Chronic Kidney Disease Epidemiology
Received: 01-May-2023 / Manuscript No. ECR-23-94344 / Editor assigned: 04-May-2023 / PreQC No. ECR-23-94344 / Reviewed: 18-May-2023 / QC No. ECR-23-94344 / Revised: 22-May-2023 / Manuscript No. ECR-23-94344 / Published Date: 29-May-2023
Abstract
Renal cell carcinoma (RCC) is specifically not one of those malignancies that is known to have a clear cause-effect aetiology. We can only make an effort to pinpoint a few clinical, occupational, and chemicals linked to carcinogenesis in RCC. Some of these variables that raise the risk of the RCC include smoking, asbestos or other chemical carcinogens, or organic solvents. Radiation therapy and viral infections have also been mentioned as risk factors. Several medications have been shown to enhance the prevalence of RCC and other neoplasms. Of fact, heredity has a significant impact on how some kidney cancer cases develop. Dialysis, hypertension, and chronic renal failure all require special consideration. Obesity, lifestyle, habits, and diet can all raise the risk of RCC. This article aims to provide an overview of the clearly identified causes of renal cell cancer.
Keywords: Chronic kidney disease; Renal replacement therapy; Risk factors; Chronic kidney failure; Epidemiology
Keywords
Chronic kidney disease; Renal replacement therapy; Risk factors; Chronic kidney failure; Epidemiology
Introduction
Not only does cell change give rise to a solid tumor's malignant potential, but also do a cancer cells intricate interplay with the stroma that supports it. The latter consists of neutrophils, monocytes, and macrophages as well as fibroblasts, endothelial cells, and an inflammation infiltrate that may be very complicated and contain other hematopoietic cells. Together, these cells create a microenvironment that enhances the potential for tumour growth and invasion [1]. A body of evidence demonstrating the significance of stromal cells for tumour formation has been gathered through analysis of stromal cells using various histology techniques or flow cytometry. Several tumour stromal cell molecular markers with strong diagnostic and prognostic potential have been found. Similar to other solid tumours, kidney tumours have a diverse microenvironment made up of both cancerous and healthy stromal cells as well as a significant number of macrophages [2].
At age 70, kidney cancer incidence reaches its peak and is twice as common in men as in women. The frequency of kidney cancer has increased globally during the last 20 years. About 25% of kidney cancers have significant metastatic potential and are already metastatic when they are diagnosed. Metastatic kidney cancer has a bad prognosis. After the discovery of metastases, the life expectancy is only 10–13 months without specialised therapy [3].
Smoking, obesity, chronic abuse of painkillers, acquired cystic kidney disease, hypertension, and other genetic illnesses are all risk factors for the development of RCC. Clear cell renal cell carcinoma (60–85%) and renal papillary carcinoma (7–14%), both arising from epithelial cells of the proximal tubule; homophobe renal cell carcinoma (4–10%); benign oncocytoma (2–5%); and collective duct cancer (1- 2%), all arising from intercalating cells of the collecting ducts of the kidney, These are the five types of kidney tumours that are currently recognized by medical science. The treatment of RCC is hindered by insufficient response to current drugs and late diagnosis. Thus, there is an urgent need for novel diagnostic and treatment approaches that go beyond the properties of cancer cells [4].
Materials and Methods
Relevant data sources should be identified to gather information on CKD epidemiology. These may include national health databases, population-based surveys, medical registries, electronic health records, and published literature. Additionally, data from international organizations, such as the World Health Organization (WHO) and the Global Burden of Disease (GBD) study, can provide valuable insights. Determine the study design based on the research objectives and available resources. For example, a cross-sectional study can be conducted to estimate CKD prevalence in a specific population, while a cohort study can examine CKD incidence and risk factors over time. Define the target population, such as adults aged 18 and above, or specific subgroups based on age, sex, or geographical location [5].
If conducting a population-based study, define the sampling strategy to ensure representative data. Random sampling techniques, such as stratified or cluster sampling can be used to select participants from the target population. Consider sample size calculations to ensure adequate statistical power for the study objectives. Determine the data collection methods based on the study design. This may involve conducting interviews, administering questionnaires, reviewing medical records, or utilizing laboratory data. Standardized protocols and validated measurement tools should be used to ensure data quality and consistency. Collect relevant demographic information, medical history, lifestyle factors, and laboratory results (e.g., serum creatinine, urine albumin-to-creatinine ratio) [6].
Analyze the collected data using appropriate statistical methods. Calculate CKD prevalence or incidence rates with confidence intervals. Perform statistical tests, such as chi-square tests or t-tests, to assess associations between risk factors and CKD. Multivariable regression models can be used to adjust for confounding variables and identify independent predictors of CKD. Consider subgroup analyses by age, sex, ethnicity, or other relevant factors. Ensure compliance with ethical guidelines and obtain necessary approvals from relevant ethical review boards. Safeguard participant privacy and confidentiality, and obtain informed consent. Adhere to data protection regulations and anonymized data when reporting results [7].
Acknowledge the limitations of the study, such as potential selection bias, information bias, or generalizability issues. Consider sources of potential confounding and attempt to address them through study design or statistical adjustment. Interpret the study findings in the context of existing literature and the study's objectives. Discuss the implications of the results for public health and clinical practice. Clearly report the study methodology, results, and limitations in a structured manner, following the guidelines of relevant scientific journals or reporting standards, such as the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) statement [8].
Results
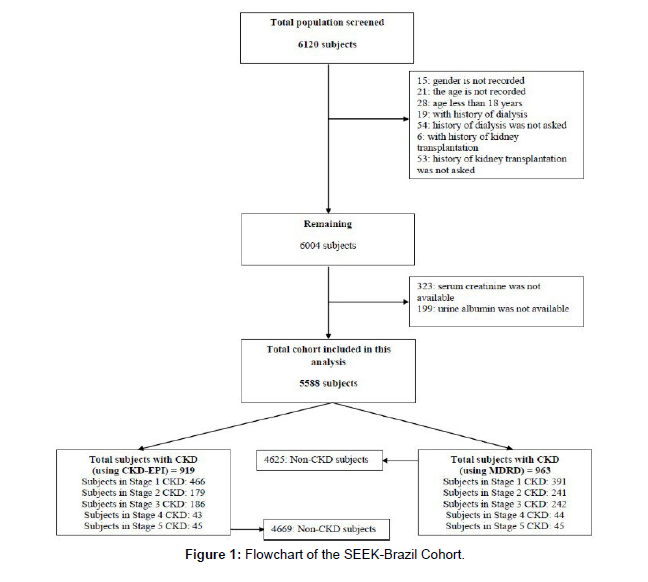
A total of 6,120 subjects were screened as part of the SEEK- Brazil project. We recruited these subjects through 53 screening camps held in 12 cities across Brazil, representing diverse regions of the country. Exclusion criteria were applied, resulting in the exclusion of 28 subjects below 18 years of age, 19 subjects with a history of dialysis, and 6 subjects with a history of kidney transplantation. Subjects with missing data for certain variables, including gender, age, history of dialysis or kidney transplantation, serum creatinine, and urine albumin, were also excluded. This analysis includes a total cohort of 5,588 subjects [9] (Figure 1).
The mean age of all participants was 45.22 ± 15.2 years (ranging from 18 to 98 years), with 55.1% being males and 44.9% females. Hypertension was observed in 43.1% of the population, while 18.8% had diabetes. The mean body mass index (BMI) was 23.91 ± 5.3 kg/ m^2. Defining overweight and obesity as BMI between 25-30 and >30 kg/m^2, respectively, the prevalence of overweight and obesity in our sample was 26.4% and 11.7%, respectively. However, 36.5% of the population had abdominal obesity, as indicated by a mean waist Renal Disease (MDRD-3) and Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equations were 111.31 ± 53 and 104.9 ± 25.52 mL/min/1.73 m^2, respectively. These values provide an indication of kidney function in the study population [10].
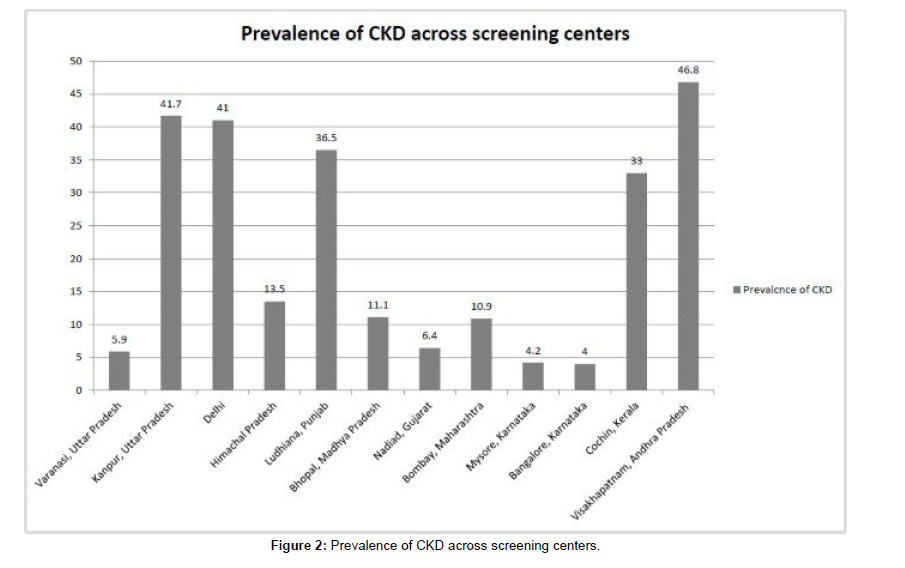
In summary, this analysis included 5,588 subjects from the SEEKBrazil project. The cohort exhibited a diverse range of demographic and clinical characteristics, including age, gender, prevalence of hypertension and diabetes, BMI, waist circumference, and eGFR. These findings provide a snapshot of the study population and lay the foundation for further investigations into the epidemiology of kidney disease in Brazil [11] (Figure 2).
Discussion
According to a widely used classification of macrophage activation, tumour related macrophages are type 2 macrophages (M2). Stein et al. first identified type 2 macrophages as variably activated macrophages in 1992. The macrophage mannose receptor, CD206, was proposed as a marker for the alternate sort of activation that IL-4 was said to cause. The subsequent research resulted in the gathering of a sizable amount of information regarding M2 cell population markers and the contributing factors. From a molecular and functional perspective, the M2 population looked to be very varied. The most significant factor in their detrimental impact on tumour development, however, continues to be their key functional characteristics linked to the inhibition of immune response, extracellular matrix remodeling, and angiogenesis stimulation. Although the M1/M2 dichotomy is currently being rethought, we will continue to utilize this nomenclature in this review for the sake of simplicity and clarity [12].
To complement the Th1/Th2 paradigm, the idea of two different forms of macrophage activation was proposed at the turn of the 20th century. M1, also known as classically activated macrophages, exhibit opsonic receptors and bactericidal effector chemicals (FcRI, II, and III). The M1 phenotype arises in response to exogenous inflammatory circumference of 83.03 ± 14 cm. The mean estimated glomerular filtration rate (eGFR) calculated using the Modification of Diet in stimuli like lipopolysaccharide (LPS) or other bacterial products as well as endogenous inflammatory stimuli like the Th1 cytokine IFN. M2, or alternatively activated macrophages, display traits such as the expression of monophonic receptors like the macrophage mannose receptor CD163 and hMARCO, the up regulation of Th2-associated cytokines and chemokines like IL-1ra or AMAC-1, and the production of extracellular matrix substances and ECM remodeling factors (fibronectin, tenascin-c, and MMP12). The functional nature and markers of M2 are similar to tumor-associated macrophages [13].
The ability of M2 to stimulate angiogenesis, which is accomplished via the production of angiogenic agents such cytokines and matrix metalloproteinase, aids in the growth of the tumour. For substantial angiogenesis to occur, the extracellular matrix must be degraded, capillary endothelial cells must migrate and proliferate, and mature capillaries must differentiate. Metastatic cells can exit the tumour and enter the bloodstream through newly formed capillaries, which also offer the tumour enough nutrition and oxygen. Cell invasion requires MMPs, or matrix metalloproteinase. The family of matrix metalloproteinase includes more than 20 enzymes that can digest the proteins that make up the extracellular matrix. In addition to metastasis, MMPs play a critical role in normal extracellular matrix remodeling and cell migratory activities, including wound healing and physiological angiogenesis [14].
It should be noted that during tumour invasion, stromal cells manufacture the majority of MMPs, whereas tumour cells just act as an inducer of this process by secreting a variety of chemokines, cytokines, and inducers. MMPs work in the activation of a few matriximmobilized growth and angiogenesis regulators in addition to breaking down extracellular matrix proteins. It has been demonstrated that MMP-9 and MMP-2 play a specialised role in the growth factor TGF's proteolytic activation. Similar to this, metalloproteinases can release angiogenic (members of the VEGF family) and antiangiogenic (thrombospondin, angiostatin) factors [15]. In practically all tumour types, overexpression of MMPs is linked to greater invasiveness and a bad prognosis. Recent research has shown that kidney cancers have higher MMPs expression, and that this increase is helpful for prognostication. For instance, the expression of the MMP-2 protein is strongly connected with the histological grade, TNM stage, tumour size, and LNM in RCC, suggesting that the MMP-2 protein may function as a biological marker for the prognosis in RCC [16].
A variety of enzyme systems are involved in the complex process of ECM remodeling. The MMPs are more crucial for the pathogenesis of tumours than the plasminogen activation system. Many clinical and experimental studies have confirmed the hypothesis that urokinase-like plasminogen activator (uPA) and its regulators are actively involved in the development of the metastatic phenotype of many tumour types. To activate its proteolytic action [17], a soluble serine protease known as uPA interacts with the uPAR on its membrane receptor. The main action of uPA is the proteolytic cleavage of plasminogen to create plasmin. Contrarily, ECM proteins like fibronectin, fibronectin, laminin, and fibrin can all be broken down by plasmin. Similar to MMPs, plasmin cleaves procollagenase, SF/HGF, FGF, and TGF by proteolytic cleavage to activate collagenase [18].
All of the uPA system's actions promote tumour growth and metastasis. Higher levels of uPA activity are linked to worse disease prognoses, and several tumour forms have been identified to produce uPA activation-related proteins more frequently. Experimental models provided more evidence for the importance of uPA for the development of tumours. Inhibiting uPA activity has been found to stop tumour invasion and metastasis. It has been established that TAMs produce uPA and its receptor in breast and other tumour types, where they contribute to the destruction of the extracellular matrix (ECM) [19], a step necessary for the growth of new blood vessels. The level of uPA receptor expression was related to the density of blood vessels in tumours and the poor prognosis for the illness. Despite the lack of distant metastases, high levels of uPA and uPAR in tumour tissue extracts are associated with a significantly shorter life expectancy for individuals with ccRCC. Our results highlight the importance of uPA regulated by tumor-associated macrophages in the remodeling of the tumor's vascular system [20].
Conclusion
The function of tumor-associated macrophages in the pathogenesis of RCC is well-known. More study is needed to develop novel and effective diagnostic or therapy strategies for RCC. The majority of traditional M2 markers (CD206, CD163) or the pan-macrophage marker CD68 has been used to measure macrophages in RCC to date. Yet, employing these markers for a simple quantification of macrophages is insufficient for predicting the clinical course of the disease and may potentially be misleading given that CD68 was demonstrated to be expressed by ccRCC cells. Other TAM markers should be investigated from the range of M2 markers that are now available. Tenascin-C, FXIIIa, fibronectin, IG-H3, Stabilin-1, YKL-39, SI-CLP, and other substances are among them.
These markers can be combined to develop a novel diagnostic approach with outstanding predictive ability. The location of the tumour where TAM analysis is done must also be taken into account. It was explained that macrophage analysis in different tumour locations has varying prognostic value and that they may express different markers. Current therapeutic targeting strategies for TAMs have been developed in a manner like that of diagnostic techniques. They employ markers like CD204, CD206, or foliate receptor beta, which aren't especially selective for macrophages associated with tumours and even less so for a specific type of tumour. Screening tests must be performed in order to detect TAM markers that are particular to tumours. It will be feasible to develop targeting techniques for reprogramming or eliminating TAM populations that promote tumours thanks to the findings of these screenings.
Acknowledgement
None
Conflict of Interest
None
References
- None Pages S, Caux V, Stoppa-Lyonnet D, Tosi M (2001) Screening of male breast cancer and of breast-ovarian cancer families for BRCA2 mutations using large bifluorescent amplicons. Br J Cancer 84:482-488.
- Jedy-Agba E, Curado MP, Ogunbiyi O (2012) Cancer incidence in Nigeria: a report from population-based cancer registries. Cancer Epidemiol 36:271-278.
- Tamimi AF, Tamimi I, Abdelaziz M (2015) Epidemiology of malignant and non-malignant primary brain tumors in Jordan. Neuroepidemiology 45:100-108.
- Beygi S, Saadat S, Jazayeri SB, Rahimi-Movaghar V (2013) Epidemiology of pediatric primary malignant central nervous system tumors in Iran. Cancer Epidemiol 37:396-401.
- Fisher JL, Schwartzbaum JA, Wrensch M, Wiemels JL (2007) Epidemiology of brain tumors. Neurologic Clinics 25:867-890.
- Li H, Mitchell P, Rochtchina E, Burlutsky G, Wong TY, et al. (2011) Retinal vessel caliber and myopic retinopathy: the Blue Mountains eye study. Ophthalmic Epidemiol 18:275-280.
- Ohno-Matsui K, Ikuno Y, Lai TYY, Gemmy Cheung CM (2017) Diagnosis and treatment guideline for myopic choroidal neovascularization due to pathologic myopia. Prog Retin Eye Res 63:92-106.
- Shinohara K, Moriyama M, Shimada N, Tanaka Y, Ohno-Matsui K, et al. (2014) myopic stretch lines: linear lesions in fundus of eyes with pathologic myopia that differ from lacquer cracks. Retina 34:461-469.
- Neelam K, Cheung CM (2012) Choroidal neovascularization in pathological myopia. Prog Retin Eye Res 31:495-525.
- Ladas ID, Moschos MM, Rouvas AA, Karagiannis DA, Kokolakis SN, et al. (2003) Lacquer crack formation after photodynamic therapy. Eur J Ophthalmol 13:729-733.
- Yanhui D, Huibin L, Zhenghe W (2017) Prevalence of myopia and increase trend in children and adolescents aged 7−18 years in Han ethnic group in China. Chin J Epidemiol 38:583-587.
- Dana R, Kathryn R, Elvis O, Annette K, Son H, et al. (2005) Visual acuity and the causes of visual loss in a population−based sample of 6−year−old Australian children. Ophthalmology 112:1275-1282.
- Troxel WM, Lee L, Hall M, Matthews KA (2014) Single-parent family structure and sleep problems in black and white adolescents. Sleep Medicine 15:255-261.
- Spiegelman D, Hertzmark E (2005) Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol 162:199-200.
- Petersen MR, Deddens JA (2006) Easy sas calculations for risk or prevalence ratios and differences. Am J Epidemiol 163:1159-1161
- Mantovani A, Allavena P, Sica A (2004) Tumour-associated macrophages as a prototypic type II polarised phagocyte population: role in tumour progression. Eur J Cancer 40:1660-1667.
- Yu MC, Mack TM, Hanisch R, Cicioni C, Henderson BE, et al. (1986) Cigarette smoking, obesity, diuretic use, and coffee consumption as risk factors for renal cell carcinoma. J Natl Cancer Inst 77:351-356.
- Novick AC (2004) Laparoscopic and partial nephrectomy. Clin Cancer Res 10:6322-6327.
- Hollingsworth JM, Miller DC, Daignault S, Hollenbeck BK (2006) Rising incidence of small renal masses: a need to reassess treatment effect. J Natl Cancer Inst 98:1331-1334.
- Miller DC, Saigal CS, Banerjee M, Hanley J, Litwin MS, et al. (2008) Diffusion of surgical innovation among patients with kidney cancer. Cancer 112:1708-1717.
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, CrossRef Indexed at
Google Scholar Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, CrossRef Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Citation: Kovesdy C (2023) Chronic Kidney Disease Epidemiology. Epidemiol Sci,13: 494.
Copyright: © 2023 Kovesdy C. This is an open-access article distributed underthe terms of the Creative Commons Attribution License, which permits unrestricteduse, distribution, and reproduction in any medium, provided the original author andsource are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 1644
- [From(publication date): 0-2023 - Feb 10, 2026]
- Breakdown by view type
- HTML page views: 1284
- PDF downloads: 360