Case Report Open Access
Chordoid Glioma of the Third Ventricle with High Mib-1 Index: A Case Report
Serdar Altınay1*, Aydın Sav2, Koray Özduman2and Türker Kılıç31Bağcılar Training and Research Hospital, Department of Pathology, Istanbul, Turkey
2Department of Neuropathology, Acıbadem University, Medical Faculty, Istanbul, Turkey
3Department of Neurosurgery, Bahçeşehir University, Medical Faculty, Istanbul, Turkey
- *Corresponding Author:
- Altınay S
Bağcılar Eğitim ve Araştırma Hastanesi
Patoloji Laboratuvarı Merkez Mahallesi Mimar
Sinan Caddesi No:6. Bağcılar, Istanbul
Türkiye
Tel: 9002124404000
Fax: 9002124404243
E-mail: drserdara@yahoo.com
Received date: March 24, 2015 Accepted date: June 25, 2015 Published date: June 27, 2015
Citation:Altinay S, Sav A, Özduman K, Kiliç T (2015) Chordoid Glioma of the Third Ventricle with High Mib-1 Index: A Case Report. J Clin Exp Pathol 5:235. doi:10.4172/2161-0681.1000235
Copyright: ©2015 Altınay S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Clinical & Experimental Pathology
Abstract
Chordoid glioma is a slowly growing and uncommon neoplasm which involves the third ventricle and is commonly seen in middle age woman. It is a novel entity which was just recently been incorporated to the World Health Organization (WHO) pathological central nervous system (CNS) tumor classification with chordoma like histologic features and glial fibrillary acidic protein immunoreactivity. Hereby we report a case of third ventricular chordoid glioma in a 32 year old man with history of headache and visual disturbances. Magnetic Resonance Imaging (MRI) imaging showed a homogeneously enhancing mass occupying in the third ventricle. The lesion underwent a subcapsular removal through craniotomy. Histologically the tumor had a uniform appearance consisting of clusters and cords of epithelioid cells embedded within a mucinous stroma, containing a lymphoplasmacytic infiltrate. Immunohistochemistry revealed diffuse expression of Glial Fibrillary Acidic Protein (GFAP), vimentin, cytokeratin and S-100 protein reactivity. An uncommonly high MIB-1 index of 10% was detected. There was no nuclear accumulation of p53 protein. Our patient, who rejected radiotherapy and chemotherapy options after surgery (at 52 months), is currently disease-free
KeyWords
Chordoid glioma; MIB-1; Suprasellar region; Third ventricle
Abbreviations:
ABC: Avidin-Biotin-peroxidase Complex; CNS: Central Nervous System; EGFR: Epidermal Growth Factor Receptor; EMA: Epithelial Membrane Antigen; F: Female; GFAP: Glial Fibrillary Acidic Protein; HPF: High Power Fields; LI: Labeling İndex; M: Male; MRI: Magnetic Resonance Imaging; NSE: Neuron Specific Enolase; PAS: Periodic Acid Schiff; RT: Radiotherapy; WHO: World Health Organization
Introduction
Chordoid glioma (CG) is a rare slowly growing tumor which is usually located in third ventricle of adults. Firstly described as a new pathologic entity by Brat et al. in 1998 [1]. It was introduced the World Health Organization (WHO) classification of nervous system tumors in 2000 [2]. On the basis of limited clinico-pathological data this neoplasm has been classified as a low grade corresponding to WHO grade II.
Chordoid glioma was so named because of its histologic features resembling a chordoma and because of its immunoreactivity to GFAP. Histologically it is characterized by clusters and cords of epithelioid tumor cells which express GFAP and which possess a mucinous stroma typically containing a lymphoplasmacytic infiltrate [1,3-6]. Actualy the first case described by Wanschitz et al. [7] was a solid, third ventricular tumor occurring in a 24 year old woman. The authors concluded that the tumor was a meningioma with peculiar expression of GFAP. Subsequent immunohistochemical and ultrastructural studies of similar cases have not supported a meningothelial derivation; rather evidence indicates that they are glial in nature [2,4,8-11]. Mitosis are rare and MIB-1 index is always lower than 5% in CG.
We describe the clinical, morphologic, and immunohistochemical features of a different case of chordoid glioma with high MIB-1 index. We believe that our case will the first patient from Turkey and will be added to the database as highest MIB-1 index in chordoid glioma case.
Material and Methods
The tumor tissue was fixed in 10% buffered formalin and embedded in paraffin wax. Sections were stained with hematoxylin and eosin, periodic acid-Schiff with or/without diastase digestion, whereas other sections were used for the immunohistochemical study. Mitoses were countered by examining 10 high power fields (HPF). Immunohistochemical analysis was performed by using avidin-biotin-complex (ABC) method. The antibodies and dilutions used were as follows: (m: monoclonal, p: polyclonal) Glial fibrillary acidic protein (GFAP) (clone ZCG29, prediluted; Zymed Laboratories, San Francisco, Calif), cytokeratin (clone AE1/AE3, 1:200 dilution; BioGenex,), Vimentin (clone V9, 1:2000 dilution; BioGenex), S-100 (p, 1:100, BioGenex), Ki-67 (clone MIB-1, 1:50 dilution; BioGenex), P53 (Clone BP53 1.100, Biogenex). TTF-1 (Clone 397A, 1.100, Biogenex) EMA (clone E29, 1:50 dilution; Dako), and CD34 (clone QBEnd710, 1:20 dilution; BioGenex). The proliferation index was calculated by estimating the percentage of Ki-67 positive neoplastic cells in the total number of tumor cells in the “hot spot” area. Proliferation index was evaluated by two pathologist using MIB-1 antibody staining and manually counting 1000 cells from at least five representative microscopic fields, at high-power magnification (400×) in all three paraffin blocks.
Case Presentation
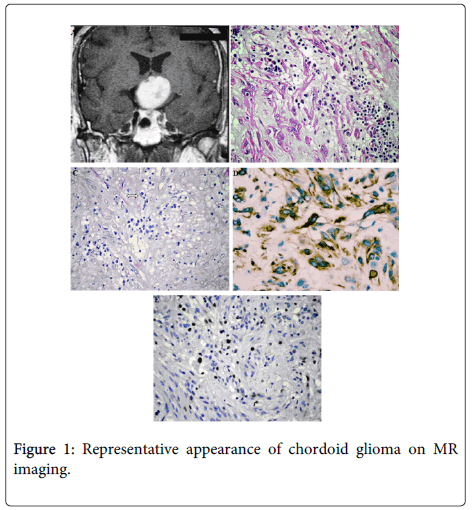
The patient is a previously healthy, 32 year old man presented to clinical attention with a 2 month history of headache and visual disturbances. Preoperative MRI examination of the patient revealed an anterior third ventricular mass. The lesion was 2.8 cm long in its largest diameter was sharply demarcated from surrounding tissue and had rounded borders. It was isointense on T1 weighted imaging and enhanced brightly and fairly homogeneously after intravenous contrast enhancement and hyperintense on T2 weighted images. Some minimal heterogeneity was noted within the lesion. There was minimal mass effect on surrounding tissues. The mass was obliterating the anterior portion of the third ventricle however no obstruction of foramina of Monroe was noted and there was no evident ventriculomegaly.
All tumour tissue was evaluated. Haematoxylin and eosin (H&E) stained sections were reviewed by an experienced neuropathologist (AS). Histologically the tumor was composed of clusters and cords of epithelioid tumor cells which were embedded in a mucinous stroma containing a lymphoplasmacytic infiltrate. The tumor was moderately cellular and consisted of round to polygonal cells with abundant eosinophilic cytoplasm. Tumor cells were arranged in a little diastase resistant PAS positive mucin rich matrix. The nuclei were large, some of them were spindle shaped and contained a fine chromatin and inconspicuous nucleoli. Mitotic figures were 0-1/10HPF. Vascular proliferation and necrosis were absent. Whorl formation, psammoma bodies, nuclear pseudoinclusions, ependymal rosettes, physaliphorous cells, chondroid metaplasia were not observed. On immunohistochemistry tumor cells showed strong and diffuse intracytoplasmic GFAP expression, and also staining for vimentin. Weakly nuclear reactivity for pan-cytokeratin and S-100 protein was also identified. The Ki-67 (MIB-1) proliferation index was 10%. TTF-1 staining was nonspecific. No immunoreactivity for EMA, or CD34 was observed. There was no reactivity of p53 mutation. Our patient, who rejected radiotherapy and chemotherapy options after surgery, (52 months) is currently disease-free (Figure 1).
Discussion
A literature search through PUBMED revealed 80 cases of chordoid glioma [12]. The clinical, histologic, and immunohistochemical features of our case is similar to those reported in previous series [1,8-11,13-19]. Buccoliero et al. [13] reviewed 32 cases of chordoid glioma reported in the literature. They showed that the lesion typically arose in the third ventricular region with frequent attachment to hypothalamic and suprasellar structures (63%) and that adults were more commonly affected (median age, 45 years; range 12-70 years) with a female predominance (F; 63% M; 37%). The 12 year old male patient who was presented by Castellano et al [20] was the first pediatric chordoid glioma case. Most cases in the literature reportedly have presented with signs and symptoms of obstructive hydrocephalus, including headache, nausea, and ataxia. Others were reported to have caused hypothyroidism and/or visual disturbances due to compression of the hypothalamus and/or the optic chiasm. Psychiatric and memory abnormalities have also been reported, presumably due to compression of the medial temporal lobes. Our patient who is 32 year old man presented to hospital with history of headache and visual disturbances.
On MRI chordoid gliomas are generally well circumscribed lesions of 2 to 4 cm diameter which are located in the suprasellar-third ventricular region. They enhance with contrast. Our neuroimaging findings were consistent with previous reports. On MRI images, chordoid glioma is more sharply circumscribed than the hypothalamic-optic glioma and does not extend to the optic chiasm or optic tracts. There may be mass effect and edema in optic glioma however enhancing tumor tissue is usually not seen [17,21]. The MRI findings characteristic of CG is an isointense ovoid mass on T1-weighted MR (63% of cases) and slightly hyperintense T2-weighted images, with homogeneous enhancement by gadolinium (70%) [22].
The strikingly chordoid appearance of these neoplasms, with their eosinophilic clustered tumor cells in a blue mucinous matrix is distinctive among other regional lesions, including pituitary adenoma, crraniopharyngioma, pilocytic astrocytoma and meningioma [2]. However the most difficult differential diagnosis is between chordoid glioma, chordoma and chordoid meningioma. Absence of physaliphorous cells and of bone infiltration and lack of psammoma bodies, whorl formation and nuclear pseudoinclusions may help in the diagnosis. We did not encounter those diagnostic difficulties in our case.
Immunohistochemistry is very helpful in the differential diagnosis and nosological classification of the CNS neoplasms. Most tumor cells of chordoid gliomas show a strong staining for VIM and GFAP and show focal reactivity to EMA and cytokeratin in some cases. In our case there was diffuse staining for GFAP and VIM,
Additionally weak coexpression of S-100 protein/EMA may be seen. Vajtai et al. [11] reported stains for EMA and Cytokeratin were non-reactive. Buccoliero et al [13] detected that their case surprisingly positive NSE immunoreactivity. This result could indicate a divergent neuronal and glial differentiation and at the same time could suggest that the immunohistochemical positivity of NSE should not exclude chordoid glioma in the differential diagnosis of a third ventricular lesion.
Histogenesis of the chordoid glioma is uncertain. Electron microscopy findings are also suggestive of glial origin, especially of ependymal cells known as tanycytes, which are located in the anterior third ventricle and cover the circumventricular organs in the anatomic vicinity of the third ventricle, the organum vasculosum of the lamina terminalis and the subfornical organ. Authors showed TTF-1 as a useful marker for the diagnosis of CG and the understanding of its oncogenesis [23]. We detected nonspecific staining with TTF-1. Nevertheless, the small number of cases studied ultrastructurally (9 of 32) some conflicting results (i.e., clear cell body zonation in 3 of 9 cases and normal and/ or abnormal cilia in 3 of 9 cases), and the possible difficulty in performing electron microscopy on routinely treated surgical specimens could reduce its definitive utility in the diagnosis [8,10,18].
Given the rarity of this tumor, optimal treatment after histologic diagnosis is unclear. Surgery followed by radiation therapy has been reported [24]. The anatomic location of chordoid glioma and the frequent adherence to the hypothalamus does not allow a complete surgical excision in the majority of cases [25]. The few cases that reported using chemotherapy and/or radiotherapy showed these therapies to be ineffective [26]. The ideal therapy is total removal of the tumor. However, according to the literature total removal of the tumor carries a high risk and conventional radiation therapy has little effect on the residual tumor [25,27]. In all chordoid gliomas the MIB-1 labeling index is uniformly low, mainly ranging 0-1.5%. The percentage of Ki-67 positive cells was 10% in our case. It was the highest rate reported so far. However four years of short duration follow-up precludes the prediction of the tumor’s behavior. Similarly the mean available follow-up (8-48 month) in the current series of 32 cases was relatively short. Although it was a gross total resection; since our patient had a high MIB-1 index it will of great importance to follow the case in the long term.
We did not see any nuclear accumulation of mutated p53 protein. On the basis of literature in molecular genetic studies, no chromosomal imbalances were detected. None of the neoplasms contained genetic aberrations of the TP53 and CDKN2A tumor supressor genes, amplification of the EGFR, CD4 and MDM2 protooncogenes [9].
Conclusion
We present a new case of chordoid glioma which is interestingly showing a relatively high MIB-1 index and different from the cases reported in previous series glioma with a review of the literature. Our case maybe a distinct entity with high MIB index in the near future. In conclusion, we propose that each report of this uncommon brain neoplasm will help to define its nature and appropriate treatment further.
Conflict of Interest
• The authors of the article certify that they have NO affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
• Written informed consent was obtained from patient.
References
- Brat DJ, Scheithauer BW, Staugaitis SM, Cortez SC, Brecher K, et al. (1998) Third ventricular chordoidglioma: a distinct clinicopathologic entity. J NeuropatholExpNeurol 57: 283-290.
- Biernat W (2000) 2000 World Health Organization classification of tumors of the nervous system. Pol J Pathol 51: 107-114.
- Dumont AS, Farace E, Schiff D, Shaffrey ME (2003) Intraventriculargliomas. NeurosurgClin N Am 14: 571-591.
- (2000) Pathology and Genetics: Tumors of the Nervous System. In: Kleihues P, Cavanee WK (eds.) World Health Organization Classification of tumors. IARC Press, Lyon, pp. 90-91.
- Kleihues P, Louis DN, Scheithauer BW, Rorke LB, Reifenberger G, et al. (2002) The WHO classification of tumors of the nervous system. J NeuropatholExpNeurol 61: 215-225.
- Radner H, Blümcke I, Reifenberger G, Wiestler OD (2002) [The new WHO classification of tumors of the nervous system 2000. Pathology and genetics]. Pathologe 23: 260-283.
- Wanschitz J, Schmidbauer M, Maier H, Rössler K, Vorkapic P, et al. (1995) Suprasellar meningioma with expression of glial fibrillary acidic protein: a peculiar variant. ActaNeuropathol 90: 539-544.
- Raizer JJ, Shetty T, Gutin PH, Obbens EA, Holodny AI, et al. (2003) Chordoidglioma: report of a case with unusual histologic features, ultrastructural study and review of the literature. J Neurooncol 63: 39-47.
- Reifenberger G, Weber T, Weber RG, Wolter M, Brandis A, et al. (1999) Chordoidglioma of the third ventricle: immunohistochemical and molecular genetic characterization of a novel tumor entity. Brain Pathol 9: 617-626.
- Sato K, Kubota T, Ishida M, Yoshida K, Takeuchi H, et al. (2003) Immunohistochemical and ultrastructural study of chordoidglioma of the third ventricle: its tanycytic differentiation. ActaNeuropathol 106: 176-180.
- Vajtai I, Varga Z, Scheithauer BW, Bodosi M (1999) Chordoidglioma of the third ventricle: confirmatory report of a new entity. Hum Pathol 30: 723-726.
- Morais BA, Menendez DF, Medeiros RS, Teixeira MJ, Lepski GA (2015) Chordoidglioma: Case report and review of the literature. Int J Surg Case Rep 7C: 168-171.
- Buccoliero AM, Caldarella A, Gallina P, Di Lorenzo N, Taddei A, et al. (2004) Chordoidglioma: clinicopathologic profile and differential diagnosis of an uncommon tumor. Arch Pathol Lab Med 128: e141-145.
- Castellano-Sanchez AA, Recine MA, Restrepo R, Howard LH, Robinson MJ (2000) Chordoidglioma: a novel tumor of the third ventricle. Ann DiagnPathol 4: 373-378.
- Cenacchi G, Roncaroli F, Cerasoli S, Ficarra G, Merli GA, et al. (2001) Chordoidglioma of the third ventricle: an ultrastructural study of three cases with a histogenetic hypothesis. Am J SurgPathol 25: 401-405.
- Galloway M, Afshar F, Geddes JF (2001) Chordoidglioma: an uncommon tumour of the third ventricle. Br J Neurosurg 15: 147-150.
- Grand S, Pasquier B, Gay E, Kremer S, Remy C, et al. (2002) Chordoidglioma of the third ventricle: CT and MRI, including perfusion data. Neuroradiology 44: 842-846.
- Ricoy JR, Lobato RD, Báez B, Cabello A, Martínez MA, et al. (2000) Suprasellarchordoidglioma. ActaNeuropathol 99: 699-703.
- Tonami H, Kamehiro M, Oguchi M, Higashi K, Yamamoto I, et al. (2000) Chordoidglioma of the third ventricle: CT and MR findings. J Comput Assist Tomogr 24: 336-338.
- Castellano-Sanchez AA, Schemankewitz E, Mazewski C, Brat DJ (2001) Pediatricchordoidglioma with chondroid metaplasia. PediatrDevPathol 4: 564-567.
- Pomper MG, Passe TJ, Burger PC, Scheithauer BW, Brat DJ (2001) Chordoidglioma: a neoplasm unique to the hypothalamus and anterior third ventricle. AJNR Am J Neuroradiol 22: 464-469.
- Tu A, Yeo T, Steinke D, Resch L, Mehta V (2010) Chordoidglioma: imaging pearls of a unique third ventricular tumor. Can J NeurolSci 37: 677-680.
- Bielle F, Villa C, Giry M, Bergemer-Fouquet AM, Polivka M, et al. (2015) ChordoidGliomas of the Third Ventricle Share TTF-1 Expression With OrganumVasculosum of the Lamina Terminalis. Am J SurgPathol 39: 948-956.
- Pasquier B, Péoc'h M, Morrison AL, Gay E, Pasquier D, et al. (2002) Chordoidglioma of the third ventricle: a report of two new cases, with further evidence supporting an ependymal differentiation, and review of the literature. Am J SurgPathol 26: 1330-1342.
- Piepmeier J, Baehring JM (2004) Surgical resection for patients with benign primary brain tumors and low grade gliomas. J Neurooncol 69: 55-65.
- Kobayashi T, Tsugawa T, Hashizume C, Arita N, Hatano H, et al. (2013) Therapeutic approach to chordoidglioma of the third ventricle. Neurol Med Chir (Tokyo) 53: 249-255.
- Nakajima M, Nakasu S, Hatsuda N, Takeichi Y, Watanabe K, et al. (2003) Third ventricular chordoidglioma: case report and review of the literature. SurgNeurol 59: 424-428.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 14747
- [From(publication date):
August-2015 - Jul 03, 2025] - Breakdown by view type
- HTML page views : 10178
- PDF downloads : 4569