Research Article Open Access
Children with Mild CAG Repeat Expansion in HTT Gene Showing Psychiatric but not Neurological Presentation: Is It One More Shade of Huntington Disease?
Massimo Marano1,2#, Simone Migliore1,2#, Sabrina Maffi1, Federica Consoli1, Alessandro De Luca1, Irene Mazzante2 and Ferdinando Squitieri1*1IRCCS Casa Sollievo della Sofferenza, San Giovanni Rotondo, Italy
2LIRH (Lega Italiana Ricerca Huntington e malattie correlate) Foundation, Rome, Italy
- Corresponding Author:
- Ferdinando Squitieri
Huntington and Rare Diseases Unit and Neurology at CSS-Mendel Casa Sollievo della Sofferenza Reserch Hospital (IRCCS)
Viale Regina Margherita, 261 00198 - Rome, Italy
Tel: +39 0644160527
E-mail: f.squitieri@css-mendel.it
Received date: April 28, 2017; Accepted date: June 13, 2017; Published date: June 20, 2017
Citation:Marano M, Migliore S, Maffi S, Consoli F, De Luca A, et al. (2017) Children with Mild CAG Repeat Expansion in HTT Gene Showing Psychiatric but not Neurological Presentation: Is It One More Shape of Huntington Disease? J Alzheimers Dis Parkinsonism 7:335. doi:10.4172/2161-0460.1000335
Copyright: © 2017 Marano M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Alzheimers Disease & Parkinsonism
Abstract
Objective: Huntington disease (HD) generally manifests in adulthood. Large mutations with CAG repeat expansion in HTT gene may rarely cause juvenile Huntington disease (JHD) in early childhood or adolescence with atypical clinical features, i.e., atypical parkinsonism, if compared to adult patients. Our objective is to characterize the rare occurrence of clinical manifestations in children carrying mutations in the low-mild size, generally causing adult HD with typical choreic movements. Methods: We are following up a subgroup of young subjects with HD mutation who manifested with disabling psychiatric condition since early childhood or adolescence. We are collecting data by the observational studies Registry and ENROLL-HD since 2004. Among 60 JHD patients we are currently following-up, we selected people who carry a mutation in the mild range of CAG expansions (i.e., expected to manifest in adulthood), psychiatric manifestations and no neurological signs or movement disorders suggestive of HD. All patients were genetically (i.e., CAG size analysis) and clinically (i.e., total motor score within the Unified HD Rating Scale) characterized. Results: We found four subjects who showed the characteristics for this analysis. All four subjects presented a CAG expansion size <45 repeats. Two patients manifested a schizophrenia-like disturbance during their adolescence, with the later appearance of motor signs after age 20. In the other two cases, patients presented symptoms of autistic spectrum disorder, since infancy. One of them showed also a schizophrenia-like disturbance and, later, HD onset with motor signs after 20. A 45 year old patient is currently manifesting an autistic disorder in absence of others neurological signs. Conclusion: The description of JHD includes sometimes children with psychiatric manifestations associated with adult motor onset. We advise to pay careful attention to such rare conditions that might represent either psychiatric conditions erroneously classified as JHD or prodromic adult HD cases.
Keywords
Juvenile Huntington disease; Autism; Schizophrenia; Age at onset; Mutation penetrance
Introduction
Huntington disease (HD) is an inherited autosomal dominant disorder, caused by an expanded mutation in the HTT gene beyond 36 CAG repeats. It manifests generally around the age of 40, with progressive behavioral, cognitive and neurological symptoms, whose involuntary choreic and dystonic movements are the main hallmark. Although the intergenerational increasing number of the CAG repeats concurs to anticipate the age at onset in affected children compared to their transmitting parent, other genetic and environmental factors influence the phenotype presentation, disease progression and the age of initial symptoms [1]. In a rare occurrence (i.e., about 10% of all HD cases) the disease may start before the age of 20, i.e., juvenile HD (JHD), by a neurological presentation, that is generally atypical compared to adult HD, with predominant dystonia and parkinsonism and a severe progression towards high disability and death [2]. The development of JHD is however hard to compare with adulthood HD, due to missing validated evaluation scales for young children. In general, JHD manifests with neurological or mixed (i.e., both motor and non motor) symptoms (i.e., learning issues, psychiatric symptoms, neurodevelopmental delay), associated with large CAG repeat expansions beyond 50- 60, sometimes overcoming 80-100 repeats in the very rare infantile cases. In these very rare conditions, the disability is often contributed by a dramatic occurrence of drug resistant epilepsy and fast mental and physical decay towards coma and cachexia in few years.
Among JHD cases, clinical conditions associated with mild CAG expansions (e.g. 40-45 repeats), non neurological/motor abnormalities and psychiatric features, are also described [3]. These conditions are difficult to detect because, according to published guidelines for genetic tests in minors, we cannot proceed with the molecular diagnosis in subjects under the majority (18 years in Italy), unless they manifest with suspicious symptoms of a given disease [4]. Therefore, in case of missing neurological manifestations, we may only recognize them by genetic confirmation after many years, once they become adult subjects.
On the other hand, adult HD patients often suffer for psychiatric symptoms. Such issues could significantly anticipate the onset of motor signs in a number of individuals, but whether and how psychiatric manifestations related to the disease itself is an open major debate. JHD children may experience the unfortunate condition to descend by a relatively young parent (e.g. frequently the father), who manifests with psychiatric abnormalities. In this case young patients may share a violent home environment with the risk to develop an antisocial behavior themselves. An obvious consequence of this is the implication for their genetic testing and, eventually, for the interpretation of a positive result [2].
In this report, we describe the case of four subjects who manifested with HTT-CAG mutation in a mild range of repeats and schizotypal or autistic behavior since the adolescent or pre-adolescent age, in total absence of any neurological manifestation. Such description raises the question of whether the timing of clinical HD features shows a continuum from birth to onset or, conversely, JHD may represent a HD variant different from adult HD, possibly contributed by diverse/ additional pathogenic mechanisms.
Methods
Patients
We have set a multidisciplinary outpatient service including professionals (e.g. neurologists, psychiatrists, psychologists, geneticists, nutritionists) with specific expertise in HD and in strict collaboration with family members, at LIRH Foundation sites in Northern, Middle and Southern Italy, since 2001 [5] and at Casa Sollievo della Sofferenza Research Hospital, CSS-Mendel Institute of Human Genetics in Rome, since July 2015. The study on the observational analysis of adults and minors with HD and the collection of data and samples, were approved by the local Ethical committee and communicated to the National Privacy Authority according to local legislation. All subjects and parents signed an informed consent.
Since 2004, we have started a prospective collection of data by contributing to REGISTRY [6] and, more recently, to ENROLL-HD programs. Currently, we have clinical and genetic data from about 60 JHD cases with onset movement disorders ≤ 20 years of age. Within this population, we selected four young patients who manifested with a severe behavioral disturbance, three of them developing neurological and motor symptoms in adulthood, later than 20 years. Neurological and psychiatric assessments were performed by the international Unified Huntington Disease Rating Scale (UHDRS) [7] and according to the Diagnostic and Statistical Manual of mental disorders V (DSM-V) criteria. Motor impairment was assessed by the Total Motor Score (TMS) assay, that is part of the UHDRS and includes items testing impaired voluntary movements (i.e., coordination, balance, gait, ocular movements, language, tongue impersistance, muscular tone) and the involuntary movement disorder (i.e. chorea, pakinsonism, dystonia) [7]. We test the confidence of motor abnormalities by the Diagnostic Confidence Level (DCL) score, that includes items starting from 0 (no abnormalities, TMS ≤ 5) to 4 (motor abnormalities that are unequivocal signs of HD with ≥ 99% confidence, TMS>10) [7].
Genetic testing
The DNA samples, collected for research purposes, were re-sized, after informed consent, at CSS-Mendel Institute of Human Genetics. We currently perform the exact determination of CAG repeat number in Exon 1 of the HTT gene on Chromosome 4p16.3 (RefSeq: NM_002111.6) by Polymerase Chain Reaction (PCR), followed by PCR product sizing through capillary electrophoresis adopting ABI3130 detection system and GeneMarker software. To confirm the presence of a very large expansion in JHD patients, that would be missed by PCR in case of homozygosity of a normal CAG repeat allele, we adopt TP-PCR procedure [8].
Results
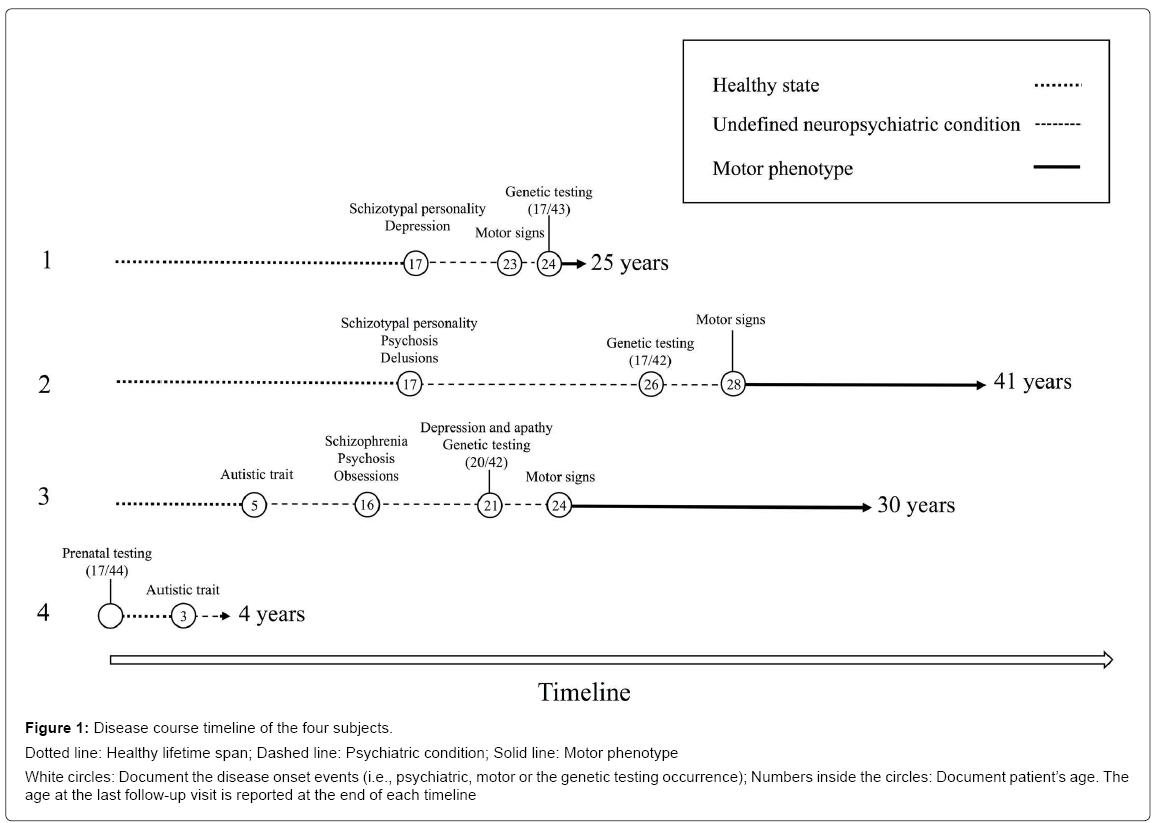
Demographic, genetic and clinical details are reported in Table 1. All subjects presented a CAG expansion size <45 repeats, i.e., with a mutation expected to manifest an adult phenotype [9]. None but one subject (patient 2) reported a suspicious family history of a psychiatric disorder independently from HD. Two patients (patients 1 and 2) manifested a schizophrenia-like disturbance during their adolescence, with later appearance of HD motor signs after the age of 20. In the other two cases, they presented a disabling psychiatric condition with autistic spectrum disorder, since infancy. One of them (patient 3) showed a schizophrenia-like disturbance and, later, an onset with motor signs suggestive of HD after the age 20. The last one (patient 4) is currently under observation, presenting an autistic spectrum disorder in absence of other neurological symptoms. All patients presented a total motor score at UHDRS less than 5 (Diagnostic Confidence Level <4), before the clinical diagnosis of HD. The disease course timeline of the four subjects is showed in Figure 1.
| Items | Patient 1 | Patient 2 | Patient 3 | Patient 4 |
|---|---|---|---|---|
| Transmitting parent | Father | Father | Father | Father |
| Expanded CAG repeat number | 43 | 43 | 42 | 44 |
| Age at the genetic testing | 24 | 26 | 21 | Prenatal test |
| Psychiatric symptoms | Schizotypal personality changes, psychosis, mood changes, suicide ideation | Schizotypal personality changes, psychosis, delusions | Schizotypal personality changes, psychosis, autistic spectrum, obsessions, mood changes | Autistic spectrum |
| Age at onset of psychiatric symptoms | 17 | 17 | 5 | 3 |
| Motor symptoms | Bradykinesia and rigidity | Severe dystonia, dysarthria and dysphagia | Bradykinesia and incoordination | No |
| Age at onset of motor symptoms | 23 | 28 | 24 | No |
Table 1: Demographic, genetic and clinical features.
Figure 1: Disease course timeline of the four subjects.
Dotted line: Healthy lifetime span; Dashed line: Psychiatric condition; Solid line: Motor phenotype
White circles: Document the disease onset events (i.e., psychiatric, motor or the genetic testing occurrence); Numbers inside the circles: Document patient’s age. The age at the last follow-up visit is reported at the end of each timeline
Patient 1
This patient manifested a schyzotypal personality disorder with auditory hallucinations and depression since the age of 17. He underwent treatment with neuroleptics and mood stabilizers (clozapine, up to 300 mg per day and valproate, up to 500 mg per day). At the age of 23, he developed bradykinesia and rigidity and at 24, he was tested positive for HD mutation (43 CAG repeats) due to a paternal inheritance. Suicidal ideation was only recently reported and the patient is therefore accurately monitored. No family history of neuropsychiatric disorder, but for HD, was reported. The affected father, who carried 38 CAG repeats in HTT gene, developed early psychiatric symptoms at the age of 48 and received the diagnosis of manifest HD at the age of 55.
Patient 2
This patient received the diagnosis of schizotypal personality disorder with psychosis and delusions at the age of 17, and a consecutive treatment with long acting neuroleptics (e.g. haloperidol decanoate, 50 mg fortnightly) and benzodiazepines. Transitorial drug abuse (marijuana, heroin and metanphetamine) and further treatments with olanzapine up to 10 mg per day, lithium carbonate 600 mg per day and valproate, were reported since age 20. The psychiatric symptoms were barely controlled by the therapy. First movement disorder was a suspicious drug induced oro-mandibular dystonia with severe dysarthria and dysphagia at age 28. The subsequent development of generalized dystonia and incoordination was suggestive of the clinical diagnosis of HD. Genetic test was performed to confirm the clinical diagnosis and revealed a mutation with 43 CAG repeats. The maternal history of unspecified psychiatric disturbance and cognitive impairment was also reported. The paternal inheritance was supported by anamnestic findings, with no further information available.
Patient 3
The patient’s family reported an early psychiatric manifestation in the autistic spectrum disorder, since the age of 5. Despite of a normal neuro-development, he presented mild psychic delay (e.g. delayed language acquisition) with autistic traits at age of four and schizotypal personality changes evolving in the following years to schizophrenia like symptoms, i.e., auditory hallucinations and psychosis, since the age of 17. Since that time, he was treated by neuroleptics (haloperidol up to 3 mg per day and olanzapine up to 10 mg per day). He developed a severe obsessive compulsive and perseverative behaviors since the age of 16 and depression and apathy since he was 21, when genetic test confirmed HD with 42 CAG repeats. He developed bradykinesia at 24 followed by muscular rigidity and gait disturbances receiving the clinical diagnosis of manifest HD. The affected father carried 42 CAG repeats and manifested first motor and psychiatric symptoms since the age of 55.
Patient 4
In this case a prenatal molecular diagnosis of HD (44 CAG repeats) was obtained through a chorionic villus sampling by the unaffected mother. By that time, the father was in a presymptomatic life stage carrying 41 CAG repeats. The patient presented suspicious psychic and motor delay (e.g. first steps since the age of 15 months and, currently, first word utterances at four years of age) and was evaluated for autism spectrum disorder since the age of 17 months. At the age of 3, he presented marked autistic traits, sometimes showing stereotyped movements. The neurological exam was otherwise normal and the case, at the age of 4, is currently under observation.
Discussion
HD is manifesting with a very heterogeneous phenotype. However, the discovery of its gene mutation opens new clues to genotypephenotype correlations in different cohorts and populations and, finally, to new therapeutic approaches. In spite of the advances in research, we still need to learn how the many shades of polymorphic CAG repeats in HTT gene (either mutated or wild type) is influencing the clinical HD presentation and progression before to fully elucidate the mystery of HD biology [9].
Our cases show severe behavioral changes occurring very early in their life. In three cases first manifestation was a schizotypal personality change evolving into full schizophrenia-like phenotype, in one case associated with autistic spectrum disorder, then developing HD after the border age of 20 years, the edge to distinguish JHD from adult HD. In the other case, the five year old child is currently manifesting with autism and no neurological symptoms. All these patients but for the last one, received the genetic test in their adult life when the suspicious of HD became visible. Patient 4 was diagnosed by a prenatal diagnosis to his mother who, then, refused the abortion.
In our cohort of Italian origin, the frequency of such unusual cases is of about 7% (4/60) of all JHD cases. The occurrence of behavioral changes before the motor onset in adult HD patients is quite common although, in our experience, most onset cases are mixed (i.e., motor, psychiatric and cognitive features). However, in case of JHD the clinical presentation is generally atypical and the overlapping motor, psychiatric and cognitive manifestations, due to the relatively severe and fast disease progression, may represent a confounding factor in the age at onset ascertainment. We think, therefore, it is important for specialists to distinguish the real JHD from conditions where the behavioral symptoms are randomly marking a life time well before the expected neurological manifestations that are likely related to the typical HD cortico-striatal circuit alteration. Our cases were missing of neurological signs as ascertained by the TMS-UHDRS, specifically the total motor score assay, that indicated a Diagnostic Confidence Level below 3 before motor signs suggestive of HD, in all cases.
Nopoulos’ group documented abnormal morphometric scores in children carrying a mutation in the range of adult patients, together with some behavioral features when compared to children from the general population [10]. Such evidence is in line with the hypothesis that HD might be associated with developmental delays, beside to the neurodegenerative process. Our findings are in line with Nopoulos’ conclusions as the autism spectrum disorders may represent a condition associated with developmental delay [11] and we found autistic features in two of our four subjects. Other JHD cohorts from different populations showed onset symptoms, other than neurological features or movement disorders [12,13]. However, the missing longitudinal follow-ups, which may eventually highlight a motor onset at young age (e.g. before age 20) in these cases, may have obscured whether they are real JHD cases or randomly occurring behavioral symptoms as happening in the general population or, conversely, a prodromal condition of adult HD or, finally, a condition that may develop in children living in an environment with other family members suffering for psychiatric disturbances.
Our analysis has some limitations. For example, we do not have any brain imaging of these subjects, although classical imaging may, by now, only offer unspecific indication and would not, anyway, document evidence of HD onset related to validated markers of psychiatric or neurodevelopmental disorder. However, an important novelty of our study is that we prospectively report, to our knowledge for first time, the case of subjects carrying a CAG mutation and showing schizophrenialike and autistic features with ascertained motor abnormalities occurring only in adulthood (age>21), at least in three out of four cases.
Our cases were followed-up during many years but for patient 4, who will be monitored during the next years and is currently 4 years old. This last case raises the question of how and whether the genetic and psychological counselling is carried out during the prenatal diagnosis. Genetic and psychological counseling should allow parents to make a proper decision whether to terminate or not the pregnancy in advance, before to perform the prenatal genetic test by chorionic villus sampling with its intrinsic miscarriage risk.
By our study, we want to recommend paying careful attention to the JHD classification that, in our opinion, is an extremely rare and particularly severe variant of HD associated with large and biologically toxic mutations. The analysis of collaborative studies on large size JHD cohorts are urgently required to address the many, yet unsolved issues (e.g. validated clinical markers including imaging variations and clinical evaluation tools), related to this condition that is rare, severe and extremely frustrating for both the families and the scientific community. Indeed, all these limitations make experimental trials unavailable to young children with HD, thus precluding their parents’ hope to stop the relentless development of the disease affecting their children. For instance, longitudinal prospective analyses of clinical symptoms and brain changes based on careful JHD patients’ stratification according to mutation size and age at onset are strongly needed and expected.
Conclusion
In conclusion, we here prospectively report, to our knowledge for first time, the case of subjects with HTT-CAG mutation showing schizophrenia-like and autistic features and follow-ups documenting the beginning of HD in adulthood (after than the age 20) in three out of four cases. Conditions with mild size CAG expansions may represent either a prodromal HD which will start in the adulthood with its typical clinical features after many years or other psychiatric pathologies occasionally associated with HD.
Acknowledgement
We are grateful to patients, their families and the family associations (i.e. LIRH Tuscany), for collaboration to the study.
References
- Wexler NS, Lorimer J, Porter J, Gomez F, Moskowitz C, et al. (2004) Venezuelan kindreds reveal that genetic and environmental factors modulate Huntington's disease age of onset. Proc Natl Acad Sci USA 101: 3498-3503.
- Quarrell O, Brewer HM, Squitieri F, Barker RA, Nance MA, et al. (2009) Juvenile Huntington disease and other trinucleotide repeat disorder. Oxford University Press Book.
- Squitieri F, Frati L, Ciarmiello A, Lastoria S, Quarrell O (2006) Juvenile Huntington's disease: Does a dosage-effect pathogenic mechanism differ from the classical adult disease? Mech Ageing Dev 127: 208-212.
- Anderson JA, Hayeems RZ, Shuman C, Szego MJ, Monfared N, et al. (2015) Predictive genetic testing for adult-onset disorders in minors: A critical analysis of the arguments for and against the 2013 ACMG guidelines. Clin Genet 87: 301-310.
- Squitieri F, Griguoli A, Capelli G, Porcellini A, D'Alessio B (2016) Epidemiology of Huntington disease: First post-HTT gene analysis of prevalence in Italy. Clin Genet 89: 367-370.
- Cubo E, Ramos-Arroyo MA, Martinez-Horta S, Martínez-Descalls A, Calvo S, et al. (2016) Clinical manifestations of intermediate allele carriers in Huntington disease. Neurology. 87: 571-578
- Huntington Study Group (1996) Unified Huntington's disease rating scale: Reliability and consistency. Mov Disord 11: 136-142.
- Jama M, Millson A, Miller CE, Lyon E (2013) Triplet repeat primed PCR simplifies testing for Huntington disease. J Mol Diagn 15: 255-262.
- Squitieri F (2013) Neurodegenerative disease: 'Fifty shades of grey' in the Huntington disease gene. Nat Rev Neurol 9: 421-422.
- Lee JK, Mathews K, Schlaggar B, Perlmutter J, Paulsen JS, et al. (2012) Measures of growth in children at risk for Huntington disease. Neurology 79: 668-674.
- Toizumi M, Nguyen GT, Motomura H, Nguyen TH, Pham E, et al. (2017) Sensory defects and developmental delay among children with congenital rubella syndrome. Sci Rep 7: 464-483.
- Gatto EM, Parisi V, Etcheverry JL, Sanguinetti A, Cordi L, et al. (2016) Juvenile Huntington disease in Argentina. Arq Neuropsiquiatr 74: 50-54.
- Ribaï P, Nguyen K, Hahn-Barma V, Gourfinkel-An I, Vidailhet M, et al. (2007) Psychiatric and cognitive difficulties as indicators of juvenile Huntington disease onset in 29 patients. Arch Neurol 64: 813-819.
Relevant Topics
- Advanced Parkinson Treatment
- Advances in Alzheimers Therapy
- Alzheimers Medicine
- Alzheimers Products & Market Analysis
- Alzheimers Symptoms
- Degenerative Disorders
- Diagnostic Alzheimer
- Parkinson
- Parkinsonism Diagnosis
- Parkinsonism Gene Therapy
- Parkinsonism Stages and Treatment
- Stem cell Treatment Parkinson
Recommended Journals
Article Tools
Article Usage
- Total views: 3303
- [From(publication date):
June-2017 - Dec 23, 2024] - Breakdown by view type
- HTML page views : 2678
- PDF downloads : 625