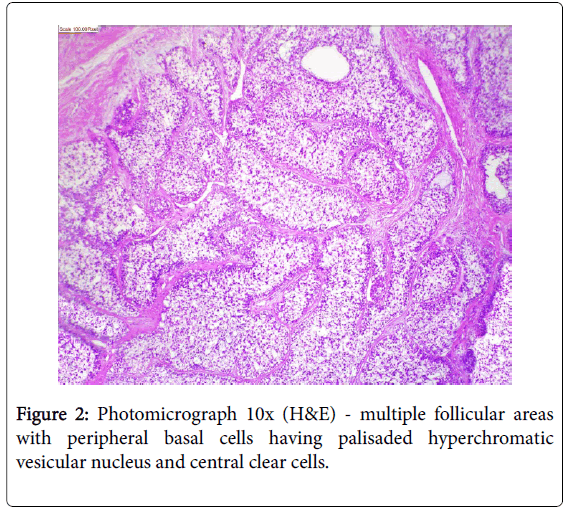
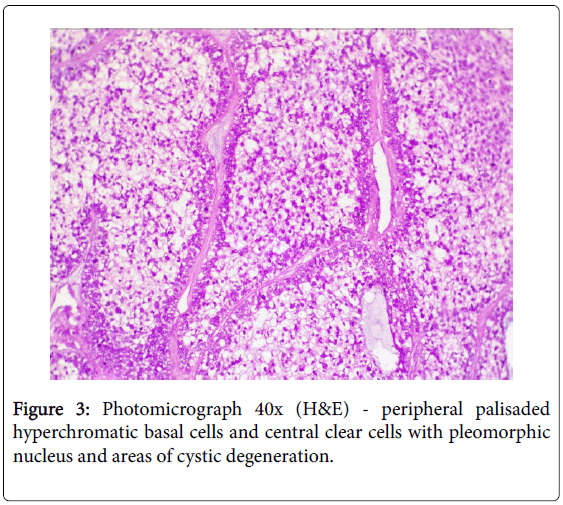
A 17-year girl reported to the Department of Oral Medicine and Radiology with dull aching right lower posterior teeth since 1 month. Routine intraoral examination revealed a good oral hygiene with no carious, missing, filled, or mobile teeth. Vertical percussion of FDI tooth number 45, 46 and 47 elicited a painful response. Palpation of the associated cortical area revealed a firm symptomatic bony buccal expansion, without ulceration, paresthesia or discoloration of the overlying mucosa. She had no known systemic disease, was not on any medications, and gave no history of trauma. The routine physical and extraoral examination was non-contributory. The regional lymph nodes were not palpable. Routine orthopantomogram (OPG) showed 4.5×3.5 cm unilocular radiolucent lesion with scalloped poorly defined non-sclerotic border involving the apical areas of non-carious right mandibular molars and premolar, and not invading into the periodontal ligament spaces (Figure 1). Chest and skull radiographs had no pathological findings. Routine hematological examinations gave normal parameters. A provisional clinical diagnosis of ameloblastoma or keratocystic odontogenic tumour/odontogenic keratocyst was given by the radiologist. Histopathological examination (HPE) of the incisional scalpel biopsy revealed multiple follicular areas with peripheral basaloid cells having non-palisaded hyperchromatic vesicular nucleus without definitive loss of polarity, and central areas of polygonal-round clear cells with sparse focal areas of cystic degeneration (Figure 2). Peripheral area of the lesion lacked true encapsulation and showed spicules of non-invading vital mature bone. A diagnosis of clear cell odontogenic carcinoma (CCOC): ameloblastomatous variant was made after assessing the probable provisional diagnoses. In due consideration of her young age, esthetics, and the variable prognosis reported in the published scientific literature a segmental osteotomy through intraoral approach and reconstruction using iliac bone graft with titanium bone plates was performed. HPE of the received segmented bone showed the same features as of incisional biopsy (Figure 3). Nuclear pleomorphism was scanty and mitotic activity was absent or Figure 4). The resected bony margins and soft tissue appeared to be free from the lesional tissue, and hence the patient was not referred for adjuvant radiotherapy or chemotherapy. The tissue sections stained negative for periodic acid-Schiff (PAS), Congo-red, mucicarmine, and human melanoma antigen (HMB-45); indicating negativity for glycogen, amyloid, mucin, and melanocyte. The case is under quarterly follow-up and has been disease-free over 1.4 years.
Discussion
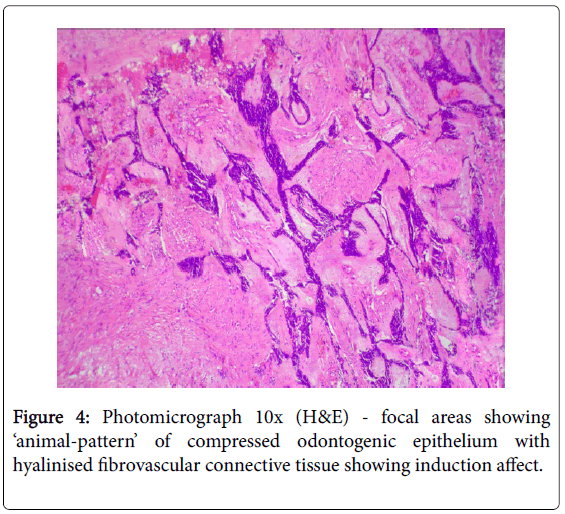
A literature review showed that the most common age of clinical presentation of CCOC were 53-56 yr. (17-89 yr.), with female predilection (1.8:1), and the mandible (62.5%) being more commonly involved than the maxilla, with preponderance for the posterior segment [1-11]. Some early reviews found preponderance for the anterior segment of the jaws [10]. The most commonly reported clinical symptoms were pain, localized jaw enlargement, and mobility of the involved teeth [5-7]. Most of the cases reported as painless slow growing swelling of several months or years [6]. Bleeding, paresthesia of lower lip, trsimus, proptosis, and non-healing ulcer were rare complaints [6,7]. Radiographically CCOC are not distinguishable from other osteolytic lesions of the jaws. They appeared as non-specific radiolucent lesions with irregular margins, often associated with root resorption and cortical bone perforation, and not associated with unerupted teeth [1,5-7]. Histopathologically the CCOC is composed predominantly of sheets, solid islands, nests or trabeculae of roundpolygonal cells with cytoplasmic clearing, separated by hyalinzed fibrous septa, and often admixed with basaloid-polygonal cells having granular eosinophilic cytoplasm [4]. Three histopathological patterns or variants have been described based on the relative proportions of these cells: (1) Monophasic – islands and nests of monomorphic clear cells with well-defined cell membrane and centrally placed round nucleus; (2) Biphasic – characterized by oval and linear nests of clear cells intermixed with islands of smaller hyper-chromic polygonalbasaloid cells with scanty eosinophilic granular cytoplasm and welldefined cell membrane. Sometimes the eosinophilic polygonal cells are arranged in double layers centrally within the clear cell cluster and appeared as duct-like structures (gland-like pseudolumina), indicating their odontogenic origin; (3) Ameloblastomatous – characterized by islands of clear cells having peripheral layer of ameloblastoid palisaded hyperchromatic cuboidal cells [1-6]. A systematic review of 67 cases by Loyola AM, et al. in 2015 reported that 79.2% demonstrated biphasic pattern, 16.9% ameloblastomatous pattern, and 3.9% monophasic pattern [7]. Mild-moderate pleomorphism, anisocytosis, anisokaryosis, gland-like pseudolumina and squamous metaplasia were observed in most cases [4,6]. Abnormal mitotic figures, necrosis, neural/vascular invasion, keratin pearl, hemorrhage, increased vascularity, and osteodentin deposition were rare [6]. Occasionally mild-moderate chronic inflammatory infiltrate of lymphocyte and plasma cells with or without giant cells were identified [6]. Rarely regional metastasis (19%) to level IB (submandibular) lymph nodes and distant metastasis (11.9%) to the lungs has been reported [6,7]. The lesional clear cells usually were PAS positive and diastase sensitive (indicating glycogen), and stained negatively for Congo-red (amyloid) and mucicarmine (mucin) [1,6]. However, none of these special stains were sufficiently specific to allow for an unequivocal diagnosis of CCOC [6]. Immuno positivity for cytokeratins (CK) 8, 13, 14, 18, 19, AE1/AE3, epithelial membrane antigen (EMA), S-100, p53, p16, antiameloblastoma antigen, vimentin and collagen type IV has been reported, while some studies gave immuno negativity for vimentin, S-100, desmin, smooth muscle actin (SMA), HMB-45, p53, enolase, calponin, chromogranin, glial fibrillary acidic protein (GFAP), CD10, CD31, CD45, and 1- antichymotrypsin [1-6,9]. The labeling indices with proliferation markers Ki-67 and proliferating cell nuclear antigen (PCNA) were low (2.73% and 8.11% respectively), suggesting the less aggressive nature of the tumor [5,6]. Mitotic index is not a reliable parameter for detecting malignancy in odontogenic tumors since many benign ameloblastomas may have an increased number of mitoses while CCOC may not show mitotic activity [12]. Our present case was reported in a 17-year old while the mean age of appearance for CCOC was 55 year and it differed from the previously reported cases in that it was PAS negative. HPE of the excised segmented bone showed focal areas of desmoplastic ameloblastoma in the associated peripheral stroma (Figure 4), which has not been previously reported in CCOC, and this helped in pointing to the odontogenic origin of the lesion.The regional lymph nodes were apparently negative clinically and there were no signs of distant metastasis to the lungs. Imunno phenotyping of our case was not performed as the literatures gave no specific diagnostic marker and most authors believed that immunohistochemical evaluation is generally not helpful in distinguishing CCOC from other clear cell odontogenic tumors [5]. The diagnosis of CCOC is made primarily on the attributes of the histopathological features [9].
Clear cells in the head and neck evoke a broad differential diagnoses that include odontogenic, salivary, metastatic, and melanotic tumors. Eversole LR, et al. proposed that the clear cells in the odontogenic tumors were the pre-secretory ameloblasts, and on electron microscopic examination they found these clear cells to be organelle poor cells that contained lysosomes, mitochondria, tonofilament bundles and desmosomes, and lacked secretory granules [3,4]. The most common odontogenic tumor with clear cell component is the clear cell variant of calcifying epithelial odontogenic tumor (CEOT) [13]. This is a benign, albeit locally aggressive neoplasm, which is composed of nests and sheets of polygonal clear cells, concentric ‘Liesegang ring’ calcifications, ghost cells and the fibrovascular stroma containing amyloid [4]. This differential diagnosis was excluded in the present case as it lacked amyloid (as indicated by Congo-red negativity), and had no areas of psammomatoid calcification or ghost cells. Waldron CA, et al. described a clear cell odontogenic carcinoma with regional metastasis which they termed as clear cell ameloblastoma (CCA) in the same year as Hansen et al. initially reported CCOT [3,5]. Most authors opine that CCA, clear cell ameloblastic carcinoma (CCAC) and CCOC are a single neoplastic entity and represent a clinicopathological continuum, while others suggest that they should be regarded as separate entities [5,13]. CCAC occurs in older individuals and is usually associated with recurrence or a carcinomatous change in a long-standing ameloblastoma [11]. CCAC histopathologically shows ameloblastoma histology with clear cells, increased mitotic activity, focal areas of necrosis, and cells with hyperchromatic pleomorphic nucleus [11]. Since our case did not have the clinical history or histopathological features of CCAC, this differential diagnosis was ruled out. The review of literature did not help resolve our nosological dilemma on reporting our case as CCA or as CCOC. In the past the terms CCA and CCOT has been used as synonyms for CCOC [10]. Werle et al. in their systematic review of 59 published cases found that 17 (29%) cases that were primarily diagnosed as ameloblastoma were later classified as CCOC [11]. Most authors including WHO considers clear cell anaplasia as dedifferentiation of odontogenic epithelium and regarded clear cells as an ominous sign that leads to local invasion, metastasis, recurrence, and sometimes fatal outcome [7,9]. Hence it was strongly advocated to consider all clear cell odontogenic neoplasms as low grade malignancies [6-9]. We concluded that caution needs to be exercised in odontogenic neoplasms with clear cells and that detailed postoperative follow-up was mandatory, and hence we felt justified in giving a HPE report of CCOC.
Hyalinizing clear cell carcinoma (HCCC) and the clear cell intraosseous mucoepidermoid carcinoma (CCMEC) were the other major consideration in our differential diagnoses. HCCC is a malignancy of the minor salivary glands, more commonly involving the soft tissues of palate and the base of tongue, and a ‘central’ variant is proposed to occur due to adjacent bone destruction and involvement [4]. Based on the current histopathological observations and immuno phenotype published in the scientific literature it is impossible to distinguish HCCC from monophasic CCOC, despite the different cell of origin [4,5,10]. The location and the primary site of the lesion is the main diagnostic criterion to distinguish the two [4,10]. Recent molecular studies have reported that both HCCC and CCOC have EWSR1-ATF1 translocation, and 83% of CCOC had EWSR1 rearrangement [10,14]. Balanced reciprocal translocations has been observed in approximately one-third of the sarcomas and EWSR1-ATF1 translocation in CCOC could signal the aggressiveness of this lesion [14]. Primary intraosseous localization, absence of any salivary gland tissue, and desmoplastic ameloblastoma-like areas helped differentiate our case from HCCC. The intra-osseous CCMEC is very rare and the triphasic architecture of mucous, epidermoid and intermediate cells along with clear cells gave these lesions a distinctive histopathological appearance [5]. Lack of cystic spaces lined by mucous cells, absence of intermediate cells, squamous differentiation, and mucin (as indicated by mucicarmine negativity) helped exclude CCMEC from our diagnosis. Other rarer salivary gland neoplasms with clear cell histopathological appearance include the clear cell myoepithelial carcinoma, clear cell oncocytoma, and clear cell acinic cell carcinoma [4]. These tumors have distinct morphological, histopathological and immunophytic appearances that help pathologists to distinguish them from CCOC [4,5].
The other consideration in our differential diagnoses was to exclude the possibility of metastatic lesions such as renal cell carcinoma, thyroid carcinoma, and clear cell breast carcinoma. Our case had no clinical signs or symptoms of renal, thyroid or breast carcinoma and was negative for any pathology under ultra-sound evaluation of these organs. Lack of prominent intra-tumoral hemorrhage, sinusoidal vascularity, and other histopathological features that characterize these metastatic carcinomas helped in excluding metastatic lesions from our diagnosis. Melanocytic tumor could be excluded from our differential diagnosis by the absence of melanoma associated antigen HMB-45, as well as from the fact that most of these tumors arise in the soft tissues and never occur as a primary intra-osseous lesion.
The clinical and biological behavior of CCOC is complicated by the different prognosis and mortality rate reported in the scientific literature. The aggressiveness of these neoplasms are documented by some authors as extensive invasion of adjacent tissues, regional metastasis to the lymph nodes, less frequent distant metastasis to the lungs, and a recurrence rate of 55% [1,4]. The management of CCOC are a challenge as the most appropriate treatment for a tumor is determined by a definitive diagnosis and adequate understanding of the biologic behavior of the tumor. Because of the insufficiency in the number of cases and the varied prognosis reported, various therapeutic approaches have been applied by the surgeons over the years including curettage, enucleation, en bloc resection, and subtotal mandibulectomy/maxillectomy [1,7,8]. Recurrence (80%) within 2 year and/or metastasis developed in cases, which underwent curettage or enucleation, and hence wide local resection with partial mandibulectomy/maxillectomy with clear margins was the treatment of choice [5-10]. Fatal clinical outcome has been reported in cases with distant metastasis [8-10]. Treatment protocol included lymph node resection and radical surgery if the nodes were positive, and adjuvant chemotherapy and/or radiotherapy for those with tumor positive margins and/or regional/neural/vascular invasion [5-8]. Adjuvant radiotherapy at carcinoma doses of 6600 cGy at 200 cGy per fraction delivered once a day or a total dose of 7440 cGy at 120 cGy per fraction delivered twice daily is advocated by some authors [7,8]. Chemotherapy is usually reserved for palliative care as there is lack of evidence for its efficacy in definitive treatment [8]. Most authors acknowledge that this rare tumour has an aggressive behavior and consider them as low grade malignant odontogenic neoplasm which require a long term follow-up [5]. In the presenting case to maintain the quality of life in the young girl and due to variable prognosis reported in the scientific literature a segmental osteotomy through intraoral approach and iliac bone graft with titanium bone plate reconstruction was performed. Lymph node resection or neck dissection was not performed as there was no clinically palpable lymphadenopathy. No radiotherapy or chemotherapy was provided as the resected margins were tumor free. Recurrence has not been identified in the 1.4 year of follow-up, and the young girl is leading a satisfactorily happy life.
Conclusion
The scientific literature has reported less than 124 cases of CCOC after 30 year of its first reporting. Because of the paucity in CCOC cases that gave varied prognosis the scientific fraternity has limited knowledge about its biological behavior and this complicates establishing a standard criteria for diagnosis and management. The authors hope that the reporting of this additional case contributes to a better characterization of the epidemiology, histopathology, and prognosis of this odontogenic carcinoma, and help the clinicians to arrive at a better informed management option. It is recommended to avoid usage of confusing terms like clear cell ameloblastoma (CCA) and clear cell odontogenic tumor (CCOT), as clear cell dedifferentiation in odontogenic neoplasms is considered as low grade malignancy.
References
- Maiorano E, Altini M, Viale G, Piattelli A, Favia G, et al. (2001) Clear Cell Odontogenic Carcinoma: Report of Two Cases and Review of the Literature. Am J Clin Pathol 116: 107-114.
- Panda S, Sahoo RS, Srivastav G, Padhiary S, Dhull KS, et al. (2014) Pathogenesis and Nomenclature of Odontogenic Carcinomas: Revisited. J Oncol 2014: 197425.
- Eversole LR, Belton CM, Hansen LS (1985) Clear-Cell Odontogenic Carcinoma: A New Case and Long-Term Follow-Up of an Old Case, and Review of the Literature. J Oral Pathol 14: 603-614.
- Bilodeau EA, Hoschar AP, Barnes EL, Hunt JL, Seethala RR, et al. (2011) Clear Cell Carcinoma and Clear Cell Odontogenic Carcinoma: a Comparative Clinicopathologic and Immunohistochemical Study. Head Neck Pathol 5: 101-107.
- Waldron CA, Small IA, Silverman H (1985) Clear cell ameloblastoma--an odontogenic carcinoma. J Oral Maxillo fac Surg 43: 707-717.
- Xavier FA, Rodini CO, Ramalho LP, Sarmento VA, Nunes FD, et al. (2008) Clear cell odontogenic carcinoma: case report with immunohistochemical findings adding support to the challenging diagnosis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 106: 403-410.
- Loyola AM, Cardoso SV, De Faria PR, Servato JS, De Paulo LB, et al. (2015) Clear cell odontogenic carcinoma: report of 7 new cases and systematic review of the current knowledge. Oral Surg Oral Med Oral Pathol Oral Radiol 120: 483-496.
- Chera BS, Villaret DB, Orlando CA, Mendenhall WM (2008) Clear cell odontogenic carcinoma of the maxilla: a case report and literature review. Am J Otolaryngol Head Neck Med Surg 29: 284-290.
- Li T, Yu S, Gao Y, Wang E (2001) Clear Cell Odontogenic Carcinoma: A Clinicopathologic and Immunocytochemical Study of 5 Cases. Arch Pathol Lab Med 125: 1566-1571.
- Richardson MS, Muller S (2014) Malignant Odontogenic Tumors: An Update on Selected Tumors. Head Neck Pathol 8: 411-420.
- Werle H, Blake FAS, Reichelt U, Schmelzle R, Heiland M, et al. (2009) Clear-Cell Odontogenic Carcinoma: A New Case and Long-Term Follow-Up of an Old Case, and Review of the Literature. J Oral Maxillo fac Surg 67: 1342-1348.
- Zurac S,Slootweg PJ (2009) Mitotic index in clear cell odontogenic tumors. Ther Pharmacol Clin Toxicol 13: 172-178.
- Braunshtein E, Vered M, Taicher S, Buchner A (2003) Clear cell odontogenic carcinoma and clear cell ameloblastoma: a single clinic opathologic entitiy? A new case and comparative analysis of the literature. J Oral Maxillofac Surg 61: 1004-1010.
- Yancoskie AE, Sreekantaiah C, Jacob J, Rosenberg A, Edelman M, et al. (2014) EWSR1 and ATF1 rearrangements in clear cell odontogenic carcinoma: presentation of a case. Oral Surg Oral Med Oral Pathol Oral Radiol 118: e115-118.
Citation: Jayapalan CS, George A, Noufal A, Pynadath MK, Mangalath U (2016) Clear Cell Odontogenic Carcinoma (CCOC): Mini-Review of Literature and Case Report of Mandibular Radiolucency in 17-year Girl. Diagn Pathol Open 1: 120. DOI: 10.4172/2476-2024.1000120
Copyright: ©2016 Jayapalan CS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 14707
- [From(publication date): 11-2016 - Jul 02, 2025]
- Breakdown by view type
- HTML page views: 13719
- PDF downloads: 988