Chlamydial Infection Mimicking Rectal Neoplasia: An Uncommon Diagnosis Complicated by the Interference of Drugs Used for the Treatment of Symptoms
Received: 13-Dec-2018 / Accepted Date: 19-Dec-2018 / Published Date: 26-Dec-2018 DOI: 10.4172/2161-0681.1000360
Abstract
Diagnosis of anogenital infections by Chlamydia trachomatis is challenging, because symptoms are mild, nonspecific and tolerated long time by patients before seeking the clinician. When diagnostic attempts are done, empiric treatment of symptoms modifies clinical presentation to such an extent that it is likely to incur a misdiagnosis. We observed a case of severe ulcerative proctitis, which in the initial phase of clinical investigation was strongly suspected for cancer. Histologic examination has ruled out the neoplastic nature of the ulcer, avoiding a diagnostic error. Diagnosis of C. trachomatis infection was achieved by amplification of DNA extracted from a thick section of biopsy and confirmed by serology. After treatment the lesion healed completely, with an initial retraction visible only to endoscopic controls. Proctitis by Chlamydia trachomatis is an emerging sexual transmitted infection demanding particular attention for diagnosis and treatment. Compulsory notification of LGV cases (caused by serovars L1-L3) should also be adopted, because it would allow discovering promptly epidemic outbreaks in risk groups.
Keywords: LGV; Ulcerative proctitis; Differential diagnosis
Introduction
Urogenital infections by Chlamydia trachomatis are common worldwide with estimated 105.7 million new cases in 2008. In endemic areas (Africa, India, Southeast Asia, South America, and the Caribbean), LGV, the invasive form of the infection caused by serovars L1-L3, presents itself in the classic or bubonic form, characterized by a genital lesion and voluminous regional lymphadenopathy. LGV can also cause severe proctitis and proctocolitis that occur as limited outbreaks outside endemic areas, primarily in MSM. Since 2003 there has been a progressive increase of cases of LGV proctitis and proctocolitis in Europe, North America and Australia [1,2].
In 2016, 2,043 cases of LGV were reported in 22 European countries, with 86% of all notified cases coming from France, the Netherlands and United Kingdom [3,4]. Almost all cases in 2016 were reported among men who have sex with men. In Italy, notification of LGV is not compulsory and the increase of case number observed in many other countries during last years has not been noticed [5-8].
Case Description
We present a case observed at INMI “L. Spallanzani”, where A.D., a 51-year-old Caucasian man, was attending regularly outpatient care for HIV infection. He was anti HCV negative and followed ART with Efavirenz 600 mg / emtricitabine 200 mg / tenofovir 245 mg (Atripla®) since 2009. He had an interesting job and he frequently travelled abroad, also in Tropical Countries.
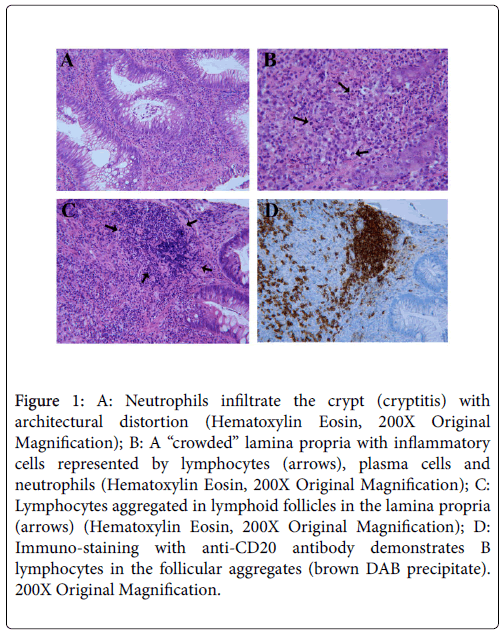
In October 2013 he developed dysuria, rectal tenesmus and hematochezia. Without consulting the clinic, he performed proctologic consultation. The rectal endoscopy has documented: “In the anal canal hemorrhoidal congestion and erosion of anal mucosa at 9 h, diameter of 4 mm. In the anterior wall of rectum, 4-8 cm far from anus, there is an ulcer, 4 cm in diameter, with elevated and irregular edges and irregular base, bleeding spontaneously and at contact”. Endoscopic aspect was suggesting rectal neoplasia, however biopsies of the two sites showed: “dense homogeneous lymphoplasmacytic inflammation expanding and thickening the lamina propria; neutrophils causing active crypt injury (cryptitis, Figure 1A)”, other morphologic features were crypts distortion and regenerative atypia of epithelial cells. Diagnosis of proctitis in the absence of features suggesting neoplasia was made. RMN of abdomen and pelvis have documented reactive lymphnodes at the level of mesorectum and thickening of rectal walls.
Figure 1: A: Neutrophils infiltrate the crypt (cryptitis) with architectural distortion (Hematoxylin Eosin, 200X Original Magnification); B: A “crowded” lamina propria with inflammatory cells represented by lymphocytes (arrows), plasma cells and neutrophils (Hematoxylin Eosin, 200X Original Magnification); C: Lymphocytes aggregated in lymphoid follicles in the lamina propria (arrows) (Hematoxylin Eosin, 200X Original Magnification); D: Immuno-staining with anti-CD20 antibody demonstrates B lymphocytes in the follicular aggregates (brown DAB precipitate). 200X Original Magnification.
The patient went at visit in our outpatient service in January 2014, depressed, suffering for chronic rectal pain, tenesmus, anal mucous discharge mixed with blood. Hematology and serology tests were done: full blood count was normal, CD4 count 1,501/mmc (46.4%); HIVRNA was detected (<40 cp/ml), (RT PCR Abbott); ESR was 16 mm/h (nv<15), CRP was 1.2 mg/dl (nv<0.6), CEA 6.1 ng/ml (<4.7); IgG anti Schistosoma spp. negative; IgG anti Entamoeba histolytica (ELISA) positive; parasitological examination of 3 stool samples was negative, occult blood research positive in two.
During February we asked to our Pathology service a second opinion about the biopsy of November 2013, with the following response: "Multiple fragments of rectal mucosa associated with fragments of granulation tissue in ulcer. It is evident a dense infiltrate superficial and profound of plasma cells and lymphocytes (Figure 1B), with sub epithelial formations of follicles (Figure 1C), that have an expanded mantle zone (lymphocytes CD5+, BCL6-, BCL2+); the network of dendritic follicular cells is conserved (as put in evidence by immunohistochemistry coloration for CD23) with germinal centers BCL6 and CD10 positive; in the mantle zone are present rare blasts CD30+, occasionally cycline D1+; the lymphatic infiltrate seems to be polytypic with mixed immunophenotype B (CD20+, CD79a+, CD23-; Figure 1D) and T (CD3+, CD56-, CD57-), the last ones are also present intra epithelium. Glandular crypts show inflammation with several crypt abscess and reactive aspects of atypia. Numerous plasma cells are present (CD138+, CD56-) with prevalence of kappa light chains and scarce expression of lambda light chains. Cycling cells percentage is variating from 10% to 40%. Immunohistochemistry for EBV-LMP1 and HHV8-LNA is negative. Coloration for CK AE1/AE3 does not evidence lympho-epithelial lesions or neoplastic epithelial cells. Comment: Morphological characteristics suggesting lymphoplasmocitoid proliferation of reactive type, polyclonal and hyperplastic (pseudo lymphoma).”
This specimen was also submitted to molecular biology tests for research of M. tuberculosis complex (nested-PCR), atypical mycobacteria and T. pallidum (PCR), which were negative; CMV DNA (QT) and EBV DNA (QT <Y899> Real-time PCR) were both detected; HPV DNA (<Q812> PCR) and HSV-2 DNA were absent. The research for CMVDNA and EBVDNA, plus HHV6 DNA, was repeated in another tertiary level laboratory (University Policlinic “Umberto I” of Rome) and resulted positive for all three viruses.
In March metronidazole 750 mg tid (three times a day) orally for 10 days was prescribed, without significant impact on clinical conditions. In April rectal endoscopy showed increase of the lesion; biopsy report was: "Fragments of rectal mucosa, characterized by edema, increase of lympho-monocytes and plasma-cells in the chorion, with a discrete presence of neutrophil granulocytes, infiltrating glandular epithelium and forming several cryptic abscess. Confirmation of acute active proctitis.” Beclomethasone enema was stopped and replaced by mesalazine 2 g enema OD (overdose) and metronidazole 750 mg tid orally for two weeks. In May the patient reported to feel better and resumed to travel for work.
In the meantime Chlamydia trachomatis DNA was found in the biopsy (Anyplex™ CT/ NG Real-time Detection 3.1 Assay, Seegene, Seoul, Korea) and Entamoeba histolytica/dispar DNA was instead absent. The patient underwent treatment with Doxycycline 100 mg bid orally for 30 days until the 10th of June. A CT scan of abdomen and pelvis (11/06) had documented: “Expansion of the anterior wall of the rectum with inhomogeneous enhancement after intravenous injection of contrast. Obliteration of anterior perirectal adipose tissue. Two perirectal lymph nodes of 6 and 7 mm diam. Volumetric increase of prostate (5 cm dm). No sign of ascitic fluid”. In July symptoms completely regressed, the patient was gaining weight. Anti-Chlamydia trachomatis antibodies IgG and IgA (ELISA) in serum were positive. Control proctoscopy confirmed the decrease in volume of rectal ulcer.
Treatment with doxycycline was repeated in October (from 19/10 to 19/11) for the reappearance of hematochezia and local discomfort. Rectal endoscopy showed mucosal folds orthogonal to rectal axis, covered by intact mucosa, slightly hyperemic but not bleeding, as for initial retraction; biopsy report was: "mild edema and chronic inflammation of submucosa. No atypical cells of the rectal mucosa." In November 2014 and March 2015 the patient was asymptomatic. Every year in 2016 and 2017 he has repeated endoscopy, which was normal.
Discussion and Conclusions
Unfortunately, due to the lack of sufficient DNA extracted to be amplified, our Microbiology Laboratory could not further characterize the Chlamydia strain responsible of the infection, but the clinical picture and anti-Chlamydia antibodies were clearly indicative of invasive C. trachomatis infection, corresponding to the definition of probable case of LGV, according to the criteria of Nieuwenhuis et al. [5]. Doxycycline therapy was started without further investigations because in the meantime the rectal lesion had enlarged and the patient was suffering, and was continued until the CT examination of 11 June was performed. The result of CT scan, resolution of symptoms, and the disappearance of the ulcer at endoscopic control, confirmed the diagnosis and allowed us to stop the treatment [6].
LGV may appear like IBD or rectal malignancy and diagnosis is easily missed unless appropriate tests are performed. LGV cases mistaken for IBD may be inappropriately treated with immunosuppressive drugs. Soni et al. [9] reported a series of 12 cases of LGV proctitis that were initially diagnosed as cases of IBD proctitis or ‘nonspecific’ proctitis: three were treated with doxycycline and recovered; nine were treated with metronidazole; 5-aminosalicylate; hydrocortisone or prednisolone without significant symptom relief. Once LGV diagnosis was made, the other 9 patients were treated with doxycycline with prompt and complete resolution of symptoms.
Symptomatic treatment may also complicate infection evolution and/or the interpretation of results of diagnostic tests. In our case, the positivity for CMV, EBV and HHV6 DNA detected with RT PCR in biopsy thick-section and confirmed in two tertiary level laboratories, may be associated to the presence of inflammatory cells infiltrate, induced by C. trachomatis , and to the effect of topic corticosteroid administration. We hypothesize that topical beclomethasone may have induced the reactivation of latent infection by herpetic viruses in epithelial cells of the rectal mucosa [3] (iatrogenic artifact), inhibiting also the immunological control of follicular lymphocytes of submucosa.
Rectal bleeding in young patients is rarely attributed to Chlamydial infection and is considered to be caused by other infectious organisms such as E. coli enterohaemorragic, Salmonella and Shigella spp, Campylobacter species . When these differentials are ruled out, patients are usually investigated for inflammatory bowel disease (IBD) or cancer, without thinking about Chlamydia proctitis that when present is associated to rectal bleeding in 50%-60% of cases [9].
Medical history about sexual habits sometime proves unreliable, because patients do not want to reveal to the physician that they have engaged in risky sexual practices. They therefore prefer to contact the surgeon for fear of having cancer, with the objective to perform colonoscopy with biopsy. In our case endoscopy strongly suggested cancer, but fortunately histological examination of the biopsy has never confirmed this hypothesis, forcing the patient to resort to his infectivologists.
The second treatment in October 2014 was based on the reappearance of same symptoms, with suspicion of new exposure. We were not sure about partner treatment. Moreover, the standard treatment of partners with azithromycin 1000 mg once may be too short if C. trachomatis infection is due to the L1-L3 serovars, as suggested by Oud et al. [6].
Finally we want emphasize the need of compulsory notification of ulcerative STIs. It is probable that seven cases of LGV proctitis by serovar L2b in HIV positive patients described by Latini et al [4], (which occurred in Rome, Italy: 3 cases in 2015 and 4 in 2016) have been epidemiologically linked to the case we have described, which preceded them of about one year. The link of ulcerative STIs to the risk of HIV and other blood borne and sexually transmitted diseases is well known. Monitoring incident cases of ulcerative STIs, including LGV, may help to control outbreaks and to protect health of young heteroand homosexual people from HIV transmission [4] Figure 1.
Funding
The work was supported by grant “Ricerca corrente” from Italian Ministry of Health.
Conflicts of Interest
The authors declare no conflict of interest.
References
- European Centre for Disease Prevention and Control. Lymphogranuloma Venereum. In: ECDC. Annual epidemiological report for 2016. Stockholm: ECDC; 2018
- C Foschi, A Marangoni, A DAntuono, P Nardini, M Compri, et al. (2014) Prevalence and predictors of Lymphogranuloma Venereum in a high risk population attending a STD outpatient’s clinic in Italy. BMC Res Notes 7: 225.
- F Goodrum, K Caviness, P Zagallo (2012) Human cytomegalovirus persistence. Cell microbiol 14: 644-655.
- Latini A, Zaccarelli M, Paglia MG, Dona MG, Giglio A, et al. (2017) Inguinal and anorectal Lymphogranuloma Venereum: a case series from a sexually transmitted disease center in Rome, Italy. BMC Infect Dis. 17: 386.
- R F. Nieuwenhuis, J M. Ossewaarde, H M. Götz, J Dees, H. Bing Thio, et al. (2004) Resurgence of Lymphogranuloma Venereum in Western Europe: An Outbreak of Chlamydia trachomatis Serovar L2 Proctitis in The Netherlands among Men Who Have Sex with Men. Clinical Infectious Diseases 39: 996-1003.
- Oud EV, de Vrieze NHN, de Meij A, de Vries HJ (2014) Pitfalls in the diagnosis and management of inguinal lymphogranuloma Venereum: important lessons from a case series. Sex Transm Infect 90: 279-282.
- Quint KD, Bom RJ, Quint WG, Bruisten SM, van der Loeff MFS, et al. (2011) Anal infections with concomitant Chlamydia trachomatis genotypes among men who have sex with men in Amsterdam, the Netherlands. BMC Infectious Diseases 11: 63.
- Somboonna N, Wan R, Ojcius DM, Pettengill MA, Joseph SJ et al. (2011) Hypervirulent Chlamydia trachomatis Clinical Strain Is a Recombinant between Lymphogranuloma Venereum (L2) and D Lineages. MBio 2: e00045–11.
- S Soni, R Srirajaskanthan, SB Lucas, S Alexander, T Wong et al. (2010) Lymphogranuloma Venereum proctitis masquerading as inflammatory bowel disease in 12 homosexual men. Aliment Pharmacol Ther 32: 59-65.
Citation: Bellagamba R, Mencarini P, Del Nonno F, Nicastri E, Antinori A, et al (2018) Chlamydial Infection Mimicking Rectal Neoplasia: An Uncommon Diagnosis Complicated by the Interference of Drugs Used for the Treatment of Symptoms. J Clin Exp Pathol 8:360. DOI: 10.4172/2161-0681.1000360
Copyright: © 2018 Bellagamba R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 4480
- [From(publication date): 0-2018 - Nov 19, 2025]
- Breakdown by view type
- HTML page views: 3582
- PDF downloads: 898