Research Article Open Access
Childhood Activities and Schistosomiasis Infection in the Kassena-Nankana District of Northern Ghana
| Francis Anto1*, Victor Asoala2, Martin Adjuik2, Thomas Anyorigiya2, Abraham Oduro2, James Akazili2, Patricia Akweongo1, Langbong Bimi3 and Abraham Hodgson4 |
||
| 1 School of Public Health, College of Health Sciences, University of Ghana, Legon, Ghana | ||
| 2 Navrongo Health Research Centre, Navrongo, Upper East Region, Ghana | ||
| 3 Department of Animal Biology and Conservation Science, University of Ghana, Legon, Ghana | ||
| 4 Research and Development Division, Ghana Health Service, Accra, Ghana | ||
| Corresponding Author : | Francis Anto School of Public Health University of Ghana, Legon Accra, Ghana Tel: +233-0244577063 E-mail: fanto@ug.edu.gh |
|
| Received May 13, 2014; Accepted June 25, 2014; Published July 05, 2014 | ||
| Citation: Anto F, Asoala V, Adjuik M, Anyorigiya T, Oduro A, et al. (2014) Childhood Activities and Schistosomiasis Infection in the Kassena-Nankana District of Northern Ghana. J Infect Dis Ther 2:152. doi:10.4172/2332-0877.1000152 | ||
| Copyright: © 2014 Anto F, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | ||
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
||
Visit for more related articles at Journal of Infectious Diseases & Therapy
Abstract
Schistosomiasis is a common cause of morbidity especially among rural children in less developed countries. The extent and distribution of schistosomiasis infection among school-age children was assessed and the association between some childhood activities and prevalence of infection was determined in northern Ghana. A cross-sectional study was conducted during which stool and urine samples were collected from children 6-15 years. Samples were analysed using the Kato-Katz technique and the 10 ml urine filtration methods respectively. Data on water contact activities were also collected. The level of infection was compared in relation to location and water contact activities. A total of 1,764 children participated in the study. Prevalence of Schistosoma haematobium infection was 18.9%. The highest level of infection (33.1%) was found among children resident in the southern part of the district, the lowest among those in the eastern (3.6%) and northern (3.8%) parts. S. mansoni infection was 10.9%. The highest level of S. mansoni infection (54.2%) was found among children resident in the central part of the district. The overall prevalence of infection (S. haematobium+S. mansoni) was moderate (27.1%). More males (32.5%) than females (20.2%) were infected (χ2=32.8, P<0.0001). Children aged 9-12 years had the highest prevalence of infection (31.8%; 95% CI: 28.4- 35.5) with the lowest among those aged 6-8 years (23.9%; 95% CI: 20.2-28.0). Swimming in the canals (χ2=404.4, P<0.0001) and working on tomato farms (χ2=37.7, P<0.0001) were risk factors for infection. Herding cattle appeared to have protected the children from infection (χ2=34.8, P=0.0001). Schistosomiasis is prevalent throughout the district with children resident in the central and southern parts of the district being more at risk of infection. There is the need to put in place an integrated and effective schistosomiasis control programme.
| Keywords | |
| Schistosomiasis; Kassena-Nankana district; Tono irrigation scheme; Water contact activities; School-age children; Herding cattle; Childhood activities | |
| Introduction | |
| Human schistosomiasis, also known as bilharzia, is a complex of acute and chronic parasitic infections caused by mammalian blood flukes belonging to the genus Schistosoma. The disease is endemic in 74 countries and considered second only to malaria in terms of the socioeconomic and public health importance in the tropics and sub-tropics. An estimated 200 million people are infected, of whom 120 million are symptomatic and 20 million have severe disease. Six hundred million people are at risk of infection [1]. | |
| There are two forms of the disease namely urogenital and intestinal schistosomiasis. Urogenital schistosomiasis is due to Schistosoma haematobium whilst the intestinal form is caused by S. mansoni, S. japonicum, S. mekongi, S. guineensis and S. intercalatum [2]. Depending on the species of parasite, schistosomiasis leads to renal and bladder damage or liver and intestinal disease and contributes to anemia, growth retardation and impaired cognitive development in children. The disease also contributes to malnutrition and disrupts school attendance. | |
| Humans become infected when the larval forms of the parasite (cercariae) – released by freshwater snails – penetrate the skin in the course of wading, bathing, swimming, washing clothes, or performing other household chores in contaminated freshwater. Bulinus globosus and B. truncatus are the intermediate host snails of S. haematobium [3] in Ghana whilst Biomphalaria pfeifferi is responsible for the transmission of S. mansoni [4,5]. | |
| Schistosoma infection is usually acquired in childhood as children tend to spend time swimming or bathing in snail infested freshwater. Prevalence and intensity of infection increase with age, peaking in the 5 to 14 year age group [6]. In older people, there is a drastic decline in intensity of infection but not in the prevalence of the disease. | |
| The present work was undertaken to assess the extent and distribution of schistosomiasis infection among school-age children and establish the association between some childhood activities and responsibilities including swimming, working on tomato farms, and herding cattle and the prevalence of infection in the Kassena- Nankana district of northern Ghana. The current paper, to the best of our knowledge is the first comprehensive parasitological report on schistosomiasis that has covered the entire district [5,7,8]. | |
| Materials and Methods | |
| Study site and population | |
| The study was conducted in the Kassena-Nankana District (KND) in northern Ghana. The district covers an area of about 1,674 km2 of Sahelian savannah with an estimated population of 144,000 [9]. The main occupation of the inhabitants is subsistence farming of millet, groundnut, rice, vegetables and livestock. The average annual rainfall is 850 mm, almost all of which occur in the months of June-October, with the rest of the year being relatively dry [10]. Most of the people live in multi-family compounds, which form the basis of the address system used in the Navrongo Health and Demographic Surveillance System (NHDSS), and are separated from one another by agricultural land. A large reservoir (Tono dam) in the middle of the district and about 90 dug-out dams provide water throughout the year for agricultural irrigation purposes [11]. | |
| Sample size estimation | |
| Two main assumptions were made during the sample size estimation. We expected a higher prevalence of schistosomiasis infection (about 80%) in non-enrolled school-age children than in the enrolled children (about 70%) [5] mainly in the irrigated area. At a confidence level of 95% and a power of 80%, a sample size of 412 each of enrolled and nonenrolled school-age children was deemed adequate to demonstrate any differences in prevalence among in-school and not-in-school children in the study area. We also assumed an overall prevalence of infection of 50% in the non-irrigated area. At a confidence level of 95% and power of 80%, a minimum sample of 434 was deemed adequate to detect any significant differences in infection in terms of location. To make room for non-response a sample size of 1,764 was considered adequate for any sub-analysis. | |
| Field procedures | |
| Stool and urine samples collection and haemoglobin measurement: Stool and urine samples were collected from children aged 6-15 years resident in the Kassena-Nankana district from March- June 2008. A simple random sampling procedure was employed in which representative sample lists of eligible participants resident in all zones of the district were generated from the NHDSS database. The eligibility criteria were: being aged 6-15 years and being resident in any of the five NHDSS designated zones in the district for at least one year. Samples were allotted proportional to the size of eligible children in the zones while making room for loss to follow-up in the generation of the random list. Participants were enrolled in a sequential manner during sample collection until the predetermined number for the particular zone was obtained. Children not in-school were traced into their homes early in the morning using compound identifiers and mother’s name. In-school children were contacted at school for sample collection and questionnaire administration. Stool sample containers were distributed to the potential study participants in their homes or at school a day before sample collection for them to provide samples the next morning. Urine sample containers were however provided on the day of sample collection. Samples were collected between the hours of 10:00 and 12:00 either at school or at home. Stool and urine samples were transported in ice chests with ice packs just after collection to the laboratory at Navrongo Health Research Centre for processing and examination. Blood haemoglobin concentration levels were determined from finger prick blood samples with an automated photometer (HemoCue AB, Sweden Instruments). | |
| Laboratory analyses | |
| The Kato-Katz technique [12] was used for the determination of prevalence of Schistosoma mansoni infection. Infection was determined using optic microscopy and counting the eggs present in 41.7 mg of faeces. One aliquot of 10 ml urine sample was filtered using a 10 ml syringe and nylon monofilament and the filter placed on a single slide labeled with the identification number of the child and date of collection. The slides were examined microscopically and the eggs count expressed as number per 10 ml of urine. All samples were processed within 24 hrs of collection. | |
| Questionnaire administration | |
| Data on water contact activities (bathing, washing of clothes and swimming in the canals or dams; working on irrigated farms) were collected using a questionnaire. Other activities like herding of cattle were also collected from the children. The questionnaire was also used to update their school enrolment status. The children were also questioned on whether they have ever seen blood in their stool and/ or urine. Their demographic data were accessed from the NHDSS database. The questionnaires were administered by trained field staffs who speak the local languages as well as English. All data forms were checked by field supervisors for completeness and thoroughness and any omissions corrected in the field before being brought to the office for batching and data entry into a computer database. | |
| Data analyses | |
| The overall mean and standard deviation of the intensity of infection was calculated. The intensity of infection with S. haematobium was classified into three groups: light (1-49), moderate (50-99) and heavy (≥ 100 eggs in 10 ml of urine). The intensity of infection with S. mansoni was expressed as eggs per gramme (epg) of faeces by multiplying the number of eggs in the 41.7 mg specimen by 24 [13]. The degree of intensity of S. mansoni was also classified into three groups: light (1- 99), moderate (100-399) and heavy (400 eggs and over per gram of faeces) [13]. The prevalence of infection was tabulated against type of childhood activity, being in-school, as well as area of residence and the chi-squared test obtained. All statistical tests were two sided and an alpha level of <0.05 was considered a statistically significant result. The 95% confidence intervals were also used where appropriate. | |
| Ethical considerations | |
| Ethical approval was obtained from the Institutional Review Board of the Navrongo Health Research Centre of the Ghana Health Service before commencement of the study (Ethics approval No: NHRCIRB049). Written informed consent was obtained from the parents of the children before participation in the study. Assent was also obtained from those children aged 10 to 15 years. Permission was obtained from the chiefs and elders of the communities in which the study was conducted. Community meetings were organized in all the communities and the study explained to them. The District Education Office was also contacted and permission sought. Children who were found to be infected were referred to the nearest health facility for treatment. They were treatment based on the recommended drug regimen of a single dose of praziquantel (40 mg/kg body weight). | |
| Results | |
| Socio-demographic, haematological and parasitological measurements | |
| A total of 1,764 school-age children (6-15 years) participated in the survey: 987 males (56.0%) and 777 females (44.0%). Overall, most of the children (74.4%) were in-school; however, in the southern and central parts of the district only 45-53% were in-school. The mean age was 10.8 years (SD: 2.9; range: 6-15) and the mean haemoglobin concentration was 11.3 g ⁄ dl (SD: 1.6; range 5.6- 16.0). Hb and age distribution were similar for children resident in all parts of the district, and no association was found between the intensity of infection and Hb concentrations (Table 1). | |
| The prevalence of Schistosoma haematobium infection was 18.9% (333 ⁄ 1764); with a geometric mean egg density of 7.1 eggs per 10 ml of urine. The intensity of infection was generally low throughout the district. The highest level of infection (33.1%) was found among children resident in the southern part of the district with the lowest among those resident in the eastern (3.6%) and northern (3.8%) parts of the district. Infection with S. mansoni was 10.9% (193/1764) with a geometric mean egg density of 69.6 eggs per gram (epg) of faeces. The highest level of S. mansoni infection (54.2%) was found among children resident in the central part of the district where the intensity of infection was also moderate. | |
| The overall prevalence of infection (S. haematobium + S. mansoni) was moderate (27.1%) (Table 2). More males (32.5%, 321/987) than females (20.2%, 157/777) were found to be infected (χ2=32.8, P<0.0001); but the geometric mean density of eggs were higher for females than males [S. haematobium: males=6.8, females 8.0 eggs per 10 ml of urine; S. mansoni: males=60, females=93.6 eggs per gram (epg) of faeces]. | |
| Children aged 9-12 years had the highest prevalence of infection (31.8%; 95% CI: 28.4-35.5) with the lowest level of infection among those aged 6-8 years (23.9%; 95% CI: 20.2-28.0). The difference in the levels of infection among children in the age group of 9-12 years and the other two age groups was statistically significant (prevalence of infection for age group 13-15 years=24.1%, 95% CI: 20.7-27.8) (Figure 1). | |
| Childhood activities and the associated risk of schistosomiasis infection | |
| Some childhood activities like swimming and washing of clothes in the irrigation canals or dams and assisting on tomato farms were major risk factors that exposed the children to schistosomiasis infection. A high percentage (59.5%) of the children indicated that they swim frequently in the canals and dams with as many as 70.5% (695/987) of the males swimming frequently. Significantly more children who swim frequently than those who do not were infected (χ2=404.4, P<0.0001). Children who assisted on tomato farms were also more likely to be infected than those who did not (χ2=37.7, P<0.0001). Other activities such as taking care of cattle in the fields appeared to have protected the children from infection (χ2=34.8, P=0.0001). Caring for or herding of cattle was a major childhood activity undertaken by male children (67.9%; 670/987) with 45.4% (353/777) of female children also engaged in this activity (Table 3). | |
| Area of residence, childhood responsibilities/activities and schistosomiasis infection | |
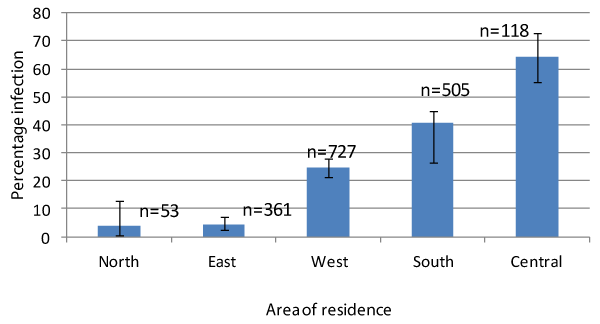
| Area of residence in the district was found to be very important in terms of exposure to schistosomiasis infection as shown in Figure 2. Children resident in the central part of the district recorded the highest level of infection of 64.4% (76/118), followed by those resident in the southern part (40.6%, 205/505). The northern part had the lowest prevalence of infection in the district (3.8%, 2/53). The level of infection among children resident in the central part was significantly higher than the levels among those resident in the other four parts of the district; similarly, the level of infection was much higher among children resident in the western part (24.6%) compared to those living in the eastern (4.4%) and northern parts (3.8%). | |
| The level of participation in the identified childhood activities/ responsibilities was also found to vary depending on which part of the district the child resides. Children resident in the central part of the district were found to engage more in bathing and washing in the canals and dams as well as working on rice and tomato farms than their counterparts residing in the other parts of the district. Working on tomato farms for example exposed the children to schistosomiasis infection (χ2=37.7, P<0.0001). In the case of children resident in the western part of the district, engaging in activities like bathing and washing in the canals and dams as well as working on rice and tomato farms exposed them to schistosomiasis infection (P<0.0001) (Table 4). | |
| Discussion | |
| A survey was conducted in the Kassena-Nankana district of Ghana to assess the extent and distribution of schistosomiasis infection among school-age children and establish the association between some childhood activities and the prevalence of infection. The study revealed that both urogenital and intestinal schistosomiasis infection were prevalent throughout the district. While the level of infection was found to be low (<10%) in the northern and eastern parts of the district, it was moderate (10-30%) in the western part, but high (>30%) in the southern and central parts of the district. The intensity of infection was generally low except for children resident in the central part of the district among whom the intensity of Schistosoma mansoni was found to be moderate. | |
| It was not surprising that schistosomiasis infection was prevalent throughout the district, with significant differences in the level of infection from one community to another as there are numerous freshwater bodies including ponds and dugouts distributed throughout the district. Such variation in the level of infection from one part of a district to another has been observed by [14] in Senegal. In the Senegal study, low prevalence of infection was observed in communities where the water bodies dried more rapidly. Most of the dugouts in the Kassena-Nankana district dry up during the dry season; the most probable reason for the low prevalence of infection in the northern and eastern parts of the district. In the central and southern parts of the district however, is located a more permanent water body, the Tono irrigation dam. The Tono irrigation scheme has a 4 km long dam and a canal system of about 42 km. This provides a favourable environment not only for the proliferation of the snail intermediate hosts of the disease but also a convenient recreational ground for children to swim and wash clothes [5,8]. | |
| The overall prevalence of infection (S. haematobium + S. mansoni) was found to be significantly higher among males than females a situation that has been observed in many other studies in schistosomiasis endemic communities [8,14-19]. The observation by Amuta and Houmsou [15] was explained in terms of higher tendency of water contact activities among males through swimming and washing of clothes. This could be partly explained by the fact that male children are usually more adventurous and therefore more likely to play in contaminated water bodies compared to female children. | |
| Some other studies however, could not establish any significant difference between the level of infection among male and female children [6,20]. In a study by Chipeta et al. [21] in Malawi, however, infection was higher in females than in males. Differences in the level of schistosomiasis infection between males and females have always been explained in terms of differences in water contact behaviours in relation to the frequency and duration of exposure to contaminated freshwater sources between the two sexes [6,16]. | |
| Our current study has also identified some childhood water contact activities and responsibilities like swimming, washing of clothes in the irrigation canals or dams and assisting on tomato and rice farms as major risk factors that expose children to schistosomiasis infection in the Kassena-Nankana district. A large proportion of the children indicated that they assist on tomato farms with many more of such children than those who do not (35.2% as against 21.8%) being infected. Most of the children also indicated that they swim frequently in the canals and dams, with over 70% of the males swimming frequently. Many more of the children who swim frequently in the canals, dams and dugouts were infected than those who do not (33.9% as against 17.2%). Our findings support a similar study by [20] in Ogun State, South-Western Nigeria, where contact with stream water was found to be associated with the prevalence of urinary schistosomiasis with over 80% of those infected having been exposed to contaminated stream water. | |
| It is important to note that some other childhood responsibilities like herding of cattle appeared to have protected the children in one way or the other from schistosomiasis infection. Caring for cattle in the field has the potential to keep children away from swimming in the canals and dams or reduce the duration of stay in the water bodies. Not much work has been done to show the relationship between herding animals in the field and schistosomiasis infection. In an earlier study by Wu et al. [22] in the lake region of China, however, herding animals was the second risk factor for schistosomiasis japonica infection after fishing. It is known that the aquatic habitats for the snail intermediate hosts for Schistosoma japonicum (Onchomelania spp) and Schistosoma mansoni and S. haematobium are not similar. Therefore, children herding cattle in China have a higher tendency to follow the animals into the marshy fields where the cattle are more likely to find fresh pasture thereby exposing the children to infection. | |
| Proximity to cercariae infested freshwater bodies has also been identified as an important risk factor in the prevalence of schistosomiasis infection [6,14]. Children resident in the central and southern parts of the district were found to be more infected than their counterparts resident in the northern and eastern parts, where contaminated water sources are more seasonal. Area of residence in the district was therefore found to be very important in terms of exposure to schistosomiasis infection. A similar study by Bogoch et al. [23] in the northern region of Ghana also identified working or playing near freshwater bodies as having a significant association with S. haematobium infection. Such association between proximity of home or school to schistosome infested water sources and infection has been reported from many other studies in schistosomiasis endemic communities including Ethiopia [6,24], Sudan [25], Côte d’Ivoire [26] and Yemen [16,27]. | |
| Several studies including Casmo et al. [28] and Deribew et al. [29] have establish an association between the intensity of Schistosoma egg count and Hb concentration in similar studies. The study by Deribew et al. in Ethiopia for example, found a significantly negative correlation between egg count/intensity and haemoglobin concentration. Our current study however, could not demonstration any association between infection intensity and Hb levels, possibly because the levels of egg intensity were low. | |
| The current study has also identified some differences in the level of infection among age groups. The level of infection was moderate (10- 30%) among the younger age (6-8 years) but increased from moderate to high (>30%) among the age group 9-12 years and decreased to a moderate level among the older children (13-15 years). Such variations in the prevalence and intensity of infection among various age groups have been reported in other studies and several explanations have been given for the differences detected. Notable among these explanations is the level of water contact [30]. We agree with Tukahebwa and colleagues assertion that exposure to contaminated water alone may not explain this age difference in infection and that this pattern could be explained by slow development of acquired immunity to schistosomiasis infection with age | |
| In conclusion, this study has revealed that schistosomiasis is prevalent throughout the district with significant variation in the level of infection among school-age children depending on where in the district one resides. The findings support the need to put in place an integrated and effective schistosomiasis control programme. This should include annual drug administration, health education and community mobilisation, provision of clean and safe water for domestic use, introduction of proper sanitation facilities in order to interrupt transmission and morbidity associated with schistosomiasis. | |
| Limitations of the Study | |
| The current level of schistosomiasis infection detected could be an underestimation of the actual prevalence of infection among schoolage children, as several studies have indicated that multiple stool and urine samples or slides are needed to improve the sensitivity of the Kato-Katz and 10 ml urine filtration methods respectively [31,32]. We could however not collect multiple samples or prepare multiple slides from the single samples collected mainly due to logistical reasons. In spite of this, our findings provide a broad picture of the distribution of schistosomiasis in the district that can guide evidence-based decision making. | |
| Acknowledgements | |
| We are grateful to the chiefs and people of the Kassena-Nankana district, the District Health Management Team and staff of the Navrongo Health Research Centre. We also acknowledge the contributions of school authorities, the children and their parents. Our thanks also go to Mr. Judas Bali, the senior technician who carried out quality control checks on the laboratory work. | |
References
- Chitsulo L, Engels D, Montresor A, Savioli L (2000) The global status of schistosomiasis and its control. Acta Trop 77: 41-51.
- World Health Organization, “Schistosomiasis, Fact Sheet No 115,” March 2013
- Paperna I (1963) Susceptibility of Bulinus (Physopsis) globosus and Bulinustruncatusrohlfsi from different localities in Ghana to different local strains of Schistosoma haematobium. Ann Trop Med Parasitol 13-26.
- Wen ST, Chu KY (1984) Preliminary schistosomiasis survey in the lower Volta River below Akosombo Dam, Ghana. Ann Trop Med Parasitol 78: 129-133.
- Amankwa JA, Bloch P, Meyer-Lassen J, Olsen A, Christensen NO (1994) Urinary and intestinal schistosomiasis in the Tono Irrigation Scheme, Kassena/Nankana District, upper east region, Ghana. Trop Med Parasitol 45: 319-323.
- Alebie G, Erko B, Aemero M, Petros B (2014) Epidemiological study on Schistosoma mansoni infection in Sanja area, Amhara region, Ethiopia. Parasit Vectors 7: 15.
- Hunter JM (2003) Inherited burden of disease: agricultural dams and the persistence of bloody urine (Schistosomiasishematobium) in the Upper East Region of Ghana, 1959-1997. SocSci Med 56: 219-234.
- Anto F, Asoala V, Adjuik M, Anyorigiya T, Oduro A, et al. (2013) Water Contact Activities and Prevalence of Schistosomiasis Infection among School-age Children in Communities along an Irrigation Scheme in Rural Northern Ghana. J BacteriolParasitol 4: 177.
- Navrongo Health and Demographic Surveillance System annual report, 2008
- Koram KA, Owusu-Agyei S, Fryauff DJ, Anto F, Atuguba F, et al. (2003) Seasonal profiles of malaria infection, anaemia, and bednet use among age groups and communities in northern Ghana. Trop Med Int Health 8: 793-802.
- Anto F, Asoala V, Anyorigiya T, Oduro A, Adjuik M, et al. (2009) Insecticide resistance profiles for malaria vectors in the Kassena-Nankana district of Ghana. Malar J 8: 81.
- WHO. Basic Laboratory Methods in Medical Parasitology(1991) Organization World Health Organization, Geneva.
- Montresor A, Crompton DW, Hall A, Bundy DA, Savioli L (1998)Guidelines for the Evaluation of Soil-Transmitted Helminthiasis and Schistosomiasis at Community Level: a guide for mangers of control programmes. Geneva: World Health Organization.
- Senghor B, Diallo A, Sylla SN, Doucouré S, Ndiath MO, et al. (2014) Prevalence and intensity of urinary schistosomiasis among school children in the district of Niakhar, region of Fatick, Senegal. Parasit Vectors 7: 5.
- Amuta EU, Houmsou RS (2014) Prevalence, intensity of infection and risk factors of urinary schistosomiasis in pre-school and school aged children in Guma Local Government Area, Nigeria. Asian Pac J Trop Med 7: 34-39.
- Al-Waleedi AA, El-Nimr NA, Hasab AA, Bassiouny HK, Al-Shibani LA (2013) Urinary schistosomiasis among schoolchildren in Yemen: prevalence, risk factors, and the effect of a chemotherapeutic intervention. J Egypt Public Health Assoc 88:130-136.
- Knopp S, Person B, Ame SM, Mohammed KA, Ali SM, et al. (2013) Elimination of schistosomiasis transmission in Zanzibar: baseline findings before the onset of a randomized intervention trial. PLoSNegl Trop Dis 7: e2474.
- Ogbonna CC, Dori GU, Nweze EI, Muoneke G, Nwankwo IE et al. (2012) Comparative analysis of urinary schistosomiasis among primary school children and rural farmers in Obollo-Eke, Enugu State, Nigeria: implications for control. Asian Pac J Trop Med 5: 796-802.
- Ahmed AM, El Tash LA, Mohamed EY, Adam I (2012) High levels of Schistosoma mansoni infections among schoolchildren in central Sudan one year after treatment with praziquantel. J Helminthol 86: 228-232.
- Morenikej OA, Idowu BA (2011) Studies on the prevalence of urinary schistosomiasis in Ogun State, South-Western Nigeria. West Afr J Med 30: 62-65.
- Chipeta MG, Ngwira B, Kazembe LN (2013) Analysis of Schistosomiasis haematobium infection prevalence and intensity in Chikhwawa, Malawi: an application of a two part model. PLoSNegl Trop Dis 7: e2131.
- Wu Z, Bu K, Yuan L, Yang G, Zhu J, et al. (1993) Factors contributing to reinfection with schistosomiasis japonica after treatment in the lake region of China. Acta Trop 54: 83-88.
- Bogoch II, Andrews JR, Dadzie RKE, Ju¨rgUtzinger J (2012) Simple questionnaire and urine reagent strips compared to microscopy for the diagnosis of Schistosoma haematobium in a community in northern Ghana Tropical Medicine and International Health
- Mengistu M, Shimelis T, Torben W, Terefe A, Kassa T, et al. (2011) Human intestinal schistosomiasis in communities living near three rivers of jimma town, South Western ethiopia. Ethiop J Health Sci 21: 111-118.
- Abou-Zeid AH, Abkar TA, Mohamed RO (2013) Schistosomiasis infection among primary school students in a war zone, Southern Kordofan State, Sudan: a cross-sectional study. BMC Public Health 13:643.
- Matthys B, Tschannen AB, Tian-Bi NT, Comoé H, Diabaté S, et al. (2007) Risk factors for Schistosoma mansoni and hookworm in urban farming communities in western Côte d'Ivoire. Trop Med Int Health 12: 709-723.
- Sady H, Al-Mekhlafi HM, Mahdy MA, Lim YA, Mahmud R, et al. (2013) Prevalence and associated factors of Schistosomiasis among children in Yemen: implications for an effective control programme. PLoSNegl Trop Dis 7: e2377.
- Casmo V, Augusto G, Nala R, Sabonete A, Carvalho-Costa FA (2014) The effect of hookworm infection and urinary schistosomiasis on blood hemoglobin concentration of schoolchildren living in northern mozambique. Rev Inst Med Trop Sao Paulo 56: 219-224.
- Deribew K, Tekeste Z, Petros B, Huat LB (2013) Urinary schistosomiasis and malaria associated anemia in Ethiopia. Asian Pac J Trop Biomed 3: 307-310.
- Tukahebwa EM, Magnussen P, Madsen H, Kabatereine NB, Nuwaha F, et al. (2013) A very high infection intensity of Schistosoma mansoni in a Ugandan Lake Victoria Fishing Community is required for association with highly prevalent organ related morbidity. PLoSNegl Trop Dis 7:e2268.
- Ebrahim A, El-Morshedy H, Omer E, El-Daly S, Barakat R (1997) Evaluation of the Kato-Katz thick smear and formol ether sedimentation techniques for quantitative diagnosis of Schistosoma mansoni infection. Am J Trop Med Hyg 57: 706-708.
- Kosinski KC, Bosompem KM, Stadecker MJ, Wagner AD, Plummer J, et al. (2011) Diagnostic accuracy of urine filtration and dipstick tests for Schistosoma haematobium infection in a lightly infected population of Ghanaian schoolchildren. Acta Trop 118: 123-127.
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 | Table 4 |
Figures at a glance
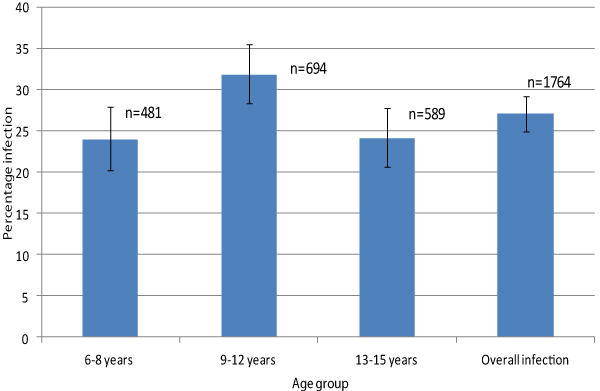 |
 |
|
| Figure 1 | Figure 2 |
Relevant Topics
- Advanced Therapies
- Chicken Pox
- Ciprofloxacin
- Colon Infection
- Conjunctivitis
- Herpes Virus
- HIV and AIDS Research
- Human Papilloma Virus
- Infection
- Infection in Blood
- Infections Prevention
- Infectious Diseases in Children
- Influenza
- Liver Diseases
- Respiratory Tract Infections
- T Cell Lymphomatic Virus
- Treatment for Infectious Diseases
- Viral Encephalitis
- Yeast Infection
Recommended Journals
Article Tools
Article Usage
- Total views: 17321
- [From(publication date):
August-2014 - Jul 01, 2025] - Breakdown by view type
- HTML page views : 12405
- PDF downloads : 4916
