Research Article Open Access
Chromium Detoxification by Using Mango (Mangifera indica) and Neem (Azadirachta indica) Leaves
Bhusari Shahin I, Pirale Gayatri D, Vitekar Aboli R, Magdum Sonali A, Suryawanshi Madhuri C, Jagtap Archana B, Wadkar Suryakant S, Shete Chidanand C and Ghosh Jai S*Department of Biotechnology, Smt. Kasturbai Walchand College, Sangli, Maharashtra, India
- *Corresponding Author:
- Jai S Ghosh
Department of Biotechnology
Smt. Kasturbai Walchand College
Sangli, Maharashtra, India
Tel: +919850515620
E-mail: ghoshjai@gmail.com
Received Date: August 24, 2017; Accepted Date: August 29, 2017; Published Date: August 31, 2017
Citation: Shahin BI, Gayatri PD, Aboli VR, Sonali MA, Madhuri SC, et al. (2017) Chromium Detoxification by Using Mango (Mangifera indica) and Neem (Azadirachta indica) Leaves. J Bioremediat Biodegrad 8: 409. doi:10.4172/2155-6199.1000409
Copyright: © 2017 Shahin BI, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
Water is one of the most vital natural resource, and is crucial for survival of all living organism. One of the major polluting sources of this water is the industrial effluents. In developing countries, most of the industries are operated in small and medium scales and some of these like electroplating and tannery industries discharge waste which is rich with Cr (VI) salts. Such untreated effluents have raised several ecological concerns. Chromium has been considered as one of the most toxic pollutant primarily because of its carcinogenic and teratogenic nature. The present work was focused on investigation into suitable biological remediation of such effluents without subsequent secondary pollution. It involved effect of concentration, contact time and pH variation on ability of the neem and mango leaves in removing chromium ions form aqueous solution. The dried leaves were washed with deionised water, air-dried and ground using ball mill. The leaf powders were added to 10 ppm stock metal ion solution made from potassium dichromate where chromium exist as Cr (VI). These mixtures were shaken at a constant rate of 150 rpm, filtered and analyzed by Atomic Absorption Spectrophotometer. The maximum adsorption was obtained at pH 5.5, agitation rate of 150 rpm, particle size, of ground dry leaves, was 5 mm, initial concentration of Cr (VI) ions of 10 mg/l and contact time 24 hrs to 48 hrs. The maximum adsorption capacity was found to be 10 ppm Cr (VI) ion concentration and 5.0 g/L of mango leaf powder (MLP) at pH 5.5. However, pH 7.0 was chosen as the optimum pH (to prevent secondary pollution due to acidic pH of treated effluent). Better adsorption did not occur at higher solution temperatures. The neem leaf powder was less effective in removing Cr(VI) ions from solutions. The adsorption mechanism followed a pseudo second order kinetics.
Keywords
Adsorption; Chromium(VI); Wastewater; Neem; Mango
Introduction
The presence of heavy metals in the environment is of major concern because of their toxicity due to bioaccumulating tendency resulting in threat to human life and the environment. It is well known that heavy metals can damage nerves, liver, and bones and could also block functional groups of essential enzymes. Conventional methods for removing dissolved heavy metal ions include chemical precipitation, chemical oxidation or reduction, filtration, ion exchange, electrochemical treatment, application of membrane technology, evaporation recovery and biological treatment.
Although all the effluent treatment techniques can be employed to remove heavy metals, they have their inherent advantages and limitations. Among all these methods, adsorption process is considered better than other methods because of convenience, easy operation and simplicity of design. Further, this process can remove/ minimize different types of pollutants and thus have wider applicability in water pollution control [1]. A fundamentally important characteristic of good adsorbents is their high porosity and consequent larger surface area with more specific adsorption sites. Activated carbons display high adsorption capacity; but the high cost presents a major drawback for practical applications, especially in the field of industrial effluent treatment where activated carbons may play a very useful role. Efforts have been made to develop low cost adsorbents for the removal of heavy metals from aqueous solutions. A number of natural and synthetic adsorbents like agricultural waste’s carbon [2], clay [3], neem leaves [4], cooked tea dust [5], zeolites [6], teak leaves activated carbon [7], cashew nut shell activated carbon [8], fly ash [9] etc., have been studied by various researchers for the removal of heavy metals [10].
The sludge removed from drinking and waste water treatment plants generates growing amounts of waste, which have to be managed. Sludge disposal is now recognized as one of the most important issues by all environmental lists. The main concern about Cr (VI) compounds is associated with their mobility, which can easily lead to the contamination of both surface and ground waters. Cr (VI) can be toxic for biological systems and water-soluble Cr (VI) is extremely irritating and toxic to human body tissue owing to its oxidizing, potential and easy permeating of biological membranes [11]. Cr (VI) is toxic to numerous plants, animals, bacteria and as a confirmed human carcinogen, poses a great threat to human health and environment [12]. Trivalent chromium on the other hand, present mainly as relatively insoluble, immobile and nontoxic hydroxides and oxides. Bioadsorption plays an important role in removal of Chromium (VI) from aqueous solutions. The main advantage of the bioadsorption technology is its effectiveness in reducing the concentration of Cr (VI) to very low levels and the use of inexpensive bioadsorbent materials [13].
Various types of biomass have been used as the biosorbent for the removal of toxic metals. Among these, plant leaves are chosen in this study as they are proposed to be natural, simple and cheap biosorbents for the efficient removal of several heavy metal ions. They can be easily found and are a renewable source. Plant leaves are also nontoxic and biodegradable. After the biosorption process, they are expected to precipitate and become sediments which can be disposed safely. A research conducted by Salim and Abu El-Halawa, used mulch plant leaves for the biosorption of cadmium, lead and copper ions. The results proved that the performance of the leaves is close to the efficiency of using activated carbon. The results also showed that oven dried leaves have better performance compared to the naturally dried ones. The functional group which is commonly found in plant leaves is carboxylate.
Available information involving the use of mango leaf powder (MLP) for the biosorption of heavy metals is quite limited. Particularly, no study involving MLP for the removal of copper ions was found. One paper was found whose the researcher” used several test plant materials including MLP as the biosorbents for the removal of lead ions. The maximum adsorption capacity for the MLP with lead was found to be 31.54 mg/g. Several researchers have also performed studies using different parts of the mango tree as biosorbents. One such study demonstrated the use of bark of mango tree as biosorbents suitable for the removal of Hg2+ and Cr3+ from aqueous solutions [14].
Materials and Methods
Preparation of adsorbent and characterization
The collected leaves of Mango and Neem were cut into average size 10 mm to 2 mm and dried in sun light for about 2 days. Then, it was pounded manually and sieved using suitable sieve particle size analyzer. These powdered leaves, were preserved in air tight containers.
Experimental procedure
All experiments were carried out in triplicate. Adsorption studies were carried out with initial Cr(VI) concentrations 10 ppm while maintaining the adsorbent dosage at 5%. The absorbent was, on an average, 5 mm in size.
Preparation of bio-absorbent
Neem and mango leaves were collected washed thoroughly with deionised water and air dried. The dried leaves were ground using and sieved to produce 5 mm particles size.
Batch sorption experiment
Batch experiments were carried out to investigate the effects of initial metal ion concentrations, temperature, pH and adsorbent dosage on the adsorption of Cr (VI) ions from aqueous solutions (of 10 ppm Cr (VI)). Absorbents (5 g) were added to 100 ml of the solution. The mixtures were stirred using a magnetic stirrer for 24 hrs and 48 hrs. The solutions were filtered using Whatman filter paper No. 41, washed with deionized water several times (to remove all dichromate which might have been attached to it loosely) and dried in oven at 120°C for 1 hour. These were then digested by using HNO3+H3PO4 i.e., 3:1 ratio then volume was made up to 100 ml with deionized water and analyzed using atomic absorption spectrophotometer (Thermo Fisher AA-203). Further Cr (VI) ions were also analyzed using diphenylcarbazide. The concentration of the analyte was determined from the calibration curve of standard solutions of Cr (VI). The effects of various parameters on efficiency of adsorption were observed by varying temperature, from 15°C, 25°C, 37°C and 45°C. pH of solution was also varied from 4.5, 5.5, 7 and 8.5 along with different adsorbent dosage.
Standard stock solution of 1 mg/mL of chromium (VI) was prepared from potassium dichromate by dissolving 2.828 g K2Cr2O7, in 1 liter deionized water. It was diluted further as per the need in different experiments.
Application to samples of effluents, from primary sedimentation pond, chromium pond and treated pond, were randomly collected, using sterile BOD bottles, for Cr (VI) removal from nearby tannery and leather industries. The analyses of samples were carried out in two steps. The first one is direct analyses to determine concentration of Cr (VI) in the wastewater and routine water testing. The second part of the analysis was carried out by treating the wastewater with the adsorbent prepared from leaves of mango and neem to remove the pollutant using the optimized parameters, keeping in mind the data from routine water testing.
Statistical analysis
Data was analyzed by one-way analysis of variance (ANOVA) with Tukey-Kramer multiple comparison test.
Results and Discussion
It should be borne in mind that chromium is a transition element and would form hydroxide complexes at neutral to alkaline pH. Usually effluents containing soluble chromium salts are acidic solutions.
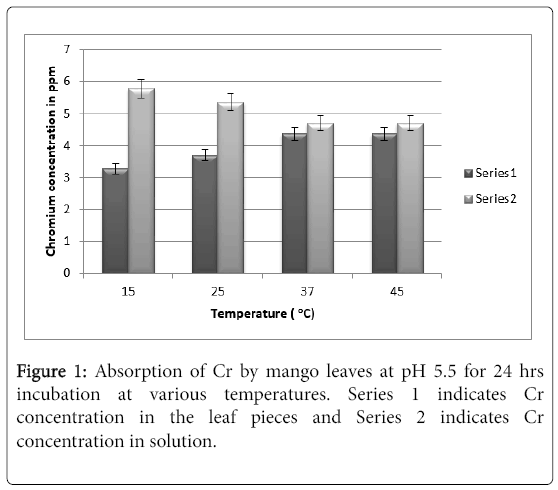
It can be seen from Figure 1 that at pH 5.5. maximum absorption was observed to be 4.5 ppm of Cr (VI) at 24 hrs at 37°C. Increasing the temperature further to 45°C does not show any increase in absorption.
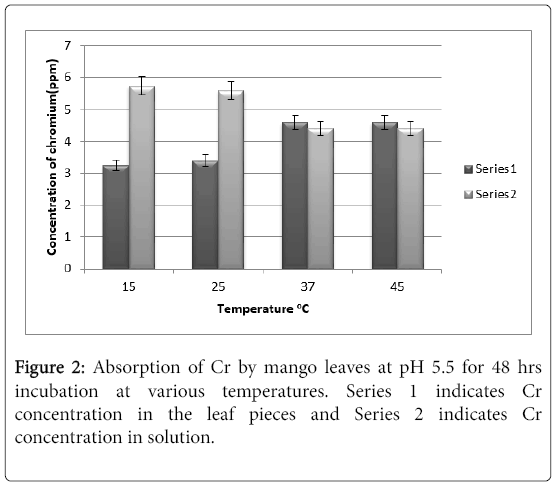
It can be noted from Figure 2 that by increasing the time from 24 hrs to 48 hrs, there is no change in absorption of chromium by the leaves.
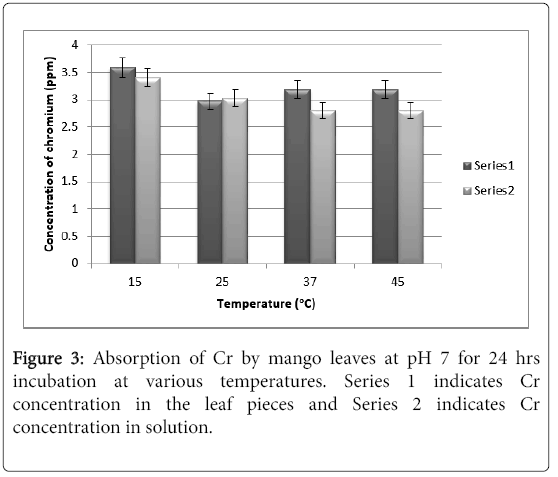
It can be seen from Figure 3 that at pH there was only 3.0 ppm of chromium absorbed by the leaves at 25°C and 3.2 ppm at 37°C, whereas at 15°C the absorption was 3.5 ppm. This erratic absorption pattern is may be due to the fact that chromium is a transition series element and tends to form water insoluble hydroxide complexes in aqueous medium at neutral to alkaline pH, which precipitates. The rate of hydroxide formation is slower at a low temperature like 15°C which shows absorption of 3.5 ppm. There is no difference in absorption pattern after 48 hrs. and hence the results are not shown.
At pH 8.5 more the 85% of the chromium gets precipitated and there is hardly any significant absorption and hence the results are not shown.
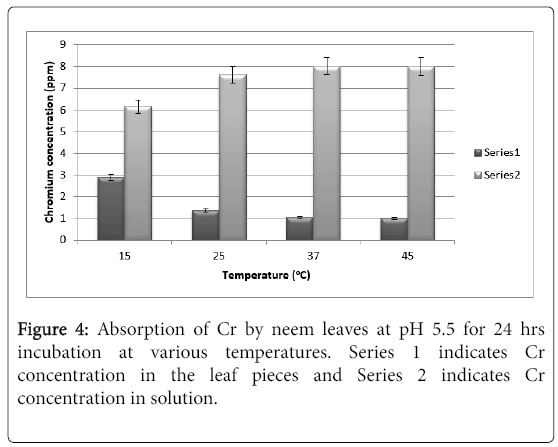
It is very evident from Figure 4 that neem leaves hardly absorb Chromium from the solution at pH 5.5. Even after 24 hrs, it can be seen that only at 15°C there is 3 ppm of absorbed chromium whereas at 25°C to 45°C there is hardly any absorption (barely 1 ppm). The results do not change any significantly by increasing the exposure to 48 hrs. and hence the results are not shown for 48 hrs exposure.
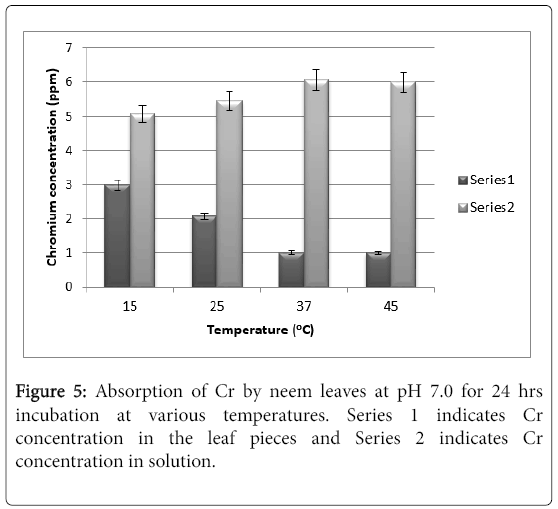
There is hardly any difference in the absorption values at pH 5.5. and that of at pH 7.0 after 24 hrs of exposure (Figure 5). Further exposure to 48 hrs also does not change the absorption and hence the results are not shown. The hydroxide formation of the element at neutral and alkaline pH does not affect significantly the absorption because it is already very poor.
The only noteworthy observation is that the absorption is virtually 0 at pH 8.5.
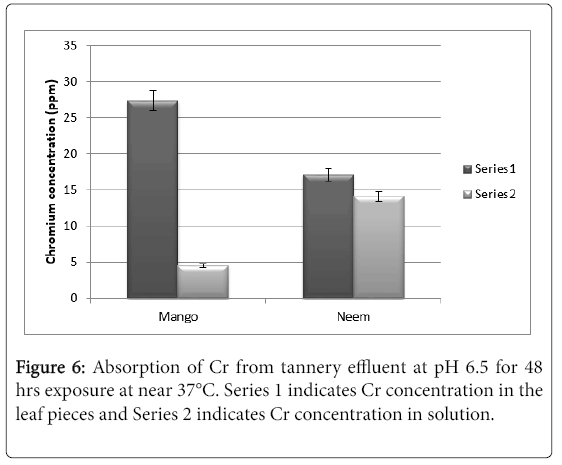
With these information, it was decided to go for effluent treatment with tannery effluent containing 32% chromium. The pH of the effluent was around 6.5. After 48 hrs of exposure the results are as shown in Figure 6. It can be seen from the results that nearly 86% of the chromium was absorbed by the mango leaf pieces as compared to 54% by neem leaf pieces.
Conclusion
The results clearly indicate that mango leaves are suitable for absorption of chromium (VI) from solutions. The experiment with actual chromium containing effluent clearly indicates that mango leaf pieces could be a potential candidate for bio-detoxification of chromium containing effluents. In order to carry out pilot plant level experiments the isotherms of this biosorption needs to be studied to determine the feasibility for scale up studies.
Acknowledgement
The authors are very grateful to the Principal of Smt. K.W. College, Sangli for being kind enough to extend the laboratory facilities for successful completion of this project.
References
- Bhatnagar A, Minocha AK (2006) Conventional and nonconventional adsorbents for removal of pollutants from water-A review. Indian Journal of Chemical Technology 13: 203-217.
- Ayyappan R, Sphia AC, Swaminathan K, Sandhya S (2005) Removal of Pb (II) from aqueous solutions using carbon derived from agricultural wastes. Process Biochemistry 40: 1293-1299.
- Saha P, Datta S, Sanya SK (2010) Application of natural clayey soil as adsorbent for the removal of copper from waste water. Journal of Environmental Engineering 136: 1409-1417
- Fu F, Wang Q (2011) Removal of heavy metal ions from waste waters: A review. Journal of Environmental Management 92: 407-418.
- Dhanakumar S, Solaraj SG, Mohanraj R, Pattabhi S (2007) Removal of Cr (VI) from aqueous solution by adsorption using cooked tea dust. Indian Journal of Science and Technology 1: 1-6
- .
- Erdem E, Karapinar N, Donat R (2004) The removal of heavy metal cations by natural zeolites. Journal of Colloid and Interface Science 280: 309-314.
- Jafar AA, Balasubramanian S (2010) Adsorption of Pb (II) ions on teak leaves activated carbon- a kinetic and equilibrium study. Der ChemicaSinica 1: 35-43.
- Tangjuank S, Insuk N, Tontrakoon J, Udeye V (2009) Adsorption of Lead(II) and Cadmium (II) ions from aqueous solutions by adsorption on activated carbon prepared from cashew nut shells. World Academy of Science, Engineering and Technology 52: 110-116.
- Alinnor IJ (2007) Adsorption of heavy metal ions from aqueous solution by fly ash. Fuel 86: 853-857.
- Demirbas E, Kobya M, Senturk E, Ozkan T (2004) Adsorption kinetics for the removal of chromium (VI) from aqueous solutions on the activated carbons prepared from agricultural wastes. Water SA 30: 533-539.
- Bagchi D, Stohs SJ, Downs BW, Bagchi M, Preuss HG (2002) Cytotoxicity and oxidative mechanisms of different forms of chromium. Toxicology 180: 5-22
- Outridge PM, Scheuhammer AM (1993) Bioaccumulation and toxicology of chromium: implications for wildlife. Review Environmental Contamination and Toxicology 130: 31-77.
- Gupta S, Babu BV (2006) Adsorption of Cr (VI) by a low-cost adsorbent prepared from neem leaves. Proceedings of National conference on environmental Conservation pp: 175-180.
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 5846
- [From(publication date):
September-2017 - Apr 04, 2025] - Breakdown by view type
- HTML page views : 4827
- PDF downloads : 1019