Research Article Open Access
Chitosan A Low Cost Adsorbent for Electroplating Waste Water Treatment
| Bhavani K1, Roshan Ara Begum E1, Selvakumar S2 and Shenbagarathai R1* | |
| 1PG and Research Department of Zoology and Biotechnology, Lady Doak College, Madurai-625 002, Tamil Nadu, India | |
| 2Department of Environmental Studies, School of Energy Environment and Natural Resources, Madurai Kamaraj University, Madurai-625 021, Tamil Nadu, India | |
| Corresponding Author : | Shenbagarathai R PG and Research Department of Zoology and Biotechnology Lady Doak College, Madurai-625 002, Tamil Nadu, India Tel: 0452-2535575 Fax: 0452-2535575 E-mail: shenbagarathai@rediff.com |
| Received February 25, 2016; Accepted April 12, 2016; Published April 18, 2016 | |
| Citation: Bhavani K, Roshan Ara Begum E, Selvakumar S, Shenbagarathai R (2016) Chitosan– A Low Cost Adsorbent for Electroplating Waste Water Treatment. J Bioremed Biodeg 7:346. doi:10.4172/2155-6199.1000346 | |
| Copyright: © 2016 Bhavani K, et al. This is an open-a ccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
In the wake of rapid industrialization, urbanization and population explosion, the basic needs of life viz. air, water and land are continuously being polluted over the period of the time. Effluent waste from Electroplating industries considerably pollutes the environment. It is of major concern as the discharged heavy metals such as Cr6+, Zn2+, Cu2+, Pb2+, Cd2+ etc. are harmful to the ecosystem. Although various techniques are used for the removal of heavy metals. Bioadsorption process is one of the mainly adopted methods to recycle and reuses the wastewater. Hence the present study focused in assessing the usage of chitosan, a marine biopolymer extracted from the locally available shells of crab Portunus pelagicus as an adsorbent. The ability of chitosan to adsorb heavy metals such as Cr6+, Zn2+, Cu2+, Pb2+, Cd2+ from the electroplating industry waste water effluents was investigated at different pH. It was found that maximum adsorption of heavy metals occurred at a pH range between 5 to 7. The study results indicate that the cost incurred towards extraction was less than that the cost incurred by the commercial treatment facilities. The -OH and –NH2 functional groups in chitosan facilitate the adsorbent function and make it an ideal adsorbent for the treatment of wastewater. This study brings to light that chitosan is function as an economically useful adsorbent for the treatment of electro plating effluent containing heavy metals.
| Keywords |
| Electroplating; Waste water; Bioadsorption; Chitosan; Heavy metals |
| Introduction |
| In the course of human development, industrialization has made possible higher standards of living in our modern Society. Such “progress” has created increased problems with waste from processing operations and their ultimate disposal creating water pollution. There are 400 registered electroplating units in the state of Tamilnadu and are located in Thiruvallur, Kancheepuram, Krishnagiri, Madurai and Coimbatore districts. These units are mainly operating in a small scale sector and 90% of them are exploiting zinc, nickel and chromium plating. In Madurai district alone 54 electroplating units are functional. While these industries economically benefit and support to the local living community it produce hazardous electroplating wastes. Moreover, these units are not only spaced inadequately and small in size, but they do not contain waste water treatment plants. Hence, the waste water effluents containing a large amount of metals and chemicals which are disposed into the mainstream water resources. These wastes contain heavy metals which are very harmful because of their non-biodegradable nature and long biological half-lives. These metals tend to accumulate in different body parts of the living organisms that cause various diseases and disorders. Hence it is imperative to control and eliminate the hazardous waste generated by the electroplating industries. Over the decade, a variety of methodologies and technologies have been developed for waste minimization and elimination from these industrial wastes [1]. |
| A wide range of physical and chemical process such as precipitation, adsorption, oxidation, coagulation, flocculation and membrane filtration processes are available for the removal of these metals from waste waters. Among them, adsorption techniques have been proved to be an excellent method to treat waste water. It is now recognized that adsorption using low cost adsorbents is an effective and economic method for the removal of heavy metals in waste water. However, low cost adsorbents with high adsorption capacities are still under development to minimize disposal problems. Much attention has recently been focused on biopolymers as bioadsorbents which are naturally produced by all living organism. The adsorbents may be of mineral, organic or biological origin, activated carbons zeolites or lowcost adsorbents (industrial by-products, agricultural wastes, biomass and polymeric materials). In the recent years, numerous studies have aimed to develop cheaper and more effective natural polymers adsorbents. Among these biopolymers, polysaccharides such as chitin and starch and their derivatives chitosan, represent an interesting and attractive alternative class of adsorbents. Their particular structure, physico-chemical characteristics, chemical stability, high reactivity and excellent selectivity towards aromatic compounds and metals, are due to the presence of chemical reactive groups (hydroxyl, acetamido or amino functions) in polymer chains. Chitosan is extracted from crustacean shell waste produced by the sea food industry. Crab shell waste is an environmental pollutant with significant health hazards. The most frequent method employed for its disposal is burning, which becomes environmentally costly, due to its low burning capacity. In such a scenario, conversion of crab shell waste to chitosan, a commercially valuable product with myriad of uses, could serve as an effective mode of shell remediation and more importantly enable waste water treatment for the electroplating units. |
| The shell fish industry produces about 60,000 tons of wastes. The disposal of such enormous amount of waste has become a serious environmental concern. Although these wastes are biodegradable but the rate of degradation of a large amount of waste generated per processing operation is comparatively slow. The immediate solution of this problem seems to recycling of the crustacean shells generated and extraction of commercially viable polysaccharide such as chitosan. It carries strong cationic charges at and below pH 6.5 and strong anionic charges above this pH. Therefore it has strong affinity for ions because it comprises of sequenced amino groups (-NH2) and hydroxyl groups (–OH) and thus it is used in many applications in waste water treatment. Hence, this study explored the possibility of using chitosan as bioadsorbent to remove the heavy metals present in the effluent of the electroplating industries. |
| Materials and Methods |
| Extraction of chitosan and sample preparation |
| Crab shells were collected from the Ramaeshwaram, Tamilnadu, India. The shells were washed and dried under hot air oven at 60°C for 24 hours to remove the viscera and tissues. The dried samples were blended and sieved. Twenty grams of shell powder was used for further analysis. The sample was then deproteinized with 1N NaOH at 80°C for 24 hours with constant stirring. It was then washed with distilled water, followed by demineralization with 2N hydrochloric acid. The sample was then washed again with distilled water so as to remove the acidic residues [2]. |
| Electro plating waste water was collected from local electro plating industry near Dindugal district, Tamilnadu. Collected waste water samples were stored in the sterile bottles that has been pre-washed with 10% nitric acid and then thoroughly rinsed with de-ionized water. |
| Deacetylation of chitin into chitosan |
| Chitin was deacetylated with 40% NaOH at 110°C, for 6 hours with constant stirring. Then 10% acetic acid was added to the sample and stored for 12 hours at room temperature. The dissolved sample was reprecipitated by adding 40% NaOH (pH 10). The sample was washed by distilled water until a pH of 6.5 was achieved. Then it was centrifuged at 10,000 rpm for 10 minutes and freeze dried subsequently. |
| Physico-chemical characterization of chitosan |
| Fourier Transform – Infra Red spectroscopy: Domszy and Roberts [3] FT-IR spectroscopy was used for the qualitative analysis of organic chemicals. The spectral location of their IR absorptions could be used to determine the general structure of compounds. FT-IR spectroscopy (Bio-Rad FTIS-40 model, USA) was used to compare and confirm the chemical conformation of standard chitosan with that of extracted chitosan. The spectra of chitosan samples were obtained with a frequency range of λ=400-4000 cm-1. |
| Yield: Chitosan yield was determined by comparing weight measurements of the raw material and of the chitosan obtained after treatment. |
| Ash content: Approximately 3-5 g of chitosan samples were accurately weighed into clean, dry, pre-weighed porcelain crucibles and charred over a Bunsen burner. The charred samples were heated in a muffle furnace and maintained at 550 ± 1°C until a gray ash was obtained. Crucibles were subsequently cooled in desiccators and weighed. Ash content was calculated as percent ratio of the mass of the ash obtained after ignition to that of the original material. |
| The ash content was calculated using the following formula: |
| Degree of deacetylation: Domszy et al. [3] the degree of deacetylation (DD) of the chitosan was calculated using the baseline by Domszy and Roberts. |
| The computation equation for the baseline is given below: |
| DD=100–[(A1655/A3450) × 100/1.33] |
| Where A1655 and A3450 were the absorbance at 1655 cm-1 of the amide-I band as a measure of the N-acetyl group content and 3450 cm-1 of the hydroxyl band as an internal standard to correct for film thickness. The factor 1.33 denoted the value of the ratio of A1655/ A3450 for fully N-acetylated chitosan. |
| Moisture Content: Moisture content of the chitosan was analysed by the gravimetric method. The water mass was determined by drying the sample to constant weight and then measuring the sample weight before and after drying. The water weight was the difference between the weight of the wet and oven dried samples. |
| Adsorption studies |
| Determination of optimum pH for adsorption: Tewari et al. [4] found optimum pH for adsorption of heavy metals (Cr6+, Zn2+, Cu2+, Pb2+, Cd2+) by chitosan was determined experimentally. 50 mL of electro plating effluent sample was dispensed in 7 conical flasks. The initial pH of the sample was adjusted in range of 2-9. Subsequently, 50 mg of chitosan powder was added to each flask. The flasks were kept for 24 hours in agitation using shaker, maintained at 25°C at 100 rpm. After 24 hours, flask content was filtered. The filtrate were analysed for heavy metal concentrations (Cr6+, Zn2+, Cu2+, Pb2+, Cd2+). Samples of the Cr6+ were analysed using UV Visible Spectrophotometer (U -2000, Hitachi) at 540 nm, according to the 1,5-diphenyl-carbazide method. The respective samples of the solution of Zn2+, Cu2+, Pb2+, Cd2+ were analysed by Atomic adsorption Spectrophotometer. The amount of metallic ion adsorbed by the chitosan was calculated using the equation |
| q-(C0-Ce)v/w |
| Co - Initial Concentration of Heavy Metals (Mg/L) |
| Ce - Final Concentration of Heavy Metals (Mg/L) |
| V- The Volume of Aqueous Solution (L) |
| W- The Mass of Chitosan (g) |
| q- The adsorbate concentration or sorption capacity of chitosan (mg/g) |
| Effect of adsorbent dose: The dependence of Cr6+, Zn2+, Cu2+, Pb2+, Cd2+ adsorption on dose was studied by varying the amount of adsorbents from 1 to 6 g/L, by keeping the other parameters (pH, contact Time) constant. 50 mL of electro plating effluent sample was dispensed in 6 conical flasks with different concentration of chitosan from 1 g/L to 6 g/L. Flasks were agitated at 150 rpm at different time intervals. The solution was filtered and the filtrate were analysed by atomic adsorption spectrophotometer Zn2+, Cu2+, Pb2+, Cd2+ and spectrophotometer for Cr6+ at 540 nm [5]. |
| Effect of contact time for adsorption: The optimum time for adsorption of heavy metals (Cr6+, Zn2+, Cu2+, Pb2+, Cd2+) by chitosan was determined experimentally. 50 mL of electro plating effluent sample was dispensed in 22 conical flasks. Contact time for treatment was varied from 40 min to 460 min. The pH of the solution was adjusted to 5. Flasks were agitated at 150 rpm at different time interval. The solution were filtered and the filtrate was analysed to atomic adsorption Zn2+, Cu2+, Pb2+, Cd2+ and spectrophotometer for Cr6+. In order to investigate the controlling mechanism of the adsorption processes, pseudo firstorder and pseudo second-order kinetics were applied in the data [6]. |
| Desorption studies |
| Determination of optimum pH for desorption study: Vieira et al. [7] After carrying out the adsorption process, the chitosan particles adsorbed with heavy metal were filtered and let a few minutes to get dry. 50 mg of dried chitosan particles dissolved with 50 mL distilled water. The pH of these solutions was adjusted from 2 to 8 with 0.1N NaOH and 0.1 M HCl. The flasks were kept for 24 hours in agitation using shaker, maintained at 25°C at 100 rpm. After 24 hours, flask content was filtered. The filtrate were analyzed for heavy metal concentrations (Cr6+, Zn2+, Cu2+, Pb2+, Cd2+). Samples of the Cr6+ were analyzed using UV Visible Spectrophotometer (Model U -2000, Hitachi) at 540 nm, according to the 1,5-diphenyl-carbazide method. The respective samples of the solution of Zn2+, Cu2+, Pb2+, Cd2+ were analyzed by Atomic adsorption Spectrophotometer. The optimum pH for desorption were ascertained from maximum metal concentration in solution. |
| Results and Discussion |
| Extraction of chitosan |
| Chitosan, the deacetylated form of chitin has found wide applications due to its versatility, non-toxicity, biodegradability, biocompatibility and bio-renewability. Keeping the importance of chitin and chitosan in mind, an attempt was made to extract chitosan from the exoskeleton of Crab (Portunus pelagicus). The yield of chitosan obtained after deacetylation of chitin was found to be 59% (Table 1). These result were in agreement with Ref. [8] reports, where the yield of chitosan from freshwater crab (Potamon potamios) and blue swimmer crab (Portunus pelagicus) was 60%. The various parameters studied after the extraction and the values obtained are tabulated in Table 1. |
| Degree of deacetylation |
| Deacetylation degree of chitosan is the most important quality parameter in this study. The degree of deacetylation showed that the percentage of acetyl groups removed from the chitin to produce chitosan. High deacetylation degree showed that the acetyl groups contained in the chitosan is low. The deacetylated chitosan enhances the interaction between the ions and hydrogen bond. Degree of deacetylation of chitosan obtained in this study ranged from 76.26- 91.60%, with an average overall deacetylation degree was 82%. |
| Ash content |
| The ash content in chitosan is an important parameter that affects its solubility, viscosity and also other important characteristics. The extracted chitosan had an ash content of less than 1% when compared to commercially available chitosan. |
| FTIR spectroscopy |
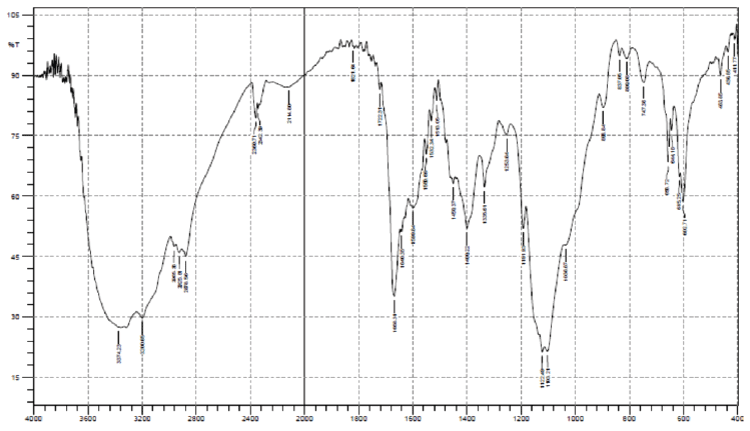
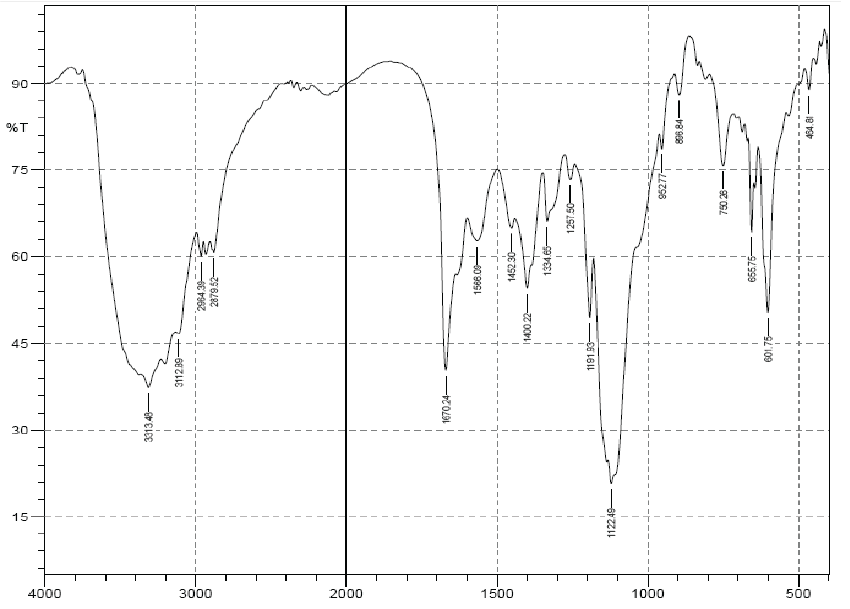
| The structure of the extracted chitosan was confirmed by FTIR analysis in this study, the IR spectra of the isolated sample of chitosan from crab shells was analyzed and compared with the IR spectrum of standard chitosan. The isolated fractions gave IR spectra similar to that of the standard chitosan (Figures 1 and 2). The results indicated a strong similarity between both the standard and extracted compound. The FTIR spectrum of the standard chitosan showed eight major peaks at the ranges of 837, 896, 1253, 1335, 1599, 1640, 2878 and 3374 cm−1, whereas the Chitosan sample from shells of P. pelagicus matched with that of seven major peaks of standard chitosan. The absorbance bands of 891, 897, 1026, 1259, 1422, 1587, 3377 indicate the HPO42-, (NH) amide III,PO4-3, PO3-4, OH group (monomer), (-NH2) Amide II, structural unit respectively. |
| Batch adsorption experiments |
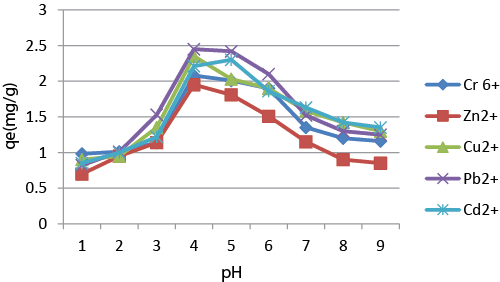
| Effect of pH: pH plays an important role in the adsorption of metal ions as it affects both the dissociation degree of the functional group from the adsorbent surface and the solubility of metal ions. The electroplating wastewater contains 2.64, 2.75, 3, 2.96, 2.9 mg/L of Cr6+, Zn2+, Cu2+, Pb2+, Cd2+ respectively. The effect of pH on adsorption of heavy metals (Cr6+, Zn2+, Cu2+, Pb2+, Cd2+) by chitosan was studied over a pH range of 2 to 9 at 25°C, at an agitation speed of 150 rpm. From the study, the adsorption capacity of chitosan for heavy metals like Cr6+, Zn2+, Cu2+, Pb2+, Cd2+) was found to be 70%, 78%, 81%, 82%, 79% respectively, at a pH 5, while adsorption was lowest at pH 2 (Figure 3). This result was in accordance with Ref. [4], who reported that adsorption of heavy metals reaches a peak at a pH range of 5 to 8. At nearly neutral pH (4 to 5) the cationic heavy metals exist as free ions and get adsorbed on to chitosan. Whereas at higher pH, adsorption performance is improved due to decrease in H+ ion that causes decrease in both binding sites and the electrostatic repulsion. Furthermore, at a lower pH, H+ ion concentration is high, so the protonation of amino group induces an electrostatic repulsion of metallic ions. |
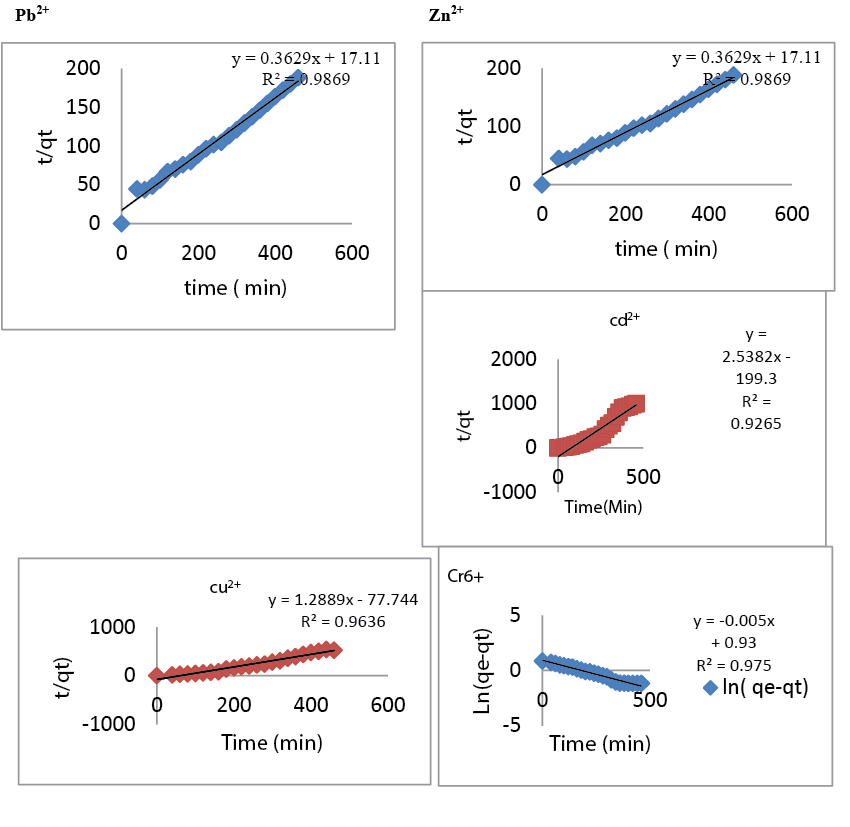
| Effect of contact time: It has been observed that at a constant concentration of metal ions and fixed amount of adsorbent, the adsorption efficiency increases with increasing the contact time up to a certain level and then it reaches the equilibrium. Figure 4 shows that adsorption rate first increased rapidly as the contact time increases, but after reaching the optimum time of about 340 min there is no significant increase. The effect may be due to the saturation of adsorption sites with metal ions on the solid particle. The optimum contact time for Cr6+, Zn2+, Cu2+, Pb2+, Cd2+ was 320 min. The slight decrease in adsorption after optimum contact time may be due to the breakage of newly formed weak adsorption bonds due to constant shaking. In order to investigate the controlling mechanism of adsorption processes such as mass transfer and chemical reaction, the pseudo first order and pseudo second order equations are applied to model the kinetics of Cr6+, Zn2+, Cu2+, Pb2+, Cd2+ adsorption onto chitosan. The pseudo first-order kinetic model did not adequately describe the adsorption results with a low correlation coefficient for the Zn2+, Cu2+, Pb2+, Cd2+ (0.8) except Cr6+ (0.975). However, the pseudo second-order kinetic model provided a comparable correlation for the adsorption of Zn2+, Cu2+, Pb2+ ions in contrast to the pseudo first-order model (0.99). The pseudo first-order rate model has been widely used for sorption of metals, which is widely used for reversible reactions with an equilibrium being established between liquid and solid phases. In many cases, the pseudo first order does not fit well to the whole range of contact time and is generally applicable over the initial stage of adsorption process. |
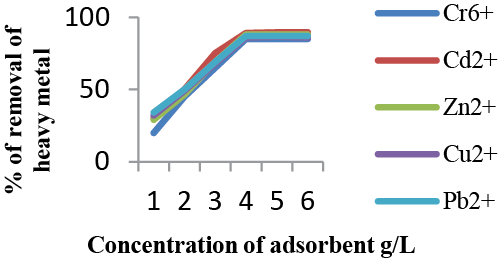
| Effect of adsorbent dose: The effect of the adsorbent dose was studied at room temperature by varying the sorbent amounts from 1 to 6 g. For all these runs, initial concentration of the metal ions was fixed. Figure 5 shows the adsorption of Cr6+, Zn2+, Cu2+, Pb2+, Cd2+ ions increase rapidly with increase in the amount of adsorbent due to greater availability of the surface area at higher concentration of the adsorbent. The significant increase in uptake was observed when the dose was increased from 1 to 4 g. Any further addition of the adsorbent beyond this did not cause any significant change in the adsorption. This might be due to overlapping of adsorption sites as a result of overcrowding of the adsorbent particles. These results indicate that removal efficiency is directly related to the number of available adsorption sites. Once equilibrium is attained, there is no effect on adsorption efficiency thus proving that even at a lower concentration of 4 g/L of the adsorbent chitosan was enough for removal of heavy metals. |
| Determination of optimum pH for desorption study |
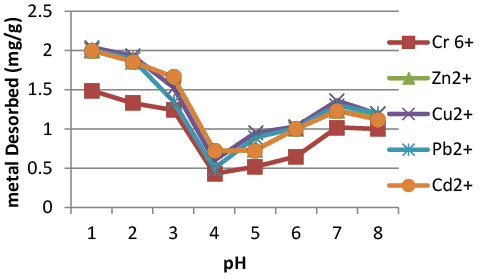
| The nature of the adsorption process and the recovery of metal ions. Regeneration of the adsorbent material is of crucial importance in economic development [9]. Regeneration must produce small volume of metal concentrates suitable for metal recovery process without damaging the capacity of the adsorbent. The effect of pH on desorption of the heavy metals by chitosan over a pH range 2 to 8, at room temperature with an agitation speed of 150 rpm is illustrated in (Figure 6). Maximum desorption occurs at pH 2. The percentage of desorption of Cr6+, Zn2+, Cu2+, Pb2+, Cd2 was found to be 70%, 78%, 81%, 82%, 79% at pH 2. The percentage of desorption was lowest at pH 5(25%) [10]. Observed that qualitative decline in the metal uptake capacity of chitin and chitosan, for an extended series of metals when the pH was reduced from neutral to acetic. Desorption study results indicate that when abundant protons compete for the adsorption site with metals at low pH, it releases the metal ions adsorbed on chitosan particle into the suspending medium, causing desorption. Hence, higher amount of metals desorbed from chitosan at lower pH values. The adsorption-desorption cycle results clearly demonstrate that the regeneration and subsequent use of the chitosan would enhance the removal of heavy metals from wastewater. |
| Thus, from the above study it is suggested that chitosan can be used as low cost adsorbent for electroplating effluent treatment. An adsorbent is considered as low cost if it requires minimal processing and is abundant in nature. It has been reported by Ref. [11], that the expense of individual adsorbent varies depending on the degree of processing required and local availability. In this study, Chitosan is extracted from the shells of P. pelagicus which is considered as a waste and is abundantly available in Rameshwaram Coast, and hence the production of which requires minimal processing and the raw materials used for chitosan extraction is freely available. Table 2 shows the cost effectiveness of the extracted chitosan [12-17]. Thus, the use of low-cost adsorbents like chitosan may also contribute to the sustainability of the surrounding environment. |
| Conclusion |
| Chitosan extracted from the locally available crab shell was utilised as an adsorbent for the removal of heavy metals from electroplating waste water. The optimum pH necessary for removing these heavy metals in solution was found to range between 5-7. Metal removal increased with an increase in pH, as there is a decrease in competition between proton and metal cation for some functional group along with a decrease in positive surface charge, resulting in a lower electrostatic repulsion between surface and metal ions. Pseudo-second order model showed the best fit to the experimental data. Thus, this study shows that the use of crab shell chitosan for heavy metal removal appears to be technically feasible, and eco-friendly with very high efficacy. Further studies would reveal the concentrations of the adsorbent required to resolve the COD and BOD level in the effluent after treatment. |
| Acknowledgements |
| Authors would like to thank DBT – BIF lab, Lady Doak College, Madurai. Supported by Ministry of Science and Technology, Department of Biotechnology, Bioinformatics Division, New Delhi (Sanc. No. BT/B I/25/001/2006). |
|
Tables and Figures at a glance
| Table 1 | Table 2 |
Figures at a glance
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 |
 |
 |
 |
| Figure 4 | Figure 5 | Figure 6 |
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 12850
- [From(publication date):
May-2016 - Jul 01, 2024] - Breakdown by view type
- HTML page views : 11801
- PDF downloads : 1049
