Research Article Open Access
Characterization of Progesterone Receptor Membrane Component 1 in Peripheral Blood Mononuclear Cells and Plasma during Pregnancy
Liping Feng1*, Desravines1, Matthew K Nazzal1, Katherine P Pryor1, Erik J Soderblom2 and Amy P Murtha11Department of Obstetrics and Gynecology, Duke University, Durham, NC, USA
2Proteomics and Metabolomics Core Facility, Duke Center for Genomic and Computational Biology, Duke University, Durham, NC, USA
- *Corresponding Author:
- Liping Feng
Department of Obstetrics and Gynecology
Division of Maternal Fetal Medicine
Duke University Medical Center
Durham, NC, USA
Tel: 919-613-1459
Fax: 919-681-9938
E-mail: feng0007@mc.duke.edu
Received date: June 27, 2016; Accepted date: July 19, 2016; Published date: July 23, 2016
Citation: Feng L, Desravines, Nazzal MK, Pryor KP, Soderblom EJ, et al. (2016) Characterization of Progesterone Receptor Membrane Component 1 in Peripheral Blood Mononuclear Cells and Plasma during Pregnancy. Adv Mol Diag 1:109.
Copyright: © 2016 Feng L, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited
Visit for more related articles at Advances in Molecular Diagnostics
Abstract
Objective: Progesterone receptor membrane component 1 (PGRMC1) is a novel membrane progesterone receptor that is highly expressed and actively regulated in gestational tissues. Research suggests that PGRMC1 plays a role in mediating progesterone function in gestational tissues and maintaining pregnancy. It has also been reported that PGRMC1 is detectable in the plasma and peripheral blood mononuclear cells (PBMCs) in patients with cancer and may serve as a biomarker. Therefore, our objective is to identify whether PGRMC1 is present in plasma and PBMCs during pregnancy and thus may be investigated as a candidate biomarker for obstetric pathology including preterm birth. Study design: Whole blood samples were collected from pregnant patients. Plasma and PBMCs were isolated. Western blot and immunoprecipitation- mass spectrometry (IP-MS) were used to investigate the presence of PGRMC1 in plasma. Flow cytometry and Western blot were used to detect PGRMC1 expression in PBMCs collected from pregnant subjects. Results: Flow cytometry and Western blot results confirmed that PGRMC1 is differentially expressed in different cell types of PBMCs. However, Western blot and IP-MS results demonstrated that PGRMC1 is undetectable in plasma samples from pregnant women. Conclusions: This pilot study characterized the presence of PGRMC1 in the bloodstream during pregnancy. PGRMC1 is differentially expressed in different cell types of PBMCs of pregnant subjects but is not detectable in the plasma of these subjects. These data provide a foundation for future evaluation of PGRMC1 in PBMCs as a potential novel biomarker for PTB.
Keywords
PBMCs; PGRMC1; Plasma; Preterm birth
Introduction
Preterm birth (PTB) remains a major public health problem worldwide [1]. PTB accounts for more than 13,000 perinatal deaths per year with an additional 30,000 infants surviving with life-long morbidities [1,2]. Development of biomarkers for identification of women at high risk for PTB has had limited success but is urgently needed.
Progesterone plays a key role in the maintenance of normal pregnancy. Clinically, 17 alpha-hydroxyprogesterone caproate reduces the risk of recurrent preterm delivery, and vaginal progesterone (P4) when used for a short cervix reduces the risk of preterm delivery [3,4]. The physiologic effects of progesterone are mediated through nuclear progesterone receptors (PGRs). However, recent studies have demonstrated that progesterone may also act independently of PGRs through non-classic/non-genomic pathways. These pathways are mediated in part by a novel progesterone receptor, progesterone receptor membrane component 1 (PGRMC1) [5].
PGRMC1 is highly expressed and actively regulated in gestational tissues [5-8]. Studies from our group and others suggest roles of PGRMC1 in mediating progesterone function in gestational tissues [5-9] and in maintaining pregnancy. It has been reported that PGRMC1 is detectable in plasma [10] and peripheral blood mononuclear cells (PBMCs) [11]. Reduced PGRMC1 levels in PBMCs in women are associated with polycystic ovarian syndrome [11] and premature ovarian failure [11,12]. Recent evidence suggests that PGRMC1 is a potential tumor and serum biomarker for human lung cancer [10]. Another study suggests that PGRMC1 is a candidate biomarker for estrogen receptor status and hypoxia in breast cancer [13]. However, PGRMC1 expression in blood during pregnancy has not been characterized. Therefore, it is imperative to evaluate the presence of PGRMC1 in the blood of pregnant women.
We hypothesize that PGRMC1 can be non-invasively detected in the bloodstream of pregnant women as a potential biomarker of PTB. The objective of this study is to investigate the presence of PGRMC1 in plasma and PBMCs during pregnancy.
Materials and Methods
This project was approved by the Duke University Institutional Review Board. Women delivering at Duke University Medical Center who completed written consent forms were prospectively enrolled in this study.
Sample collection
Healthy pregnant women were enrolled in the first trimester. At least one blood sample was collected during each trimester from each subject. Whole blood samples were collected in Monovette EDTA tubes (BD Vacutainer, Franklin Lakes, NJ) with plasma isolated by centrifugation. PBMCs were isolated by density gradient centrifugation using Ficoll (GE Healthcare Life Sciences, Pittsburgh, PA) according to the manufacturer’s instructions. PBMCs were cryopreserved in cryopreservation solution (CPS) consisting of 90% fetal bovine serum (FBS) and 10% dimethyl sulfoxide (DMSO) and stored in liquid nitrogen for subsequent flow cytometry or Western blotting.
Immunoprecipitation (IP) using HTR8/SVneo cells
PGRMC1 is known to be highly expressed in a first-trimester human placenta cytotrophoblast cell line, HTR8/SVneo (a gift from Dr. C. H. Graham, Queen’s University, Kingston, ON) [9]. Antibodies were first validated using this cell line. Cell lysates were harvested using cell lysis buffer (Cell Signaling Technology, Beverly, MA). Primary rabbit anti-human PGRMC1 monoclonal antibody (Cell Signaling Technology, Beverly, MA) was mixed with cell lysate and gently rocked overnight at 4°C. Protein A/G agarose beads (Pierce) were then added to the mixture and gently rocked for 3 hours at 4°C. The beads were pelleted by centrifuge and washed with cell lysis buffer. The bead pellet was resuspended with sodium dodecyl sulfate (SDS) sample buffer (Invitrogen, Carlsbad, CA) and heated at 70°C for 10 minutes. Samples were then ready for Western blot analysis.
IP for plasma
Twenty μg of primary rabbit anti-human PGRMC1 monoclonal antibody (Cell Signaling Technology, Beverly, MA) were added to 30 μl Protein A/G beads (Pierce) in 1ml PBS and incubated with rocking overnight at 4°C. Rabbit IgG was used in parallel as negative control. Agarose beads coupled with antibodies were washed three times with 0.2 M sodium borate (pH 9.0). One mL of freshly made 20 mM DMP solution was immediately added to the coupled agarose beads. The mixture was rocked at room temperature for 40 minutes, washed once with 0.2 M ethanolamine (pH 8.0), then rocked in 1 mL of 0.2 M ethanolamine (pH 8.0) at room temperature for one hour. The beads were then washed three times with 1 mL 0.58% v/v acetic acid +150 mM NaCl followed by three washes in cold PBS.
After plasma samples were cleared by Separopore blue CL-6B slurry, 1 mL of supernatant was added to 25 μL of uncoupled A/G beads and rocked for 30 minutes to further purify the plasma samples. One ml of supernatant was then added to 25 μL of coupled beads. The mixture was rocked overnight at 4°C. The beads were washed three times with buffer containing 150 mM NaCl, 50 mM Tris, 10 mM EGTA, and 0.2% NP40, then eluted with 50 μl 2 LDS loading buffer (Invitrogen) and boiled for 10 minutes at 70°C. The beads were pelleted by centrifugation and the supernatant analyzed by Western blot and mass spectrometry.
Liquid chromatography and tandem mass spectrometry analysis (LC-MS/MS)
IP samples in LDS loading buffer were submitted to the Duke Proteomics and Metabolomics Core Facility. Samples were reduced with 10mM DTT at 70°C for 10 minutes prior to SDS-PAGE separation on a 4-12% bis-tris acrylamide gel (NuPAGE, Invitrogen) with colloidal coomassie staining. Bands corresponding to approximately 25 kDa were excised and subjected to standardized ingel trypsin digestion. Extracted peptides were lyophilized to dryness and re-suspended in 20 μL of 0.2% formic acid/2% acetonitrile.
Each sample was subjected to chromatographic separation on a Waters NanoAquity UPLC equipped with a 1.7 μm BEH130 C18 75 μm I.D. 250 mm reversed-phase column. The mobile phase consisted of (A) 0.1% formic acid in water and (B) 0.1% formic acid in acetonitrile. Following a 2 μL injection, peptides were trapped for 3 minutes on a 5 μm Symmetry C18 180 μm I.D. 20 mm column at 5 μl/ minute in 99.9% A. The analytical column was then switched in-line, and a linear elution gradient of 5% B to 40% B was performed over 60 minutes at 400 nL/minute. The analytical column was connected to a fused silica PicoTip emitter (New Objective, Cambridge, MA) with a 10 μm tip orifice and coupled to a Waters Synapt G2 QToF mass spectrometer through an electrospray interface operating in a datadependent mode of acquisition. The instrument was set to acquire a precursor MS scan from m/z 50-2000 with MS/MS spectra acquired for the three most abundant precursor ions. For all experiments, charge dependent CID energy settings were employed, and a 120 s dynamic exclusion was employed for previously fragmented precursor ions.
Raw LC-MS/MS data files were processed in Mascot distiller (Matrix Science) and then submitted to independent Mascot searches (Matrix Science) against a SwissProt database (Human taxonomy) containing both forward and reverse entries of each protein (20,322 forward entries). Search tolerances were 10 ppm for precursor ions and 0.04 Da for product ions using trypsin specificity with up to two missed cleavages. Carbamidomethylation (+57.0214 Da on C) was set as a fixed modification, whereas oxidation (+15.9949 Da on M) and deamidation (+0.98 Da on NQ) were considered dynamic mass modifications. All searched spectra were imported into Scaffold (v4.3, Proteome Software), and scoring thresholds were set to achieve a phosphopeptide false discovery rate of 0% using the Peptide Prophet algorithm.
Western blotting
For plasma samples, plasma was centrifuged at 13,000 g for 10 minutes, and the supernatant was diluted 1:1 in 20 mM Tris, pH 7.4, and incubated with 200 μl of Separopore blue CL-6B (Bio- world, Dublin, OH) slurry (equilibrated in 20 mM Tris, pH 7.4) for 30 minutes. The slurry was then clarified by centrifugation at 1,000 g for 3 minutes, and 20 μl of the supernatant was analyzed by Western blot. In detail, supernatant was mixed with SDS sample buffer (Invitrogen, Carlsbad, CA) and heated at 70°C for 10 minutes. The mixture was then loaded into 4-12% Bis-Tris NuPAGE precast gels (Invitrogen, Carlsbad, CA). The separated proteins were transferred to polyvinylidene difluoride (PVDF) membranes and blocked with 5% non-fat milk in 1 Tris buffered saline-Tween 20 (TBS-T) buffer for 1 hour at room temperature. The blots were probed with primary and secondary antibodies, and the latter was detected using the enhanced Super Signal ® West Pico Chemiluminescent system (Thermo Scientific, Rockford, IL) in accordance with the manufacturer’s protocol. The primary antibody used was rabbit anti-human PGRMC1 antibody (Sigma, St. Louis, MO) at 1:2000 dilution. Secondary mouse anti-rabbit IgG (Conformation Specific) horseradish peroxidase (HRP)-linked antibody (Cat# 5127s, Cell Signaling Technology, Beverly, MA) was used at 1:2000 dilution. This secondary antibody only reacts with native IgG and does not bind to the denatured and reduced rabbit IgG heavy chain and light chain. The non-conformation specific secondary goat anti-rabbit IgG HRP-conjugated antibody (Cat# 7074s, Cell Signaling Technology, Beverly, MA), routinely used in our laboratory, was used to compare with the aforementioned secondary antibody.
For PBMC samples, total protein was harvested using radioimmunoprecipitation assay (RIPA) buffer (Sigma, St Louis, MO) containing 1 protease inhibitor (Thermo Scientific, Rockford, IL). Protein concentrations of the supernatants were determined by Bradford assay (Bio-Red, Hercules, CA). 10 μg of protein was analyzed by Western blotting as described above.
Measurement of PGRMC1 in plasma by ELISA
Human Membrane-associated progesterone receptor component 1 (PGRMC1) ELISA kit (Cusabio biotech, Hubei Province, China) was used to measure plasma PGRMC1 protein following manufacturer’s instruction. Samples without dilution were run with serial dilutions of recombinant human PGRMC1 as standards. Samples were run in duplicate, and the absorbance was measured at optical density (OD) of 450 nm with correction at OD of 540 nm. Four replicates were performed using plasma from 5 subjects. The lower limit of sensitivity is 47 pg/ml.
Flow cytometry
Cryopreserved PBMCs were thawed and counted following previously published procedures [14]. Two million viable PBMCs were stained according to published methods [15] using manufactured recommended amounts of fluorescently labelled antibodies against CD3 FITC (clone SK7, BD Biosciences), CD16 PE-Cy7 (clone 3G8, BD Biosciences), CD19 APC (HIB19, BD Biosciences), CD56 PE-Cy7 (B159, BD Biosciences), CD95 BV605 (DX2, BioLegend), CD14 BV510 (M5E2, BioLegend), and Agua Fixable LIVE/DEAD cell stain (Invitrogen). PGRMC1 PE (polyclonal, Bioss) was included in the staining cocktail at a saturating concentration as determined by cell titration. HTR8/SVneo cells were used as positive control for PGRMC1 staining. After resuspending cells in 1% formaldehyde, cells were acquired within 6 hours of staining using a Special Order Research Product 20-parameter LSRII analyzer (Becton Dickinson, San Jose, CA). FlowJo Flow (Version 9.7.6 (TreeStar) was used to analyze flow cytometry standard data files. Anti-mouse CompBeads (BD Biosciences) were stained using the same conjugated antibodies described above and used to generate fluorescence compensation matrix. Immunological subsets were defined as follows: T cells (CD3+ lymphocytes), B cells (CD3-CD19+ lymphocytes), NK cells (CD3- CD56+CD16+), and Monocytes (CD14+). PGRMC1 and CD95 expression was measured across the immunogical subsets. Antibody Binding Capacity (ABC) was measured using Quantibrite PE (BD Biosciences) following manufacturer recommended procedures.
Results
PGRMC1 is undetectable in plasma during pregnancy
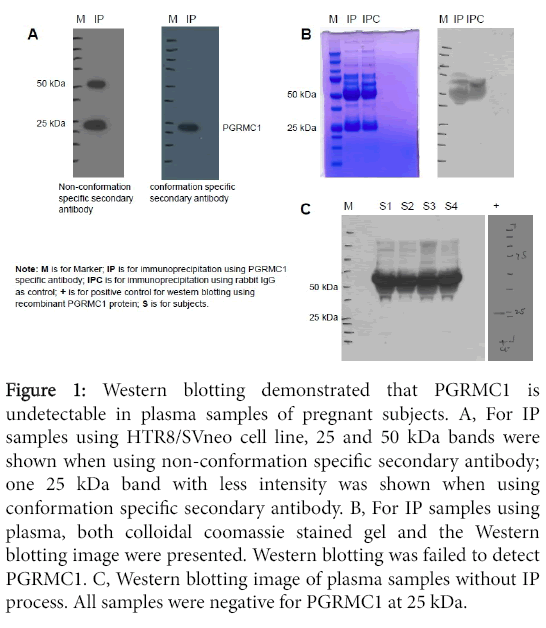
IP and Western blotting: Figure 1A shows two Western blotting images for the same IP samples obtained using HTR8/SVneo cells. The same amount of IP samples were loaded on each gel. Western blot was performed in duplicate, one using non-conformation specific secondary antibody (cat#7074s) and the other using conformation specific secondary antibody (cat#5127s). Using the nonconformational secondary antibody, two bands at 25 and 50 kDa were identified compared to only one 25 kDa band with less intensity identified using conformation secondary antibody. The conformation specific secondary antibody only reacts with native IgG and does not bind to the denatured and reduced rabbit IgG heavy chain (50 kDa) and light chain (25 kDa). Therefore, using conformation specific secondary antibody, the one 25 kDa band demonstrated in the image is specific to PGRMC1 given its known molecular weight of 25kDa. These results suggest that it is importance to use the conformation specific secondary antibody for Western blotting when analyzing PGRMC1 in blood samples that contain high levels of IgG.
Figure 1: Western blotting demonstrated that PGRMC1 is undetectable in plasma samples of pregnant subjects. A, For IP samples using HTR8/SVneo cell line, 25 and 50 kDa bands were shown when using non-conformation specific secondary antibody; one 25 kDa band with less intensity was shown when using conformation specific secondary antibody. B, For IP samples using plasma, both colloidal coomassie stained gel and the Western blotting image were presented. Western blotting was failed to detect PGRMC1. C, Western blotting image of plasma samples without IP process. All samples were negative for PGRMC1 at 25 kDa.
Figure 1B demonstrates the gel with colloidal coomassie staining for plasma IP samples as well as the Western blot image for the same IP samples. Western blot failed to detect PGRMC1 in these IP samples. Both IP samples using rabbit anti-human PGRMC1 monoclonal antibody and IP samples using rabbit IgG as negative control were negative at 25 kDa.
Western blot of plasma without IP process from four pregnant subjects also failed to detect PGRMC1 at 25 kDa (Figure 1).
IP and mass spectrometry: Three plasma samples from three different pregnant subjects were used for IP. Following IP, samples were subjected to standardized in-gel trypsin digestion and LC-MS/MS analysis. A positive protein identification required that at least two unique peptides were identified, yielding a false discovery rate of 0.0%. The total number of spectral counts for each sample varied from 128-216. There were no significant differences in the total number of spectral counts among the samples (P>0.05). Within the molecular weight region of the excised gel bands, a total of 288 peptides belonging to 39 proteins were identified from the combined 3 runs. PGRMC1 was not among those 39 proteins. The proteins identified were Ig kappa, lambda and gamma chains, keratin, ubiquitin-protein ligase E3B, glutary1-CoA dehydrogenase, complement C1q subcomponent subunit C, apolipoprotein A-I and serum amyloid p component. These data support the Western blotting data which failed to detect PGRMC1 in either IP or plasma samples.
ELISA
PGRMC1 protein was under detection in tested plasma samples. These data support the Western blotting and IP-LC-MS/MS results.
PGRMC1 is expressed in PBMCs during pregnancy
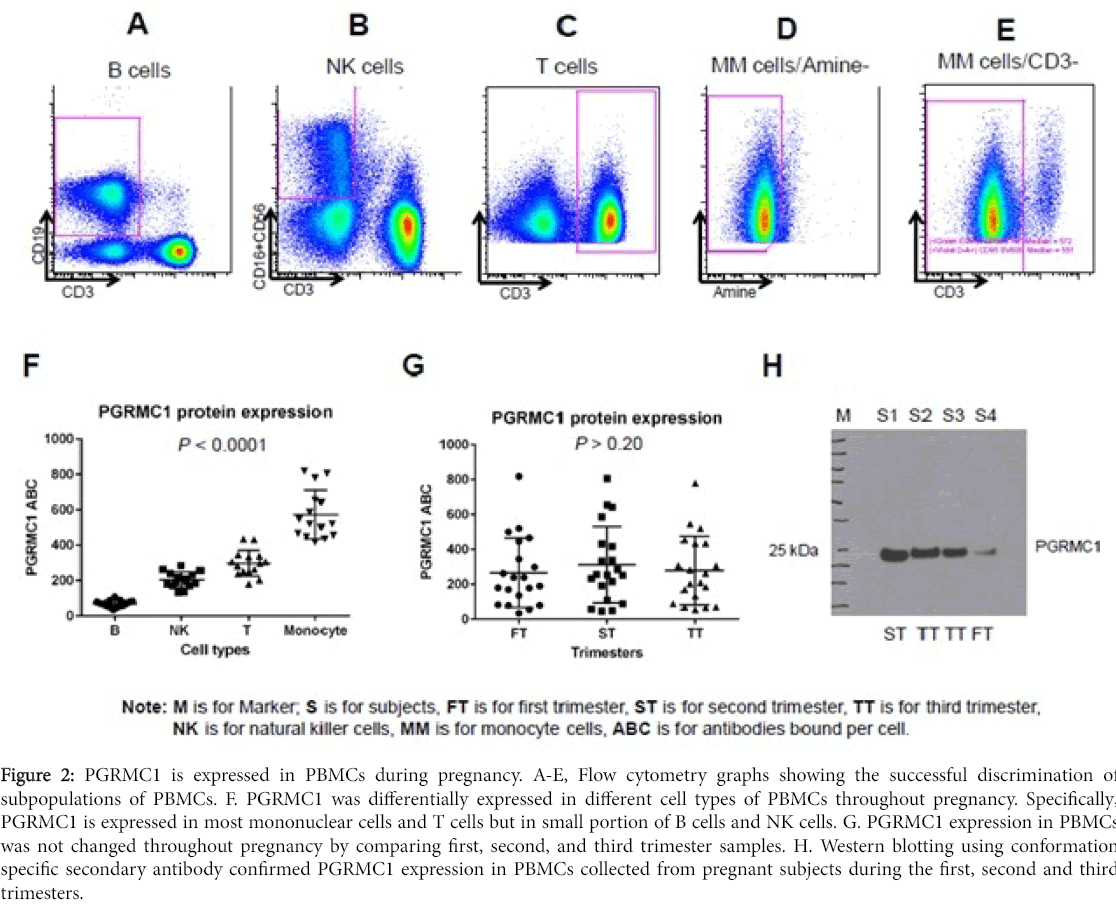
This protocol allows for determination of PGRMC1 expression in cell subpopulations of PBMCs. Successful discrimination of cell subpopulations of PBMCs was demonstrated in Figure 2A-E using cell markers described in methods. Flow cytometry results showed that PGRMC1 was differentially expressed in different cell types of PBMCs throughout pregnancy including the first, second, and third trimesters. Specifically, PGRMC1 is expressed in most mononuclear cells and T cells but only in a small portion of B cells and NK cells. In addition, PGRMC1 expression in PBMCs did not change throughout pregnancy, as demonstrated by comparing first, second, and third trimester samples. Western blotting using conformation specific secondary antibody confirmed PGRMC1 expression in PBMCs collected from pregnant subjects during the first, second and third trimesters (Figure 2).
Figure 2: PGRMC1 is expressed in PBMCs during pregnancy. A-E, Flow cytometry graphs showing the successful discrimination of subpopulations of PBMCs. F. PGRMC1 was differentially expressed in different cell types of PBMCs throughout pregnancy. Specifically, PGRMC1 is expressed in most mononuclear cells and T cells but in small portion of B cells and NK cells. G. PGRMC1 expression in PBMCs was not changed throughout pregnancy by comparing first, second, and third trimester samples. H. Western blotting using conformation specific secondary antibody confirmed PGRMC1 expression in PBMCs collected from pregnant subjects during the first, second and third trimesters.
Discussion
In this study, we characterized the presence of PGRMC1 in the bloodstream of pregnant women. PGRMC1 was differentially expressed in different cell types of PBMCs but not present in plasma during pregnancy.
PGRMC1, which plays important roles in pregnancy, is actively regulated in gestational tissues. PGRMC1 is a single transmembrane protein with high affinity for P4 and a low affinity for other steroid hormones [16,17]. PGRMC1 mediates P4 functions during pregnancy. PGRMC1 also appears to act independently of P4 to reduce myometrial contractility, prevent cytokine-induced inflammation and apoptosis of the fetal membranes, and mediate cell homeostasis and stress responses [5,6,18]. It is well established that PGRMC1 expression changes in human myometrium during pregnancy and labor at term or preterm [8]. PGRMC1 is actively regulated in the decidua at the maternal-fetal interface, as well as in the embryonic and fetal trophectoderm [7]. We previously found that PGRMC1 was decreased in fetal membranes in the setting of PPROM [6]. Additionally, recent animal studies have suggested that the effects of progesterone on PBMCs may be mediated by PGRMC1 during gestation [19,20]. PGRMC1’s functions in PBMCs during pregnancy are unclear and warrant further investigation.
Several recent studies have suggested that PGRMC1 is important for maintaining normal reproductive function. For instance, PGRMC1 levels are reduced in peripheral blood cells in women with polycystic ovarian syndrome [11] and in some women with premature ovarian failure [11,12]. In contrast, PGRMC1 over-expression is associated with impaired follicular development in women induced to undergo ovulation as part of their infertility treatment [20].
Beyond its roles in reproduction and pregnancy, PGRMC1 is upregulated in many types of cancers including breast, colon, ovarian, thyroid, lung, and cervix. PGRMC1 expression is induced by carcinogens [21,22] and promotes cell survival following DNA damage [23]. Given its role in cell survival and cancer progression, PGRMC1 is emerging as a potential biomarker and therapeutic target for certain cancers. For example, Craven et al demonstrated that PGRMC1 phosphorylation and expression patterns in breast cancer were predictive of estrogen receptor status and hypoxia in breast cancer [13]. Additionally, over-expression of PGRMC1 correlated with ovarian cancer progression, and experimental PGRMC1 knock-down promoted ovarian cancer cell apoptosis in response to chemotherapy [23]. Finally, PGRMC1 is highly expressed in squamous cell carcinoma of the lung and in a significant proportion of lung adenocarcinomas [10]. Specifically, PGRMC1 localized to secretory vesicles and was secreted by lung cancer cells, and plasma concentrations of PGRMC1 were elevated in subjects with lung cancer compared to healthy controls [10].
In contrast to the findings in lung cancer patients [10], we did not detect PGRMC1 in the plasma of any of our subjects. It may be that PGRMC1 is secreted by tumor cells but not by reproductive or gestational cells. However, given that Mir et al also detected PGRMC1 in the plasma of non-cancerous controls, it may be that the secondary antibody used in our study was more specific and reacted only with native IgG rather than with denatured and reduced rabbit IgG heavy chain (50 kDa) and light chain (25 kDa). Since the PGMRC1 band on Western blot is located at approximately 25 kDa, it is essential to use a conformation specific secondary antibody when analyzing PGRMC1 in blood samples. The other strength of this study is that IP and MS data corroborated our negative findings. Furthermore, PGRMC1 was not in the protein list of several previous proteomic analysis of human plasma including two studies using plasma from pregnant subjects [24-30]. However, PGRMC1 was detected in one human plasma proteome study [31]. Besides possible false positive or contamination from blood cells, the positive detection of PGRMC1 in Liu’s study might be due to the extensive fractionation of the plasma samples prior to MS analysis. Low-abundance proteins can often be accessible with multiple sample fractionation steps. However, the technical difficulties for PGRMC1 detection in the plasma samples might be the barrier for future biomarker development based on the assumption of PGRMC1 is one of the low-abundance proteins in plasma.
Nevertheless, blood is the most preferred and used human body fluid to be measured in routine clinical practice including monitoring pregnancy. Current data provide a solid foundation for future evaluation of PGRMC1 in PBMCs as a novel biomarker for PTB risk. Correlating PGRMC1 expression of PBMCs in the first, second, and third trimesters with subsequent delivery outcomes will be essential in determining the viability of this protein as a biomarker for PTB risk. The identification of such a biomarker could facilitate the early identification of patients at higher risk for PTB who could benefit from early targeted prophylaxis.
Conclusion
PGRMC1 is differentially expressed in different cell types of PBMCs of pregnant subjects but is not detectable in the plasma of these subjects. Current data provide a solid foundation for future evaluation of PGRMC1 in PBMCs as a novel biomarker for preterm birth risk.
Acknowledgement
This study was funded by The Biomarker Factory. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
References
- Mercer BM (2003) Preterm premature rupture of the membranes. Obstet Gynecol 101: 178-193.
- Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Mathews TJ, et al. (2010) Births: final data for 2008. Natl Vital Stat Rep 59: 3-71
- Meis PJ, Klebanoff M, Thom E, Dombrowski MP, Sibai B, et al. (2003) Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesteronecaproate. N Engl J Med 348:2379-2385.
- Hassan SS, Romero R, Vidyadhari D, Fusey S, Baxter JK, et al. (2011) Vaginal progesterone reduces the rate of preterm birth in women with a sonographic short cervix: a multicenter, randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol 38:18-31
- Pru JK, Clark NC (2013) PGRMC1 and PGRMC2 in uterine physiology and disease. Front Neurosci 7:168.
- Feng L, Antczak BC, Lan L, Grotegut CA, Thompson JL, et al. (2014)Progesterone receptor membrane component 1 (PGRMC1) expression in fetal membranes among women with preterm premature rupture of the membranes (PPROM). Placenta 35:331-333.
- Zhang L, Kanda Y, Roberts DJ, Ecker JL, Losel R, et al. (2008) Expression of progesterone receptor membrane component 1 and its partner serpine 1 mRNA binding protein in uterine and placental tissues of the mouse and human. Mol Cell Endocrinol287:81-89.
- Wu W, Shi SQ, Huang HJ, Balducci J, Garfield RE (2011) Changes in PGRMC1, a potential progesterone receptor, in human myometrium during pregnancy and labour at term and preterm. Mol Hum Reprod 17:233-242.
- Allen TK,Feng L, Grotegut CA, Murtha AP (2014) Progesterone receptor membrane component 1 as the mediator of the inhibitory effect of progestins on cytokine-induced matrix metalloproteinase 9 activity in vitro. Reprod Sci21:260-268.
- Mir SU, Ahmed IS, Arnold S, Craven RJ (2012) Elevated progesterone receptor membrane component 1/sigma-2 receptor levels in lung tumors and plasma from lung cancer patients. Int J Cancer 131:1-9.
- Schuster J, Karlsson T, Karlstrom PO, Poromaa IS, Dahl N (2010) Down-regulation of progesterone receptor membrane component 1 (PGRMC1) in peripheral nucleated blood cells associated with premature ovarian failure (POF) and polycystic ovary syndrome (PCOS). Reprod Biol Endocrinol8:58.
- Mansouri MR, Schuster J, Badhai J, Stattin EL, Wehling M, et al. (2008) Alterations in the expression, structure and function of progesterone receptor membrane component-1 (PGRMC1) in premature ovarian failure. Hum Mol Genet 17:3776-3783.
- Craven RJ (2008) PGRMC1: a new biomarker for the estrogen receptor in breast cancer. Breast Cancer Res 10:113.
- Murdoch DM, Suchard MS, Venter WD, Mhlangu P, Ottinger JS, et al. (2009)Polychromatic immunophenotypic characterization of T cell profiles among HIV-infected patients experiencing immune reconstitution inflammatory syndrome (IRIS). AIDS Res Ther6:16.
- Murdoch DM, Staats JS, Weinhold KJ (2012) OMIP-006: phenotypic subset analysis of human T regulatory cells via polychromatic flow cytometry. Cytometry A81:281-283.
- Meyer C,Schmid R, Scriba PC, Wehling M (1996) Purification and partial sequencing of high-affinity progesterone-binding site(s) from porcine liver membranes. Eur J Biochem 239:726-731.
- Thomas P (2008) Characteristics of membrane progestin receptor alpha (mPRalpha) and progesterone membrane receptor component 1 (PGMRC1) and their roles in mediating rapid progestin actions. Front Neuroendocrinol29:292-312.
- Luo G, Abrahams VM, Tadesse S, Funai EF, Hodgson EJ, et al. (2010) Progesterone inhibits basal and TNF-alpha-induced apoptosis in fetal membranes: a novel mechanism to explain progesterone-mediated prevention of preterm birth. Reprod Sci 17:532-539.
- Ndiaye K, Poole DH, Walusimbi S, Cannon MJ, Toyokawa K, et al. (2012) Progesterone effects on lymphocytes may be mediated by membrane progesterone receptors. J Reprod Immunol 95:15-26.
- Elassar A, Liu X, Scranton V, Wu CA, Peluso JJ (2012) The relationship between follicle development and progesterone receptor membrane component-1 expression in women undergoing in vitro fertilization. FertilSteril97:572-578.
- Rohe HJ, Ahmed IS, Twist KE, Craven RJ (2009) PGRMC1 (progesterone receptor membrane component 1): a targetable protein with multiple functions in steroid signaling, P450 activation and drug binding. Pharmacol Ther 121:14-19.
- Nie AY, McMillian M, Parker JB, Leone A, Bryant S, et al. (2006) Predictive toxicogenomics approaches reveal underlying molecular mechanisms of nongenotoxic carcinogenicity. Mol Carcinog 45:914-933.
- Peluso JJ, Liu X, Saunders MM, Claffey KP, Phoenix K (2008) Regulation of ovarian cancer cell viability and sensitivity to cisplatin by progesterone receptor membrane component-1. J Clin EndocrinolMetab 93:1592-1599.
- Cao Z, Yende S, Kellum JA, Robinson RA (2013) Additions to the Human Plasma Proteome via a Tandem MARS Depletion iTRAQ-Based Workflow. Int J Proteomics 2013:654356.
- Gautam P, Nair SC, Ramamoorthy K, Swamy CV, Nagaraj R (2013) Analysis of human blood plasma proteome from ten healthy volunteers from Indian population. PLoS One 8: 72584.
- Jin WH, Dai J, Li SJ, Xia QC, Zou HF et al. (2005) Human plasma proteome analysis by multidimensional chromatography prefractionation and linear ion trap mass spectrometry identification. J Proteome Res 4:613-619.
- Farrah T, Deutsch EW, Omenn GS, Campbell DS, Sun Z, et al. (2011) A high-confidence human plasma proteome reference set with estimated concentrations in PeptideAtlas. Mol Cell Proteomics 10:M110 006353.
- LoicDayon MK (2013) Proteomics of human plasma: A critical comparison of analytical workflows in terms of effort, throughput and outcome. Eupa Open Proteomics8-16.
- Robert J, Phillips KJH, Johanna T, Andres LB (2014) Human maternal plasma proteomic changes with parturition. EuPA OPEN PROTEOMICS 5:10-20.
- Michel PE, Crettaz D, Morier P, Heller M, Gallot D, et al. (2006) Proteome analysis of human plasma and amniotic fluid by Off-Gel (TM) isoelectric focusing followed by nano-LC-MS/MS. Electrophoresis 27:1169-1181.
- Liu X, Valentine SJ, Plasencia MD, Trimpin S, Naylor S, et al. (2007) Mapping the human plasma proteome by SCX-LC-IMS-MS. J Am Soc Mass Spectrom 18:1249-1264
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 12205
- [From(publication date):
August-2016 - Apr 03, 2025] - Breakdown by view type
- HTML page views : 11237
- PDF downloads : 968