Chelation and Gadolinium: How Effective is it?
Received: 10-Jan-2019 / Accepted Date: 25-Jan-2019 / Published Date: 01-Feb-2019 DOI: 10.4172/2476-2024.1000151
Abstract
Gadolinium-Based Contrast Agents (GBCA) are intravenous drugs used in diagnostic imaging procedures to enhance the quality of magnetic resonance imaging (MRI) or magnetic resonance angiography (MRA). Recent FDA alerts concerning potential side effects increased patient and medical concerns. We checked if gadolinium remains in the body system longer than pharmaceutical information states and since a growing number of chelation therapists uses chelating agents to remove gadolinium that may have been stored in the body, we checked how effective chelation agents are. Our studies indicate that in the case of gadolinium, chelation does not seem to be an option.
Keywords: Gadolinium; Gadolinium-based contrast agents; GBCA; Chelation; DMPS; DTPA; EDTA
Introduction
According to the FDA, “Gadolinium-Based Contrast Agents (GBCA) are intravenous drugs used in diagnostic imaging procedures to enhance the quality of magnetic resonance imaging (MRI) or magnetic resonance angiography (MRA).” These contrasting agents have long been considered a harmless alternative to X-rays as tumors and inflammation are detected without radiation. Now, the FDA is warning doctors and issued a statement on July 27, 2017 concerning data “evaluating the risk of brain deposits with repeated use of gadolinium-based contrast agents.” Apparently, all GBCAs are associated with higher retention of gadolinium (Gd) in the brain and other body tissues and are considered harmful. Thus, a rising number of medical doctors are using chelation in an effort to reduce the body’s Gd-burden.
Chemical structure and use of GBCA
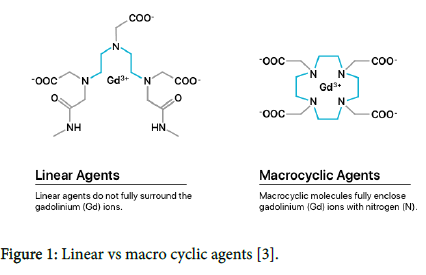
According to their chemical structure, the Gd-containing contrast agents are subdivided into ionic and nonionic, macro cyclic and linear contrast agents (Figure 1). The cyclic structure creates a strong bond to gadolinium. In contrast, the linear contrast agents are so-called Gd chelates with open, mobile chains that have no strong binding to the toxic Gd 3+ ion [1,2]. Frequently used and FDA-approved GBCAs are listed in Table 1.
Figure 1: Linear vs macro cyclic agents [3].
| Brand name | Generic name | Structure |
|---|---|---|
| Ablavar | Gadofosveset Trisodium | Linear |
| Dotarem | Gadoterate Meglumine | Macro cyclic |
| Eovist | Gadoxetate Disodium | Linear |
| Gadavist | Gadobutrol | Macro cyclic |
| Magnevist | Gadopentetate Dimeglumine | Linear |
| MultiHance | Gadobenate Dimeglumine | Linear |
| Omniscan | Gadodiamide | Linear |
| OptiMARK | Gadoversetamide | Linear |
| ProHance | Gadoteridol | Macro cyclic |
Table 1: FDA-approved GBCAS [4].
According to information from the European Medicines Agency and the Federal Institute for Drugs and Medical Devices dated January 2018, the long-term risks of gadolinium contrast agent administration are still unknown. In the EU the suspension of approvals for intravenous linear gadolinium-containing contrast media has been suspended with the exception of the gadoxetic and gadobenic acid drugs. The German arrangement applies since February 2018, and in March 2018, the withdrawal of certain gadolinium-containing contrast agents for magnetic resonance imaging (MRI) was recommended. There are currently no restrictions in the US, according to the Drug Safety Communication. Janet Woodcock, M.D. and director of the FDA’s Center for Drug Evaluation and Research stated, "The FDA will continue to assess the safety of GBCAs, and to that end, we are requiring GBCA manufacturers to conduct further studies to assess the safety of this class of contrast agents" (FDA 3/16/2018).
Gadolinium side effects and toxicity
While free gadolinium is considered highly toxic, GBCAs have been listed as nontoxic. However, in 2006, gadolinium-containing contrast agents were first mentioned as a cause of Nephrogenic Systemic Fibrosis [5,6]. Nephrogenic Systemic Fibrosis (NSF) is a potentially fatal disease that causes hardening and thickening of the skin and internal organs. In patients with advanced renal insufficiency, NSF symptoms were seen within days to months after administration (Nephro-News, issue 1/08). Among other health effects that have been reported after GBCA administration are nausea, headaches, dizziness, brain fog, and pain in the skin, bones or joints. The severity of symptoms seems to vary widely [7].
Gadolinium and chelation
In medicine, chelation has been used to treat metal poisoning and chronic metal overexposure. It is a chemical process by which a chemical chelating agent is used to bind metal ions, forming metal chelates that are then eliminated by the body. Thus, the use of chelating agents has been introduced to bind gadolinium that has been stored in the human body. By some, it is considered a promising therapy for patients who have received GBCA. GBCA are gadolinium complexes, and the molecular structure of each GBCA determines its stability. For Gadopentetat Diglumin, Gd is complexed with DTPA (Table 2). This strong molecular complex is considered nontoxic. GdDTPA or Gadopentetate Dimeglumin is a stronger complex than the chelating agents ZnDTPA, CaDTPA or CaEDTA, all of which are presently used to ‘chelate’ gadolinium i.e. break the GdDTPA complex. If gadolinium would have been freed from its DTPA bond, it would then be available as free gadolinium, which is toxic.
| Chemical Name | Chemical Formula | |
|---|---|---|
| GdDTPA | Gadopentetat Dimeglumin | C28H54GdN5O20 |
| ZnDTPA | Zink-Trinatrium-pentetat | Na3ZnC14H18N3O10 |
| CaDTPA | Calcium-Trinatrium-pentetat | C14H18CaN3Na3O10 |
| CaEDTA | EDTA Mono Calcium | C10H14CaN2O8 |
Table 2: Chemical Comparison of GBCA and Chelating Agents.
Gadolinium in urine
In our laboratory, we observed over time elevated gadolinium levels in urine before chelation. We also noticed varying gadolinium test values in patients who had received GBCAs prior to MRI or MRA. In some patients, the gadolinium contact had been months ago, yet the gadolinium concentration in urine (before chelation) remained high. According to product information, patients with healthy kidney function excrete GBCAs within a short time. Gadodiamide, for example, is a linear and thus less stable Gd chelate. One ml of gadodiamide contains 287 mg of the Gd complex. According to the information provided by the Swiss pharmaceutical manufacturer GE HEALTHCARE, "the recommended dose is usually 0.1 mmol/kg bw (equivalent to 0.2 ml/kg bw) for patients with a body weight up to100 kg. If the body weight is more than 100 kg, the administration of 20 ml remains sufficient to obtain a desired contrast for the diagnosis". With the administration of 20 ml Gadodiamide 5740 g of the GBCA is injected. Considering the half-life of Gadodiamide, 20 ml of this contrast agent should be eliminated without the help of a chelating agent as shown in Table 3. Theoretically, 32.5 hours after injection, only 0.2 μg gadodiamide should be detectable in the body. After 3 days, Gadodiamide should no longer be detectable in the urine. A 20 ml ampule of Gadodiamide contains 5740 g of this contact agent. This corresponds to 78.67 mg of elemental gadolinium per ml or 1573 mg Gd/20 ml. At present, our ICP-MS has a detection limit for Gadolinium in urine of 0.05 μg/L. It is assumed that 3 days after the Gadodiamide has been administered, the element Gadolinium can no longer be detected.
| Hours after iv-injection | µg contrast agent eliminated |
|---|---|
| 0.0 | 5740000.0 |
| 1.3 | 2870000.0 |
| 2.6 | 1435000.0 |
| 3.9 | 717500.0 |
| 5.2 | 358750.0 |
| 6.5 | 179375.0 |
| 7.8 | 89687.5 |
| 9.1 | 44843.8 |
| 10.4 | 22421.9 |
| 11.7 | 11210.9 |
| 13.0 | 5605.5 |
| 14.3 | 2802.7 |
| 15.6 | 1401.4 |
| 16.9 | 700.7 |
| 18.2 | 350.3 |
| 19.5 | 175.2 |
| 20.8 | 87.6 |
| 22.1 | 43.8 |
| 23.4 | 21.9 |
| 24.7 | 10.9 |
| 26.0 | 5.5 |
| 27.3 | 2.7 |
| 28.6 | 1.4 |
| 29.9 | 0.7 |
| 31.2 | 0.3 |
| 32.5 | 0.2 |
Table 3: Theoretical elimination after 20 ml omniscan (gadodiamide).
Gadolinium in baseline urine
Data from 550 randomized baseline urinary specimens, taken before chelation, showed a mean Gd-value of 5.76 μg/L with a standard deviation of 128 μg/L. The maximum value was 2990 μg/L. No information was given regarding the time the GBCA was administered (Source: Micro Trace Minerals Laboratory 2006). Further statistical surveillances were carried out in 2011 and 2017, indicating similar mean Gd values and equally high standard deviation. In 2018, statistical monitoring of more than 12,000 baseline urines showed a mean below the detection limit of 0.05 μg/L with a standard deviation of 2605 μg/L. This indicates that extreme urine Gd concentrations were part of this sample contingent. From that large sample contingent, we randomly selected 80 urine samples that showed a test value of more than 100 μgGd/l. Of those, 11 showed a Gd value >1000 μg/L. The maximum gadolinium value of this sample quota was 290,000 μg/L (two hundred and ninety thousand), corresponding to 290 mg/L. We suspect that this may have been a sample that was taken shortly after the GBCA had been administered. No information had been supplied by the submitting clinic. All of the extreme test values came from urine samples that had been collected before chelation. Thus, the extremely high levels of Gd in baseline urine can be attributed to the body's own excretory mechanism.
Gadolinium in urine before and after chelation
In 2018, we evaluated data from our database of 2007 to mid-2018. We compared baseline values with urine samples collected after chelation. We selected sample pairs i.e. urine collected before and after chelation, all taken by experienced medical chelation therapist. For each sample pair, the pre-chelation and post chelation urine was collected on the same day. We compared baseline urine values with those obtained after chelation with intravenous DMPS ((RS)-2,3-Bis (sulfanyl)propane-1-sulfonic acid). We also compared baseline values with those from combination therapies involving the intravenous administration of DMPS plus CaEDTA (calcium ethylenediamine tetra-acetic acid), and DMPS plus ZnDTPA. The reason: at present, some environmental physicians assume that the contemporaneous administration of two powerful, yet differently acting chelators could be more effective in metal binding and hence metal elimination. In the case of Gadolinium, this does not seem to be the case. We also checked pre and post urines that involved chelation with oral DMSA (Dimercaptosuccinic acid). Table 4 indicates that DMPS does not show the ability to bind gadolinium, a fact that had been previously pointed out by Dr. Johann Ruprecht of Heyl, Berlin, the manufacturer of DMPS (brand name Dimaval®). Of the 25 sample pairs, consisting of pre- and post-chelation urine, none showed a higher gadolinium concentration after chelation.
| Urine Test Value before Chelation Values in mcg/g Creatinine | Urine concentration after DMPS iv, 250 mg, Values in mcg/g Creatinine | Chelation Assessment |
|---|---|---|
| 3096 | 2340 | No success |
| 563 | 536 | dto |
| 525 | 507 | dto |
| 766 | 574 | dto |
| 3703 | 2186 | dto |
| 238 | 63 | dto |
| 11 | 10 | dto |
| 97 | 97 | dto |
| 91 | 65 | dto |
| 40 | 35 | dto |
| 112 | 76 | dto |
| 230 | 138 | dto |
| 31 | 32 | dto |
| 74 | 52 | dto |
| 21 | 20 | dto |
| 189 | 178 | dto |
| 21 | 21 | dto |
| 109 | 101 | dto |
| 77 | 60 | dto |
| 15 | 13 | dto |
| 494 | 449 | dto |
| 383 | 318 | dto |
| 63 | 29 | dto |
| 11 | 10 | dto |
| 97 | 97 | dto |
Table 4: Gadolinium in urine before and after chelation with dmps iv, 250 mg (1 ampule).
Since the majority of physicians using the combination treatment DMPS+CaEDTA or DMPS+ZnDTPA, do not collect baseline urines for comparison, we could select few pairs of pre and post chelation samples. For the combination treatment DMPS and CaEDTA, we located only six pairs. For the combination treatment DMPS and ZnDTPA, only two pairs were found. None showed the ability to bind gadolinium (Table 5). We also checked if oral DMSA would have the ability to bind gadolinium. Of the 34 pairs, 24 of the unchallenged urine samples showed higher Gd concentrations than the samples after chelation. In ten pairs, the post chelation urine showed near equal gadolinium concentrations, which are due to mathematical conversion factors.
| Urine Test Value before Chelation in mcg/g Creatinine | Urine concentration after Chelation in mcg/g Creatinine | Chelating Agent DMPS iv, 250 mg + CaEDTA, 1.9 g iv Assessment |
|---|---|---|
| 189 | 178 | No success |
| 1424 | 1284 | dto |
| 46 | 29 | dto |
| 586 | 281 | dto |
| 1865 | 1788 | dto |
| 189 | 178 | dto |
| Urine Test Value before Chelation in mcg/g Creatinine | Urine concentration after Chelation in mcg/g Creatinine | Chelating Agents DMPS+ZnDTPA, 1 Amp each ivAssessment |
| 696 | 512 | No success |
| 8 | 5 | dto |
| Urine Test Value before Chelation inmcg/g Creatinine | Urine concentration after Chelation in mcg/g Creatinine | Chelating Agents DMSA oral Assessment |
| 696 | 550 | No success |
| 735 | 552 | Dto |
| 768 | 610 | Dto |
| 37 | 11 | Dto |
| 9 | 5 | Dto |
| 20 | 21 | See note |
| 24 | 19 | Dto |
| 6 | 6 | Dto |
| 13 | 8 | Dto |
| 293 | 187 | Dto |
| 40 | 30 | Dto |
| 108 | 80 | Dto |
| 9 | 9 | Dto |
| 29 | 30 | Dto |
| 195 | 174 | Dto |
| 696 | 551 | Dto |
| 303 | 317 | See note |
| 104 | 87 | Dto |
| 93 | 97 | See note |
| 9 | 7 | Dto |
| 344 | 328 | Dto |
| 23 | 17 | Dto |
| 9 | 10 | See note |
| 9 | 10 | See note |
| 16 | 17 | See note |
| 11 | 12 | See note |
| 10 | 7 | Dto |
| 9 | 10 | See note |
| 189 | 217 | See note |
| 13 | 8 | Dto |
| 8 | 6 | Dto |
| 21 | 17 | Dto |
| 18 | 11 | Dto |
| 7 | 8 | See note |
Table 5: Gd in urine before and after chelation with DMPS+CaEDTA. Note: Urine creatinine levels are used to mathematically convert mcg/l values to mcg/g creatinine. This conversion is commonly done, because it reduces the potentially great margin of error which results from an incorrect sample volume given. A low urine creatinine level of 0.3 g/l or less affects the mathematical conversion factor and thus elevates test results. Low urine creatinine levels are generally the result of over hydration.
It should be noted that the urine collection time for oral DMSA is around 4 hours, during with time the patient may drink more fluid than required for that period, resulting in a drop of urine creatinine levels. For the intravenous administration of DMPS, EDTA and ZnDTPA shorter urine collection times are used. Tables 4 and 5 clearly indicate that the diagnostic assessment of Gd in urine necessitates a comparison with a urine sample taken before chelation or else the Gd concentration of the post urine sample leads to a misinterpretation of results i.e. might relate chelation therapy success when in fact the elimination of Gd is due to the body’s own excretion ability.
Conclusion
From our data, we can conclude that GBCAs remain in the body longer than pharmaceutically claimed. We have demonstrated that gadolinium is eliminated without the use of chelating agents. Our data also suggests that chelation does not seem a treatment of choice for the elimination of gadolinium. We recommend that for a definite conclusion, more data is warranted. It should include the type of GBCA used and a time table when it was used.
Competing Interests
The authors declare no competing interests.
Consent for Publication
Not applicable.
References
- Hemsen J (2012) Einfluss der MR-Kontrastmittel MultiHance, Omniscan und Teslascan auf humane embryonale Lungenfibroblasten und humane Nabelschnurenendothelzellen. Dissertation zur Erlangung des Doktorgrades der Medizin. Med. Fakultät Erlangen 1: 1-97.
- Marckmann P, Skov L (2006) Nephrogenic systemic fibrosis: suspected causative role of gadodiamide used for contrast-enhancing magnetic resonance imaging. J Am Soc Nephrol 17: 2359-2362.
- Rogosnitzky M, Branch S (2016) Gadolinium-based contrast agent toxicity: a review of known and proposed mechanisms. Biometals 29: 365-376.
- FDA Bulletin (2007) FDA Drug Safety Communication: FDA identifies no harmful effects to date with brain retention of gadolinium-based contrast agents for MRIs; review to continue. U S Food and Drug Administration.
- Agarwal R, Brunelli SM, Williams K, Mitchell MD, Feldman HI, et al. (2009) Gadolinium-based contrast agents and nephrogenic systemic fibrosis: a systematic review and meta-analysis. Nephrol Dial Transplant 24: 856-863.
- Grobner T (2006) Gadolinium – a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant 21: 1104-1108.
Citation: Blaurock-Busch E (2019) Chelation and Gadolinium: How Effective is it? Diagn Pathol Open 4: 151. DOI: 10.4172/2476-2024.1000151
Copyright: © 2019 Blaurock-Busch E. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 12208
- [From(publication date): 0-2019 - Jan 30, 2026]
- Breakdown by view type
- HTML page views: 11023
- PDF downloads: 1185