Research Article Open Access
Cisplatin-Induced Ovarian Cytotoxicity and the Modulating Role of Aqueous Zest Extract of Citrus limonium (AZECL) in Rat Models
Akunna GG1*, Nwafor J1, Egwu OA1, Ezemagu UK1, Obaje G1, Adepoju LH1 and Akingbade AM2
1Department of Anatomy, Federal University Ndufu-Alike Ikwo (FUNAI), Ebonyi State, Nigeria
2Department of Anatomy, Afe Babalola University, Ado Ekiti, Nigeria
- *Corresponding Author:
- Akunna GG
Department of Anatomy, Faculty of Basic Medical Sciences
Federal University Ndufu-Alike Ikwo (FUNAI)
Ebonyi State, Nigeria
Tel: +23408038619526
E-mail: ggakunna@gmail.com, gabriel.akunna@funai.edu.ng
Received date: June 29, 2017; Accepted date: July 11, 2017; Published date: July 17, 2017
Citation: Akunna GG, Nwafor J, Egwu OA, Ezemagu UK, Obaje G, et al. (2017) Cisplatin-Induced Ovarian Cytotoxicity and the Modulating Role of Aqueous Zest Extract of Citrus limonium (AZECL) in Rat Models. J Tradit Med Clin Natur 6:228.
Copyright: © 2017 Akunna GG, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Traditional Medicine & Clinical Naturopathy
Abstract
Cisplatin is a prominent member of the effective broad-spectrum antitumor drugs. However, its clinical usage is restricted due to some adverse side effects, such as testiculototoxicity, hepatotoxicity and nephrotoxicity. The aim of this study is to evaluate the effect of Aqueous Zest Extract of Citrus limonium (AZECL) on the ovary of female Wistar rat treated with Cisplatin. Twenty adult female Wistar rats were divided into four groups (A-D) containing five rats each. Group A rats served as negative control and were treated orally with 2.5 ml/kg body weight of normal saline, group B rats served as positive control group and were treated intraperitoneally with a single dose of 10 mg/kg body weight of Cisplatin, group C rats were treated orally with 50 mg/kg body weight of AZECL and group D rats were treated intraperitoneally with a single dose of 10 mg/kg body weight of Cisplatin and two weeks later treated orally with 50 mg/kg body weight of AZECL. Result showed a significant (p<0.01) decrease in primary follicles, secondary follicles, graafian follicles and a significant (p<0.01) increase in atretic follicles, PAS positive reaction and reduction in the total carbohydrate contents of the stromal cells in the positive control group. Also there was a significant (p<0.05) decrease in the activity level of FSH, LH and a significant (p<0.05) increase in Malondialdehyde when compared to rats in group A and C. The group post-treated with the extract had remarkable normalization of the histo-morphometric, histochemical and biochemical parameters when compared to the positive control group. Aqueous zests extract of C. limonium has a curative effect on cisplatin-induced cytotoxicity on the ovary.
Keywords
Citrus limonum zest; Cisplatin; Ovary; Histo-morphometry
Introduction
Cisplatin is a chemotherapeutic agent used for the treatment of a wide variety of cancers. Cisplatin have been implicated in premature ovarian failure, changes in the estrous cycle, increased follicular apoptosis, and a reduction in the number of Anti-Mullerian Hormone (AMH) secreting follicles among several women undergoing chemotherapy [1,2]. Ovarian failure is attributed to the inability of primordial follicular cells to regenerate [3]. Cisplatin treatment has also been associated with conditions such as nephrotoxicity, neurotoxicity, and reproductive toxicity among others [4].
Lipid peroxidation, mitochondrial damage, and DNA injury has been associated with increased generation of Reactive Oxygen Species (ROS), a major mechanism pathway for cisplatin-induced toxicity [5-9].
Citrus limonium (Lemon) is an important food source of the plant family Rutaceae. Generally, antibacterial, antifungal, antidiabetic, anticancer and antiviral activities of citrus flavonoids have been shown [10-12]. Peel, flowers and leaves of Citrus aurantium have been employed in minimizing central nervous system disorders in traditional medicine [13]. Flavonoids, a major phytochemical in citrus fruits has been shown to act as direct antioxidants and free radical scavengers together with modulating enzymatic activities and inhibiting cell proliferation. It has also been reported that fiber of citrus fruit contains bioactive compounds, such as ascorbic acid. In this study, we aimed to investigate modulating role effect of AZECL on the ovary of female Wistar rat treated with anticancer agent Cisplatin. We believed that AZECL will attenuate cisplatin-induced toxicities by reducing number of atretic follicules, lipid peroxidation by measures of malondialdehyde, total carbohydrate contents of the stromal cells through PAS positive reactions and increase in activity level of FSH, LH.
Materials and Methods
Plant materials
Plant source and identification: Fresh lemon fruits (Citrus limonum) were obtained from a farmland, identified and authenticated at the Department of Plant Science and Biotechnology, Faculty of Science, Ebonyi state University.
Preparation of aqueous zest extract of Citrus limonum: Lemon fruits were peeled with a zester or grater [14]. The zests were rinsed in clean water and dried at room temperature for about 2 weeks. It was grinded and reduced to a powdered form.
Calculated amount of volume of distilled water and powdered sample were mixed and the mixture was allowed to stand for 30 min before filtration. The mixture was then centrifuged and supernatant were collected, cleaned of particles by suction filtration using Whatmann no 1. Filter paper and cellulose filter paper. The extracts was then concentrated to dryness in vacuum at 40°C using a rotary evaporator and stored in a desiccator. Fresh solution of the different extract was then prepared in normal saline as vehicle when required [15,16].
Phytochemical analysis of the extracts: The phytochemical analysis to determine the presence of Alkaloids, Flavonoids, Saponins and Tannins was done as describe by Akunna et al. [16]
Animals
Twenty female Wistar rats (mature) weighing 100-150 g were obtained from Department of Pharmacology, Faculty of Pharmacy, University of Nigeria Nsukka (UNN).
The animals acclimatize for 2 weeks and were fed freely on standard commercial mouse cubes from Federal University Ndufu-Alike Ikwo (FUNAI).
Constant environmental condition were maintained (12 h light-12 h dark and 24°C ± 30°C). The weights of the animals were estimated using an electronic analytical and precision balance (PA, 4102).
Permission from the departmental ethical committee on animal research was gotten before the start of the experiment.
Experimental procedures involving the animals and their care were conducted in conformity with International, National and institutional guidelines for the care of laboratory animals in Biomedical Research and Use of Laboratory Animals in Biomedical Research as promulgated by the Canadian Council of Animal Care.
Animal groupings and treatments
This study was conducted in four (A, B, C and D) undisturbed cages. Twenty female rats were divided into four groups of five rats each. Group A rats served as the negative control group and were treated with 5 ml/kg body weight of normal saline daily for 3 weeks. Group B rats served as positive control and were treated intraperitoneally with a single dose of 10 mg/kg body weight of Cisplatin. Group C rats were treated orally with 50 mg/kg body weight of AZECL per day for 3 weeks while Group D rats were treated with a single dose of 10 mg/kg body weight of cisplatin and 10 days later were orally treated with 50 mg/kg body weight of AZECL per day for 11 days.
Animal sacrifice and sample collection
The rats were weighed and sacrificed by cervical dislocation. Blood samples were collected from the heart of each rat immediately after sacrifice with the aid of a 21G needle mounted on a 5 mL syringe (Hindustan Syringes and Medical Devices Ltd., Faridabad, India). This was inserted into the heart based on prior palpation of the apex beat. At least about 5 ml of blood was aspirated after which the thoracic cage was opened to allow direct access and more blood collected under adequate direct visualization of the heart. The blood obtained into tubes containing 2% sodium oxalate and centrifuged (3000 rpm for 10 min) accordingly using a table top centrifuge (P/C 03) and the serum extracted. The abdominal cavity was opened up through a midline abdominal incision and the ovaries were excised and one of the ovaries from each animal was fixed in Bouin’s fluid for histomorphometric analysis. Serum and the remaining ovary homogenate of each animal were stored at -25oC for biochemical assays.
Determination of biochemical parameters
Serum hormonal assays-Luteinizing Hormone (LH), Follicle Stimulating Hormone (FSH) and progesterone (PROG). The assays were done according to the procedure adapted by Amballi et al. [17]. Briefly, one aliquot of each centrifuged specimen was taken at a time, to avoid repeated freezing and thawing, and the samples were analyzed for hormone estimation using Enzyme Immunoassay (EIA), according to the World Health Organization (WHO) matched reagent programme protocol (manual) for EIA kits (protocol/version of December 1998 for LH, FSH). Serum progesterone was determined by ELISA using MAP LAB PLUS (Biochemical systems international, RM 2060) according to the manufacturer’s direction.
Estimation of lipid peroxidation (Malondialdehyde)
Lipid peroxidation in the ovarian tissue was estimated colorimetrically by thiobarbituric acid reactive substances (TBARS) method of Buege and Aust as described by Saalu [18]. It was expressed as nmol/mg protein.
Tissue preparation for histology and histochemistry
The fixed tissues were processed for histological and histochemical analysis as described by Akunna et al. [19]. Sections were stained with H&E and Periodic Acid-Schiff (PAS) reaction with hematoxylin counterstaining for histological and histochemical study respectively. The slides were viewed under a research microscope connected to a computer monitor for qualitative and quantitative evaluation.
Morphometric analysis
Ovarian follicles were identified and classified as described by Peters and Natty [20] while atretic follicles were identified following morphological criteria described by Greenwald and Roy [21].
Statistical Analysis
The results gotten from this study were expressed as mean ± SD of different groups. The differences between the mean values were evaluated by ANOVA followed by Student’s “t” test using SPSS (version 20).
Results
Results of phytochemical analysis
We observed the presence of saponins, tannins and flavonoids, with tannins constituting the highest while Saponin was noted to be the lowest. Alkaloids were not detected in the extract as shown in Table 1.
| Parameters | Citrus limone | mg/g |
|---|---|---|
| Saponin | +ve | 0.81 ± 0.00 |
| Tannins | +ve | 47.0 ± 31.05 |
| Alkaloids | -ve | - |
| Flavonoids | +ve | 11.4 ± 33.05 |
Table 1: Phytochemical constituents of AZECL.
Body Weight of Rats
The results of the body weight were outline. This result showed a progressive increase in body weight of rats used in this experiment throughout the period of study. However, there was a non-significant (p>0.05) decrease in body weight of the animals in group B at 2nd week of administration as shown in Table 2.
| Treatment Groups | Initial Weight | Weight 1 | Weight 2 | Weight 3 |
|---|---|---|---|---|
| Group A | 113.4 ± 8.3 | 127.8 ± 9.9 | 136.6 ± 10.7 | 146.0 ± 11.1 |
| Group B | 131.0 ± 18.6 | 145.0 ± 21.1 | 143.7 ± 10.2 | 145.6 ± 8.1 |
| Group C | 118.0 ± 13.5 | 125.4 ± 14.7 | 127.0 ± 17.1 | 128.7 ± 17.7 |
| Group D | 110.4 ± 14.5 | 117.8 ± 14.9 | 124.6 ± 14.3 | 125.0 ± 16.3 |
Table 2: Effect of cisplatin and AZECL on body weights (g) of female Wistar rats.
Histomorphometry of the Ovary
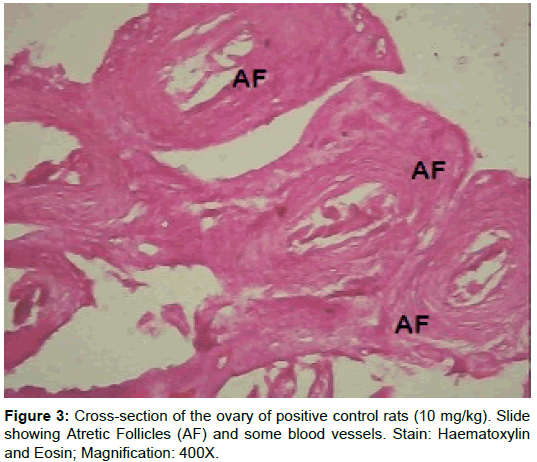
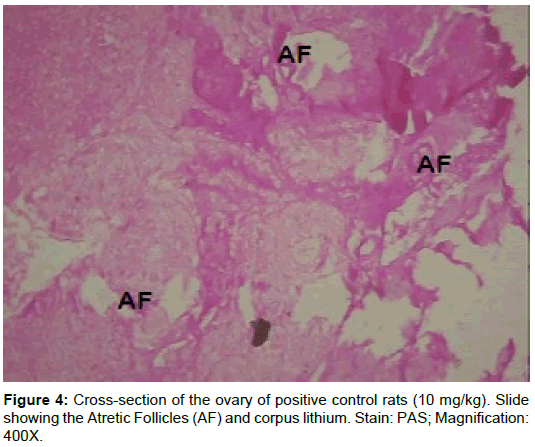
The morphology of the ovaries was verified on the following: Primary follicles, secondary follicles, graffian follicles and atretic follicles (Table 3). Results showed a significant (p<0.01) decrease in primary follicles, secondary follicles, graffian follicles and a significant (p<0.01) increase in atretic follicles of rats treated with cisplatin-alone (group B). The rats in group C had geometric values comparable to that of the negative control group. There was a significant (p<0.05) increase in primary follicles, secondary follicles and graftable fian follicles in rats post-treated with AZECL (group D) and a significant (p<0.01) decrease in atretic follicles when compared to the rats treated with only cisplatin (group B).
| Treatment Groups | Primary Follicles | Secondary Follicles | Graffian Follicles | Atretic Follicles |
|---|---|---|---|---|
| Group A | 10.2 ± 2.1 | 5.8 ± 0.8 | 4.2 ± 0.4 | 5.0 ± 0.7 |
| Group B | 4.0 ± 0.7** | 2.4 ± 0.5** | 1.8 ± 0.4** | 21.8 ± 3.2** |
| Group C | 10.6 ± 1.8 | 6.2 ± 0.8 | 4.4 ± 0.5 | 5.2 ± 0.8 |
| Group D | 7.8 ± 1.6a | 4.4 ± 0.5a | 4.2 ± 0.4a | 11.2 ± 1.6aa |
a,aaRepresent significant increases or decreases at p<0.05 and (p<0.01) respectively when compared to group C Values are means ± SD; n=5 in each group.
Table 3: Effect of cisplatin and AZECL on primary follicles, secondary follicles, graffian follicles and atretic follicles of female Wistar rats.
Histochemical Observations
Total carbohydrates
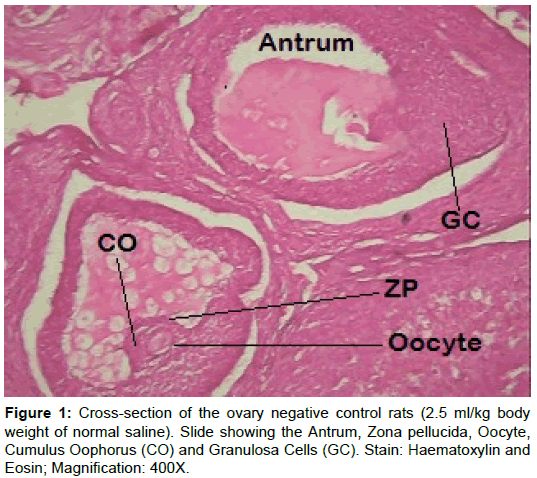
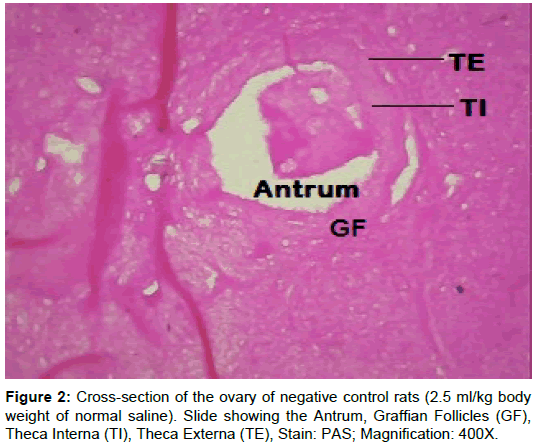
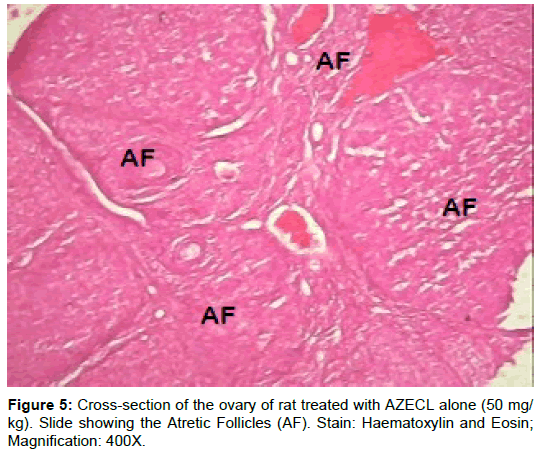
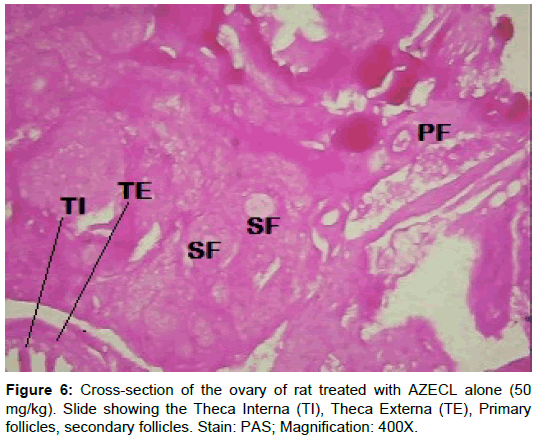
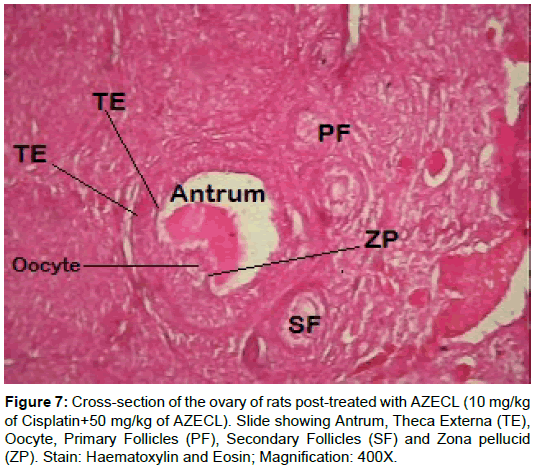
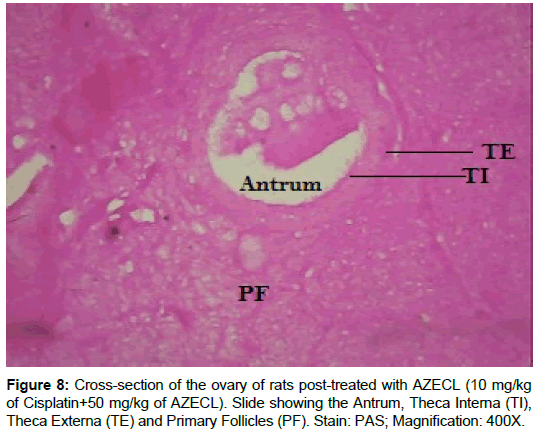
Examination of ovary of control rats revealed that the germinal epithelial cells and the stromal cells showed a slight PAS positive reaction. The ovum of primary, secondary and Graafian follicles showed a moderate reactivity while the cytoplasm of their granulosa was slightly stained. The Zona pellucida encircling the oocyte in the different types of the follicles had a marked reaction. The corona radiata and the theca folliculi showed slightly positive PAS-reaction whereas the antrum of the follicles was negatively stained. The luteal cells of the corpora lutea appeared slightly reactive with PAS-reaction. The core of the atretic follicles showed a moderate PAS-positive reaction. Examination of ovary of rats treated with cisplatin and AZECL showed reduction in the total carbohydrate contents of the stromal cells, the ovum of primary, secondary and Graafian follicles compared with those of cisplatin alone treated animals (Figures 1-8).
Biochemical Results
The effect of cisplatin and AZECL on the three main female reproductive hormones namely FSH, LH and PROG activities and MDA levels were verified as shown in Table 4.
| Treatment Groups | FSH (mlU/ml) |
LH (mlU/ml) |
PROG (ng/ml) | MDA (nmol/mg) |
|---|---|---|---|---|
| Group A | 3.8 ± 0.2 | 2.3 ± 0.11 | 51.8 ± 0.7 | 0.5 ± 0.07 |
| Group B | 1.9 ± 0.03* | 0.7 ± 0.09* | 30.6 ± 0.8** | 3.8 ± 0.12** |
| Group C | 3.2 ± 0.16 | 2.5 ± 0.06 | 50.3 ± 1.4 | 0.5 ± 0.03 |
| Group D | 2.4 ± 0.77 | 1.5 ± 0.07 | 37.8 ± 5.1 | 2.7 ± 0.5* |
Table 4: Biochemical results.
The rats treated with cisplatin alone had a significant (p<0.05) decrease in the level of FSH, LH and a significant (p<0.01) decrease in the activity level of PROG when compared with those of the negative control.
The rats in group C had a fluctuation in the level of FSH, LH and PROG but geometric values comparable to that of rats in group A. There was a significant (p<0.05) improvement in the activity level of FSH, LH and PROG in rats post-treated with the extract (group D) rats when compared to rats in group B. In the level of MDA the animals in group B showed a significant (p<0.01) increase when compared to animals in group A while the animals in group D showed a significant (p<0.05) decrease in the level of MDA when compared to animals in group B.
Discussion
General considerations
The use of cisplatin has been reduced due to severe cytotoxic side effects [22-24]. Due to elevated mitotic activities, gonads are one of the main targets [25].
Chemotherapy treatment has long been associated with premature ovarian failure and infertility in premenopausal patients. It is often assumed that chemotherapy drugs directly damage oocytes in the primordial follicle reserve and that it is this loss that leads to premature ovarian failure [26,27].
Animal-based researches have improved our understanding the underlying processes and a way forward to restoring the reproductive potential [28-34].
Gross Anatomical Parameters
In this study, the results show a progressive increase in body mass of experimental models throughout the duration of the study. Nevertheless, there exists a non-significant decrease in body weight of the animals in group B at 2nd week of administration. Our results in this regard were not in line with that of Atessahin et al. This could mean that the experimental animals have not passed their active growth phase or that the feeding habit was increased as a result of cisplatin toxicity [35,36].
Although Saalu et al.; Akunna and Ogunmodede; Akunna; Akunna and Saalu [32-40]; Akunna et al. [39-41]; Akingbade et al. [42]; Malarvizhi and Mathur [43], Setchell [44] and Creasy [45] have associated gonadal weight to infertility, it is important to state at this juncture that we couldn’t determine the weight of the ovary because of the surrounding fats and adipose tissue which may influence the results.
The Morphological Effects
From our study, the follicular count showed a significant decrease in primary follicles, secondary follicles, graffian follicles and a significant (p<0.01) increase in atretic follicles of rats in group B when compared to the corresponding control (Group A). Data here are consistent with these findings, showing an increase in unhealthy follicles, and a reduction in total follicle number, following treatment with Cisplatin [27,46].
According to Perez [47], mice exposed to chemotherapeutic agents displayed apoptosis of granulosa cells in primordial follicles. Meirow [26] also evaluated human ovarian slices post-cisplatin exposure in vitro and his reports were consistent with that of Perez [48]. Our results were also in line with these findings as revealed by the impaired integrity of the surrounding granulosa layer in most of the follicles examined and significantly reduced number of primordial cells in cisplatin alone treated rats. Of course the damaging effects of cisplatin on ovarian follicles have been previously reported [49,50]. Yucebilgin et al. [1] reported a significantly damage primordial follicles in rats after a single dose of cisplatin (5 mg/kg) and paclitaxel (7.5 mg/kg) [1]. The geometric results in our study are in line with other reports on cisplatin-induced injury [51,52].
Histochemical results showed that rats treated with cisplatin alone had an increase in carbohydrate content when compared to the negative control as shown by histochemistry. The increased carbohydrate contents may be due to disturbance in carbohydrate metabolism. A significant increase in both blood glucose and liver glycogen in experimental animals have been reported.
The biochemical effects
The ovarian toxicity recorded in this study might be due to increase in lipid peroxidation resulting from the toxicity of cisplatin or its metabolite as shown previously in our laboratory [53]. Mathews [54] reported that the damage occurred in the cell membrane by hydroxyl radicals induced oxidation. This could also explain the marked degeneration, atrophied ovary and decrease number of viable follicles reported in this study which are in line with several reports [23,25,55-57].
Oxidative stress, a condition of an imbalance between free radicals and antioxidant defense system, is an important factor in the pathogenesis system that has high content of polyunsaturated membrane lipid [58]. Recent study indicated that cisplatin led to neurotoxic effects in human and animals via induction of lipid peroxidation [59]. Our present work showed that cisplatin produced a significant increase in MDA level that induced lipid peroxidation in the brain tissues accompanied with suppression in SOD activity [60]. On the other hand, therapeutic effects of cisplatin based on the interaction with DNA in the cell, preventing proliferation and inducing apoptosis in tumor cells. According to Mukhopadhyay et al. [61]; cisplatin induced mitochondrial ROS generation triggered inflammatory response, cell death and ovarian dysfunction.
It has also been suggested that cisplatin is able to generate reactive oxygen species by inducing glucose-6-phosphate dehydrogenase and hexokinase activity and inhibit the activity of antioxidant enzyme in tissue such as SOD, CAT, and GSH-Px [62].
MDA, an aldehyde product of lipid peroxidation, is commonly used as a marker of oxidative stress in cells and in the present study; we observed a significant increase in ovary MDA levels in cisplatin-alone treated. Our result is line with that of Atasayar et al. [63].
The rat models post-treated with AZECL had remarkable normalization of these parameters. Due to abundant phytochemicals in plants, they have been utilized for various ailments traditionally and cisplatin induced toxicity [64,65]. Improved ovarian histology and function post-antioxidants treatment has been reported severally [66,67].
In this study, we observed a significant decrease (p<0.05) in the level of FSH, LH and PROG in rat treated with cisplatin. FSH stimulates the growth and maturation of ovarian follicles by acting directly on the receptors which are located on the granulosa cells. As indicated in our study, the reduction in the levels of FSH by the cisplatin might have hampered folliculogenesis and delayed maturation of the follicle in the pre-ovulatory phase. It is also likely that that cisplatin might have had effect on the hypothalamus since the secretion of FSH is regulated by the hypothalamus through gonadotropic releasing hormone secreted.
It has been indicated that LH release surges at the proestrus stage initiates ovulation. Hence any substance capable of preventing the release of LH could interfere with ovulation by decreasing the number of mature follicles [68]. The group of rat that where post treated with AZECL had a significant (p<0.05) improvement in the level LH. Alkaloids and flavonoids from plants have been shown to reduce plasma concentrations of LH, estradiol and FSH [69]. Therefore, the presence of these phytochemicals (flavonoids) in AZECL in may account in part for the improvements observed in our study.
The decreased level of MDA after AZECL treatment may be a defense mechanism against oxygen free radical damage. Concerning the effect of Citrus limonum, when animals treated with cisplatin followed by AZECL marked improvement in the histological picture of ovary and the number of healthy follicles was seen as compared with ovary of animals treated with cisplatin alone.
Plants such as Zingiber officinale, Hibiscus sabdariffa and Curcuma longa have been reported to reduce CIS-induced toxicity [70-74].
The curative potential of AZECL might be attributed to these active molecules individually or synergistically indirectly through pituitary-gonadal axis or directly by sensitizing the follicular receptors to the available gonadotrophins. Also, the reduction in follicular atresia by AZECL may be due to availability of extra ovarian regulators, the gonadotrophins (FSH and LH). The active substances in the AZECL might have stimulated follicular growth in the ovaries by interfering at the level of receptors and mRNA expression of these intra ovarian and intra follicular regulating factors. Post-treatment with AZECL could have attenuated the cisplatin ovarian toxicity through a reduction of free radicals.
Conclusion
This study has demonstrated that post-treatment with aqueous zest extract of Citrus limonum containing powerful antioxidant vitamins and citrus bioflavonoids exerted a potent protective activity against cisplatin-induced morphological and biochemical impairment of the ovary of Wistar rats.
Limitation of the Study
We couldn’t isolate the active ingredients from AZECL hence structural elucidation was out of the picture. This could have provided more insight into the possible mechanism of action of AZECL.
References
- Yucebilgin MS, Terek MC, Ozsaran A (2004) Effect of chemotherapy on primordial follicular reserve of rat: an animal model of premature ovarian failure and infertility. Aust N Z J Obstet Gynaecol 44: 6-9.
- Yeh J, Kim SB, Peresie J (2011) Reproductive toxic effects of cisplatin and its modulation by the antioxidant sodium 2-mercaptoethanesulfate (Mesna) in female Rats. Reprod Biol Insights 5: 17-27.
- Jeruss JS, Woodruff KT (2009) Preservation of fertility in patients with cancer. N Engl J Med 360: 858-911.
- Boulikas T, Vougiouka M (2003) Cisplatin and platinum drugs at the molecular level. Oncol Rep 10: 1663-1682.
- Deavall DG, Martin AE, Horner MJ, Roberts R (2012) Drug-induced oxidative stress and toxicity. J Toxicol 2012: 645460.
- Saalu LC, Oyewopo AO, Enye LA, Ogunlade B, Akunna GG, et al. (2012) The hepato-rejuvinative and hepato-toxic capabilities of Citrus paradisi Macfad fruit juice in Rattus Norvegicus. Afr J Pharm Pharmacol 14: 1056-1063.
- Ogunlade B, Akunna GG, Fatoba OO, Ayeni OJ, Adegoke AA, et al. (2012) Aqueous extract of vernonia amygdalina protects against alcohol-induced hepatotoxicity in Wistar Rats. World J Young Res 2: 70-77.
- Ogunlade B, Saalu LC, Ogunmodede OS, Akunna GG, Adeeyo OA, et al. (2012) The salutary role of Allium cepa extract on the liver histology, liver oxidative status and liver marker enzymes of rabbits submitted to alcohol-induced toxicity. J Lipid Res 2: 67-81.
- Ogunmodede OS, Saalu LC, Ogunlade B, Akunna GG, Oyewopo OA et al. (2012) An Evaluation of the Hypoglycemic, Antioxidant and Hepatoprotective Potentials of Onion (Allium cepa L.) on Alloxan-induced Diabetic Rabbits. Environ Toxicol Pharmacol 8: 21-29.
- Kawaii S, Tomono Y, Katase E, Ogawa K, Yano M, et al. (2000) Quantitative study of flavonoids in leaves of Citrus plants. J Agric Food Chem 48: 3865-3871.
- Burt SA (2004) Essential oils: their antibacterial properties and potential applications in foods: a review. Inter J Food Microbiol 94: 223-253
- Ortuno A, Baidez A, Gomez P, Arcas CM, Porras I, et al. (2006) Citrus paradise and Citrus sinensis flavonoids: Their influence in the defence mechanism against Penicilliumdigitatum. Food Chem 98: 351-358.
- Pultrini AM, Galindo LA, Costa M (2006) Effects of the essential oil from L. in experimental anxiety models in mice. Life Sci 78: 1720-1725
- Liogier HA (1990) Plantas medicinales de Puerto Ricoydel Caribe. San Juan, Puerto Rico p: 566.
- Akunna GG, Saalu LC, Ogunlade B, Akingbade AM (2013) Tackling infertility with medicinal plant. Planta Med 1: 93-105.
- Asuquo OR, Edet AG, Mesembe O, Atanghwo JI (2010) Ethanolic extracts of Vernonia amygdalina and Ocimum gratissimum enhance testicular improvement in diabetic Wistar rats. Internet J Altern Med.
- Amballi AA, Dada OA, Adeleye AO, Jide S (2007) Evaluation of the determination of reference ranges for reproductive hormones (prolactin, FSH, LH, and testosterone) using enzyme immuno assay method. Sci Res Essays 2: 135-138.
- Saalu LC (2012) The hepato-protective potentials of Moringa oleifera leaf extract on alcohol-induced hepato-toxicity in Wistar rat. Am J Biotechnol Mol Sci 2: 6-14.
- Akunna GG, Obikili EN, Anyanwu EG, Esom AE (2017) Protective and curative role of citrus sinensis peel in cadmium-induced testicular and spermatic damage: a morphometric and immunohistochemical evaluation using monoclonal antibodies against Ki-67 and proliferating cell nuclear antigen. Eur J Anat 21: 19-30
- Peters H, Natty KP (1980) The ovary: A correlation of strucure and function in Mammals. Granda Publishing, London, Toronto, Sydney and New York
- Greenwald GS, Roy SK (1994) Follicular development and its control. In the physiology of reproduction (2nd edn.). Raven Press Ltd., NY, USA.
- Cohen SM, Lippard SJ (2001) Cisplatin: from DNA damage to cancer chemotherapy. Prog Nucleic Acid Res 67: 93-130.
- Victoria C, Fuertes AM, Castilla J, Alonso C, Quevedo C, et al. (2007) Biochemical mechanisms of cisplatin cytotoxicity. Anticancer Agents Med Chem 7: 3-18.
- Noori S, Yeow S, Ziauddin SW, Xin FM, Tran H, et al. (2008) Cisplatin enhances the antitumor effect of tumor necrosis factor-related apoptosis-inducing ligand gene therapy via recruitment of the mitochondria-dependent death signaling pathway. Cancer Gene Ther 15: 356-370.
- Atessahin AI, Karahan G, Turk S, Yilmaz S, Ceribasi AO, et al. (2006) Protective role of lycopene on cisplatin induced changes in sperm characteristics, testicular damage and oxidative stress in rats. Reprod Toxicol 21: 42-47.
- Meirow D (2000) Reproduction post-chemotherapy in young cancer patients. Mol Cell Endocrinol 169: 123-131.
- Lopes M, Anderson G, Spears (2013) Cisplatin and doxorubicin induce distinct mechanisms of ovarian follicle loss, imatinib provides selective protection only against cisplatin. J Pone 8: 701-717
- Akingbade MA, Saalu LC, Oyebanji OO, Oyeniran AD, Akande OO, et al. (2014) Rhodinol-based incence testiculotoxicity in Albino rats: Testicular histology, Spermatogenic and Biochemical Evaluations. J Pharmacol Toxicol 9: 68-81.
- Ogunlade B (2013) Haematological indices and splenic histo-architecture of Wistar rat treated with aniline: supplementary role of Moringa oleifera leave extracts. Int J Biotechnol Allied Field 1: 136-144.
- Ogunlade B (2013) Highly active antiretroviral therapy: effects on foetal parameters heaematological indices and the histomorphology of the liver of the dams. Orient J Sci Res 2: 1
- Saalu LC (2013) The Comparison of three experimental rat Varicocele models: Their effects on Histomorphometry, Spermiogram and Oxidative Status. J Exp Clin Anat 12: 22-30.
- Saalu LC, Akuna GG, Enye LA (2013) Pathophysiology of varicocele: Evidences for oxidative stress as a mechanism pathway. Eur J Anat 17: 82-91.
- Saalu LC, Akunna GG, Ogunmodede OS (2013) Evidences for deleterous role of free radicals in experimental varicocele using animal model. Br J Med Med Res 3: 1125-1143.
- Akunna GG (2016) Spermiographic, 2 and 3-dimensional quantitative analysis of testicular tissues of rat submitted to citrus paradisi waste extract and cisplatin-induced cytotoxicity. Int J Cancer Res 12: 176-187.
- Akunna GG (2011) Effect of two Nigerian made perfumes on the liver of adult Wister rat. J Med Sci 11: 220-225.
- Akunna GG, Ogunmodede OS (2012) Laurus nobilis extract preserves testicular functions in cryptorchid Rat. World J Life Sci Med 2: 91.
- Akunna GG (2012) Ameliorating effect of Moringa oleifera leaf extract on chromium-induced testicular toxicity in rat. World J Life Sci Med Res 2: 20-26.
- Akunna GG, Saalu LC (2013) Anti-fertility role of allethrin based-mosquito coil on animal models. Int J Biol Pharm Allied Sci 2: 192-207.
- Akunna GG (2014) Spermatotoxicity in animal models exposed to fragrance components. J Med Sci 14: 46-50.
- Akunna GG, Akingbade AM, Faeji CO, Oni OI, Akande OO (2016) Reactive oxygen species generation could be a causative factor for perfume–induced testicular toxicity in male rat. Intl J Med Health Res 2: 28-34.
- Akunna GG, Saalu LC, Ogunlade B (2015) Histo-morphometric evidences for testicular derangement in animal models submitted to chronic and sub-chronic inhalation of fragrance. Am J Res Commun 3: 85-101.
- Akingbade AM, Akunna GG, Faeji CO (2015) Histomorphometric and spermatogenic evaluation of musk based-incense induced testisculotoxicity in adult Albino rats. Sch J App Med Sci 3: 2111-2117.
- Malarvizhi D, Mathur PP (1995) Effect of cisplatin on physiological status of normal rat testis. Indian J Exp Biol 33: 281-283
- Setchell BP (1998) Anatomy, vasculature, innervations and fluids of the male reproductive tract. New York, USA pp: 753-836.
- Creasy DM (2003) Evaluation of testicular toxicology: A synopsis and discussion of the recommendations proposed by the society of toxicologic pathology. Birth Defects Res B Dev Reprod Toxicol 68: 408-415.
- Shugaba AI, Asala AS, Gambo IM, Mohammed BM (2012) The effects of physical and oxidative stress on the ovary of the female Wistar rat. An Int J Life Sci Chem 29: 326-335.
- Perez GI (2000) Identification of potassium-dependent and-independent components of the apoptotic machinery in mouse ovarian germ cells and granulose cells. Biol Reprod 63: 1358-1369.
- Perez GI (1997) Apoptosis-associated signaling pathways are required for chemotherapy-mediated female germ cell destruction. Nat Med 3: 1228-1232.
- Yeh JB, Kim B, Liang YJ, Peresie J (2006) Müllerian inhibiting substance as a novel biomarker of cisplatin-induced ovarian damage. Biochem Biophys Res Commun 348: 337-344.
- Ozcelik B, Turkyilmaz C, Ozgun MT, Serin IS, Batukan C (2010) Prevention of paclitaxel and cisplatin induced ovarian damage in rats by a gonadotropin-releasing hormone agonist. Fertil Steril 93: 1609-1614.
- Nakai M (1995) Deformation of the rat Sertoli cell by oral administration of carbendazim (methyl 2-benzimidazole carbamate). J Androl 16: 410-416.
- Narayana K (2005) The antiviral drug ribavirin reversibly affects the reproductive parameters in the male Wistar rat. Folia Morphol 64: 65-71.
- Akunna GG (2013) Pyrethroid-based insecticide induces testicular toxicity via oxidative pathway: study suggest. Oriental J Sci Res 2: 1.
- Mathews CK (2000) Electron transport, oxidative phosphorylation and oxygen metabolism. Virginia 3: 523-557.
- Scholzen T, Gerdes J (2000) The Ki-67 protein: from the known and the unknown. J Cell Physiol 182: 311-322.
- Zhang X (2001) Cisplatin-induced germ cell apoptosis in mouse testes. Arch Androl 46: 43-49.
- Ferrara D (2006) Acute and long-term effects of in utero exposure of rats to di(n-butyl) phthalate on testicular germ cell development and proliferation. Endocrinol 147: 5352-5362.
- Boekelheide K, Schoenfeld HA, Hall SJ, Weng CC, Shetty G, et al. (2005) Gonadotropin-releasing hormone antagonist (Cetrorelix) therapy fails to protect nonhuman primates (Macaca arctoides) from radiation-induced spermatogenic failure. J Androl 26: 222-234.
- Acar N, Berdeaux O, Grégoire S, Cabaret S, Martine L, et al. (2012) Lipid composition of human eye: Are red blood cells a good mirror of retinal and optic nerve fatty acid? PLoS ONE 7: e35102.
- Kamisli S, Ciftci O, Kaya K, Cetin A, Kamisli O, et al. (2013) Hesperidin protects brain and sciatic nerve tissues against cisplatin-induced oxidative, histological and electromyographical side effects in rats. Toxicol Ind Health 31: 841-851.
- Mukhopadhyay P, Horváth B, Zsengellér Z, Zielonka J, Tanchian G, et al. (2011) Mitochondrial-targeted antioxidants represent a promising approach for prevention of cisplatin-induced nephropathy. Free Radic Biol Med 52: 497-506.
- Naziroglu M, Simsek M, Simsek H, Aydilek N, Ozcan Z, et al. (2004) The effects of hormone replacement therapy combined with vitamin C and E antioxidants level and lipid profiles in postmenopausal woman with type 2 Diabetes. Clin Chem Acta 344: 63-71
- Atasayar S, Orhan H, Gürel B, Girgin G, Özgüne√?¬? H, et al. (2009) Preventive effect of aminoguanidine compared to vitamin E and C on cisplatin-induced nephrotoxicity in rats. Exp Toxicol Pathol 61: 23-32.
- Yang HS, Han DK, Kim JR, Sim JC (2006) Effects of alpha- tocopherol on cadmium-induced toxicity in rat testis and spermatogenesis. J Korean Med Sci 21: 445-451.
- Azu OO, Duru FIO, Osinubi AA, Noronha CC, Elesha SO, et al. (2010) Protective agent, Kigelia Africana fruit extract, against cisplatin-induced kidney oxidant injury in Sprague–Dawley rats. Asian J Pharma Clin Res 3: 84-88.
- Peng SJ, Lu RK, Yu LH (1997) Effect of Semen cuacutae, Rhizoma curculiginis, Radix morindae, Officinalis on human spermatozoa’s motility and membrane function in vitro. Zhongguo Zhong Xi Yi Jie He Za Zhi 17: 145-147.
- Lirdi LC, Stumpp T, Sasso-Cerri E, Miraglia SM (2008) Amifostine protective effect on cisplatin-treated rat testis. Anat Rec Hoboken 291: 797-808.
- Benie T, Duval J, Thieulant ML (2003) Effect of some traditional plant extracts on rat oestrous cycle compared with clomid. Phytother Res 17: 748-755.
- Amin A, Hamza A (2006) Effects of ginger and roselle on cisplatininduced reproductive toxicity in rats. Asian J Androl 8: 607-612.
- Yüce A, Atessahin A, Ceribas AO, Aksakal M (2007) Ellagic acid prevents cisplatin-induced oxidative stress in liver and heart tissue of rats. Basic Clin Pharmacol Toxicol 101: 345-349.
- Ilbey YO, Ozbek E, Cekmen M (2009) Protective effect of curcumin in cisplatininduced oxidative injury in rat testis: mitogen-activated protein kinase and nuclear factor-kappa B signaling pathways. Hum Reprod 24: 1717-1725.
- Amin A, Ghoneim MD, Syam MI, Daoud S (2012) Neural network assessment of herbal protection against chemotherapeutic-induced reproductive toxicity. Theor Biol Med Model 24: 1.
- Vernet P, Aitken RJ, Drevet JR (2004) Antioxidant strategies in the epididymis. Mol Cell Endocrinol 216: 31-39.
- Kalender Y, Yel M (2005) Doxorubicin hepatotoxicity and hepatic free radical metabolism in rats. The effects of vitamin E and catechin. Toxicology 209: 39-45.
Relevant Topics
- Acupuncture Therapy
- Advances in Naturopathic Treatment
- African Traditional Medicine
- Australian Traditional Medicine
- Chinese Acupuncture
- Chinese Medicine
- Clinical Naturopathic Medicine
- Clinical Naturopathy
- Herbal Medicines
- Holistic Cancer Treatment
- Holistic health
- Holistic Nutrition
- Homeopathic Medicine
- Homeopathic Remedies
- Japanese Traditional Medicine
- Korean Traditional Medicine
- Natural Remedies
- Naturopathic Medicine
- Naturopathic Practioner Communications
- Naturopathy
- Naturopathy Clinic Management
- Traditional Asian Medicine
- Traditional medicine
- Traditional Plant Medicine
- UK naturopathy
Recommended Journals
Article Tools
Article Usage
- Total views: 3497
- [From(publication date):
August-2017 - Nov 21, 2024] - Breakdown by view type
- HTML page views : 2663
- PDF downloads : 834