Research Article Open Access
Characterization of Human Aortic Endothelial Cells, Endothelial Progenitor Cells, and Cardiomyocytes
Ching-Hung Chen1 and Chan-Yen Kuo2*1Department of Anesthesiology, Show Chwan Memorial Hospital, Changhua, Taiwan
2Graduate Institute of Systems Biology and Bioinformatics, National Central University, Chung-li, Taiwan
- Corresponding Author:
- Chan-Yen Kuo
Graduate Institute of Systems Biology and Bioinformatics
National Central University, Chung-li, Taiwan
Tel: +886-3- 4227151
Fax: +886-3-4226062
E-mail: cykuo@thu.edu.tw
Received Date: November 30, 2016; Accepted Date: December 09, 2016; Published Date: December 14, 2016
Citation: Chen CH, Kuo CY (2017) Characterization of Human Aortic Endothelial Cells, Endothelial Progenitor Cells, and Cardiomyocytes. J Tradit Med Clin Natur 6:203.
Copyright: © 2017 Chen CH, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Traditional Medicine & Clinical Naturopathy
Abstract
Despite advances in therapy, heart failure remains a significant disease burden, with poor outcomes, worldwide. Reactive Oxygen Species (ROS) damage cardiomyocytes. Endothelial progenitor cells promote the repair of the endothelium of arteries damaged by ROS. However, gene expression profiles of Human Aortic Endothelial Cells (HAECs), Endothelial Progenitor Cells (HEPCs), and Cardiomyocytes (HCMs) are unclear. In the present study, we determined the expression profiles of different genes in HAECs, HEPCs, and HCMs by performing quantitative PCR. Results showed that p53 and Cx37 were up-regulated, but VEGF, Cx43, and eNOS were down-regulated in HEPCs. Cx40 and eNOS were up-regulated in HAECs. Moreover, we determined the effect of hydrogen peroxide-derived ROS on HCMs. Results showed that Cx40, Cx45, VCAM-1, ICAM-1, p53, and p21 were up-regulated, but E-cadherin was down-regulated after high concentration of hydrogen peroxide treatment.
Keywords
Human aortic endothelial cells (HAECs); Endothelial progenitor cells (HEPCs); Cardiomyocytes (HCMs); Reactive oxygen species (ROS)
Introduction
Coronary artery disease is a large disease burden in several countries. Endothelial dysfunction caused by oxidative stress and inflammation is an essential process underlying the progression of heart failure [1-3]. Tissues engineering aims to apply the principles of engineering and life science in developing biological substitutes that maintain, restore, or improve tissues. In clinical, new drugs and vascular bypass have improved the quality of life of patients with Cardiovascular Disease (CVD) but have not decreased morbidity or mortality [4]. Tateishi- Yuyama et al. reported that autologous transplantation of bone marrow-derived progenitor cells is a potential therapy of angiogenesis for patients with limb ischemia [5]. Autologous cell therapies involving bone marrow or circulating blood-derived progenitor cells are safe and exert beneficial therapeutic effects by inducing angiogenesis/ vasculogenesis in patients with ischemic diseases [6,7]. In addition, human embryonic stem cell (hESC)-derived endothelial cells could be beneficial for potential applications such as engineering of new blood vessels, endothelial cell transplantation into the heart for myocardial regeneration, and induction of angiogenesis for treating regional ischemia [8]. However, because of ethical issues associated with ESCs, peripheral blood-derived epithelial progenitor cells (EPCs) are used for cell therapy [9]. EPCs are a potential inexhaustible source of functional vascular cells that have important features of mature ECs for regenerative medicine. However, it is difficult to define EPCs generated from different sources because they lack a unifying phenotype [10]. Glaser et al. suggested that different types of EPCs include colonyforming unit Hill cells, circulating cells, and endothelial colonyforming cells [11]. Therefore, it is very important to functionally characterize EPCs.
Gap junctions form conduits between adjacent cells that are composed of connexin subunits; these conduits allow direct intercellular communication [12]. Gap junctions also promote intercellular communication in the cardiovascular system and are essential for normal vascular function [13,14]. Connexins expressed in the vascular wall include Cx37, Cx40, Cx43, and Cx45 and those expressed by endothelial cells include Cx37 and Cx40 [12,14]. However, the role of these connexins in Human Aortic Endothelial Cells (HAECs), Human Endothelial Progenitor Cells (HEPCs), and Human Cardiomyocytes (HCMs) is unclear. Nitric Oxide (NO) is very important for regulating endothelial function. Increasing in NO production is either increased by Endothelial Nitric Oxide Synthase (eNOS) enzymes [15-17] or reduced by Reactive Oxygen Species (ROS) [18]. Ischemic preconditioning causes ROS overproduction in the mitochondria under hypoxia [19,20]. However, the effect of hypoxia on Cx37, Cx40, Cx43, and Cx45 is unclear. In this study, we characterized HAECs, HEPCs, and HCMs. In addition, we examined the effect of hypoxia on the expression of the abovementioned connexins in each cell model.
Materials and Methods
Cell lines and cell culture
HAECs (PromoCell GmbH, Heidelberg, Germany) were cultured in T-25 flasks (Corning Glassworks, Corning, NY, USA) containing endothelial cell growth medium MV (PromoCell GmbH) supplemented with 0.05 ml/ml fetal calf serum, 0.004 ml/ml endothelial cell growth supplement, 10 ng/ml epidermal growth factor, 90 μg/ml heparin, and 1 μg/ml hydrocortisone at 37°C and in an atmosphere of 5% CO2/95% air.
HEPCs (Amsbio, UK) were cultured in T-25 flasks containing EPC growth medium (Cat#Z7030073; Bio Chain Institute Inc., CA, USA) at 37°C and in an atmosphere of 5% CO2/95% air.
HCMs (PromoCell GmbH) were cultured in T-25 flasks containing myocyte growth medium (PromoCell GmbH) supplemented with 0.05 ml/ml fetal calf serum, 0.5 ng/ml epidermal growth factor, 2 ng/ml basic fibroblast growth factor, and 5 μg/ml insulin at 37°C and in an atmosphere of 5% CO2/95% air.
Culture medium was replaced every 2 days. After reaching 70–80% confluency, the cells were trypsinized and were seeded in six-well plastic dishes for performing subsequent experiments. Passages 3–6 HAECs, 3–10 HEPCs, and 3–9 HCMs were used in subsequent experiments.
RNA isolation and quantitative PCR
Total RNA was isolated from the cells by using TRIzol reagent (Invitrogen, Thermo Fisher Scientific, CA, and USA). Primer sequences and procedure used for quantitative PCR (qPCR) analysis of genes encoding vascular endothelial growth factor (VEGF), p53, p21, Cx37, Cx40, Cx43, Cx45, eNOS, VE-cadherin, vascular cell adhesion molecule-1 (VCAM-1), intercellular adhesion molecule-1 (ICAM-1), and β-actin. cDNAs of genes encoding VEGF, p53, p21, Cx37, Cx40, Cx43, Cx45, eNOS, VE-cadherin, VCAM-1, ICAM-1, and β-actin were synthesized using the following primer sets: 5ʹ-TGC AGA TTA TGC GGA TCA AAC C-3ʹ and 5ʹ-TGC ATT CAC ATT TGT TGT GCT GTA G-3ʹ for the gene encoding VEGF, 5ʹ-GCC CAA CAA CAC CAG CTCC T-3ʹ and 5ʹ-CCT GGG CAT CCT TGA GTT CC-3ʹ for the gene encoding p53, 5ʹ-GAG GCC GGG ATG AGT TGG GAG GAG- 3ʹ and 5ʹ-CAG CCG GCG TTT GGA GTG GTA GAA-3ʹ for the gene encoding p21, 5ʹ-GTT GCT GGA CCA GGT CCA GG-3ʹ and 5ʹ-GGA TGC GCA GGC CAC CAT CT-3ʹ for the gene encoding Cx37, 5ʹ-GTA CAC AAG CAC TCG ACC GT -3ʹ and 5ʹ-GCA GGG TGG TCA GGA AGA TT-3ʹ for the gene encoding Cx40, 5ʹ-CAA TCA CTT GGC GTG ACT TC-3ʹ and 5ʹ-GTT TGG GCA ACC TTG AGT TC-3ʹ for the gene encoding Cx43, 5ʹ-GGA GCT TTC TGA CTC GCC TG-3ʹ and 5ʹ-CGG CCA TCA TGC TTA GGT TT-3ʹ for the gene encoding Cx45, 5ʹ-CGG CAT CAC CAG GAA GAA GA-3ʹ and 5ʹ-CAT GAG CGA GGC GGA GAT-3ʹ for the gene encoding eNOS, 5ʹ-CAT GAG CCT CTG CAT CTT CC-3ʹ and 5ʹ-ACA GAG CTC CAC TCA CGC TC-3ʹ for the gene encoding VE-cadherin, 5ʹ-GAT ACA ACC GTC TTG GTC AGC CC-3ʹ and 5ʹ-CAG TTG AAG GAT GCG GGA GTA TAT G-3ʹ for the gene encoding VCAM-1, 5ʹ-CGA TGA CCA TCT ACA GCT TTC CGG-3ʹ and 5ʹ-GCT GCT ACC ACA GTG ATG ATG ACA A-3ʹ for the gene encoding ICAM-1, and 5ʹ-TCC ACC TTC CAG CAG ATG TG-3ʹ and 5ʹ-GCATTTGCGGTGGACGAT-3ʹ for the gene encoding β-actin. qPCR was performed using Platinum SYBR Green qPCR kit (Invitrogen, Thermo Fisher Scientific) in iCycler (Bio-Rad).
Statistical analysis
Data are shown as mean ± SEM. Treatment groups were compared using a two-tailed t-test by using a SAS software.
Results and Discussion
Gene profiles of HAECs, HEPCs, and HCMs
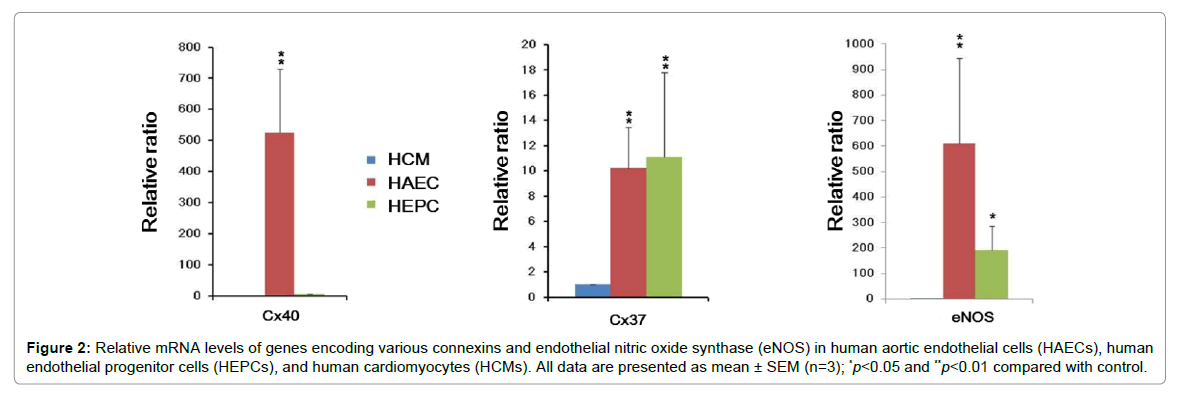
We validated the expression of genes encoding Cx37, 40, 43, 45, eNOS, VEGF, p21, and p53 by performing qPCR (Figures 1 and 2). EPCs play a critical role in neovascularization and reendothelialization after ischemia and endothelial injury, respectively [21,22]. Interestingly, we observed that the expression of VEGF was decreased in HAECs and HEPCs (Figure 1). Previous studies have reported that VEGF is expressed in cardiac myofibroblasts, nonendothelial cells with the morphological features of fibroblasts in rat myofibroblasts isolated from heart infarcts [23,24]. However, limited anti-VEGF antibody-based therapeutic approaches are available for preventing cellular senescence in patients with CVD because these approaches exert different therapeutic effects in animal experiments and clinical trials [25]. Therefore, it is important to determine the gene expression profiles of HAECs, HEPCs, and HCMs.
Figure 1: Relative mRNA levels of genes encoding different connexins, vascular endothelial growth factor (VEGF), p21, and p53 in human aortic endothelial cells (HAECs), human endothelial progenitor cells (HEPCs), and human cardiomyocytes (HCMs). All data are presented as mean ± SEM (n=3); *p<0.05 and **p<0.01 compared with control.
Figure 2: Relative mRNA levels of genes encoding various connexins and endothelial nitric oxide synthase (eNOS) in human aortic endothelial cells (HAECs), human endothelial progenitor cells (HEPCs), and human cardiomyocytes (HCMs). All data are presented as mean ± SEM (n=3); *p<0.05 and **p<0.01 compared with control.
Effect of hypoxia on HCMs
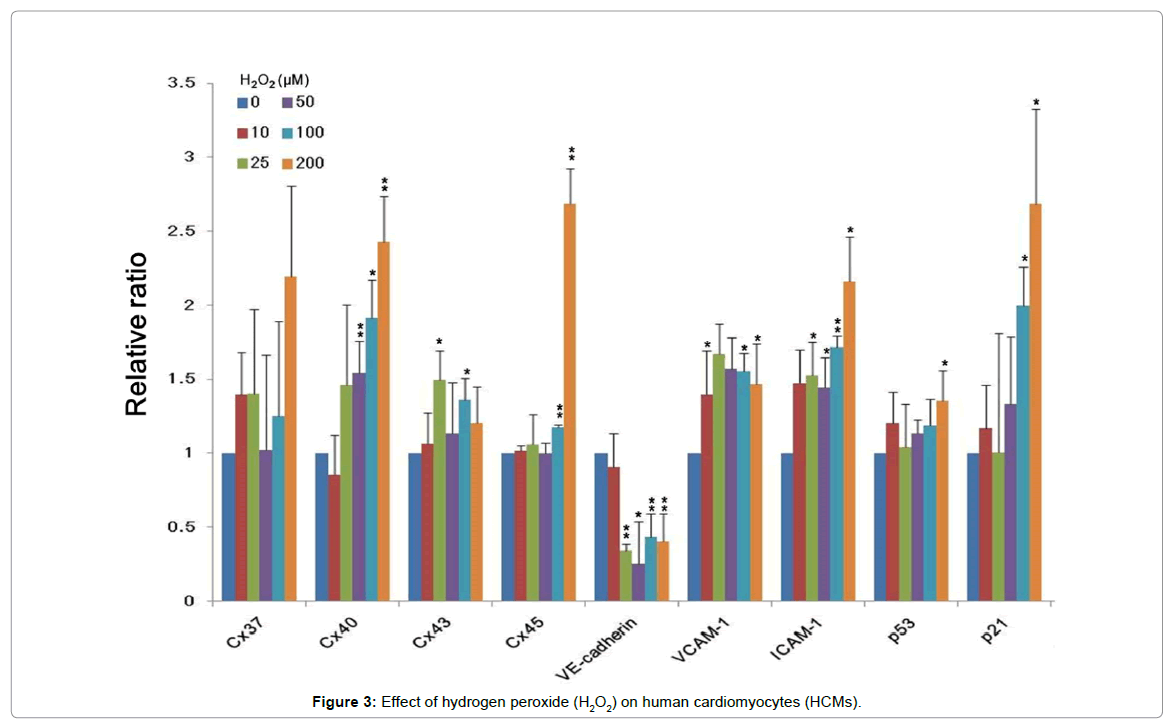
Mitochondria play a crucial role in regulating intrinsic pathways of apoptosis or programmed cell death [26]. Mitochondria are the major source of endogenous ROS in cells because they contain the electron transport chain required for oxidative phosphorylation [27,28]. However, the effect of hypoxia on HCMs is limited. We used hydrogen peroxide (H2O2) to mimic hypoxic condition [29] and determined the gene expression profiles of HAECs, HEPCs, and HCMs under hypoxia. Our results showed that the expression of genes encoding Cx40, Cx45, VCAM-1, ICAM-1, p21, and p53 was upregulated and that of the gene encoding E-cadherin was downregulated in cells treated with high concentration of H2O2 (200 μM) (Figure 3).
ROS upregulate VCAM-1 expression in endothelial cells [30]; however, this was not observed in HCMs. H2O2 increases the secretion of ICAM-1 in canine myocytes [31], which is consistent with the results of the present study. Long et al. reported that the expression of p21/ WAF-1/CIP-1, a well-characterized target of p53 transactivation, also increases under hypoxia. Furthermore, hypoxia-induced rat cardiac myocytes to apoptosis via p53 activation [32]. Thus, our results suggest that H2O2-derived ROS trigger the apoptosis of HCMs. However, further studies should be performed to confirm this hypothesis.
Interestingly, ST2 (suppression of tumor formation) is a receptor for the interleukin-33 and critical to coronary artery disease. Marzullo et al. suggested that ST2/IL-33 pathway may play a central role in the novel mechanism of plaque development and eventually rupture [33].
Acknowledgement
This study was supported by grant RD105056 from Show Chwan Memorial Hospital, Changhua, Taiwan.
References
- Scott J (2004) Pathophysiology and biochemistry of cardiovascular disease. Curr Opin Genet Dev 14: 271-279.
- Sun HJ, Hou B, Wang X, Zhu XX, Li KX, et al. (2016) Endothelial dysfunction and cardiometabolic diseases: Role of long non-coding RNAs. Life Sci.
- Jensen HA, Mehta JL (2016) Endothelial cell dysfunction as a novel therapeutic target in atherosclerosis. Expert Rev Cardiovas Ther 14: 1021-1033.
- Nugent HM, Edelman ER (2003) Tissue engineering therapy for cardiovascular disease. Circul Res 92: 1068-1078.
- Tateishi-Yuyama E, Matsubara H, Murohara T, Ikeda U, Shintani S, et al. (2002) Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: a pilot study and a randomised controlled trial. The Lancet 360: 427-435.
- Li Z, Han Z, Wu JC (2009) Transplantation of human embryonic stem cell�?�derived endothelial cells for vascular diseases. J Cell Biochem 106: 194-199.
- Huang PP, Li SZ, Han MZ, Xiao ZJ, Yang RC, et al. (2004) Autologous transplantation of peripheral blood stem cells as an effective therapeutic approach for severe arteriosclerosis obliterans of lower extremities. Thrombosis and Haemostasis 91: 606-609.
- Levenberg S, Golub JS, Amit M, Itskovitz-Eldor J, Langer R (2002) Endothelial cells derived from human embryonic stem cells. Proceedings of the National Academy of Sciences 99: 4391-4396.
- Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, et al. (1997) Isolation of putative progenitor endothelial cells for angiogenesis. Science 275: 964-966.
- Hirschi KK, Ingram DA, Yoder MC (2008) Assessing identity, phenotype, and fate of endothelial progenitor cells. Arteriosclerosis, Thrombosis, and Vascular Biology 28: 1584-1595.
- Glaser DE, Gower RM, Lauer NE, Tam K, Blancas AA, et al. (2011) Functional characterization of embryonic stem cell-derived endothelial cells. J Vasc Res 48: 415-428.
- Sohl G, Willecke K (2004) Gap junctions and the connexin protein family. Cardiovasc Res 62: 228-232.
- Michela P, Velia V, Aldo P, Ada P (2015) Role of connexin 43 in cardiovascular diseases. Eur J Pharmacol 768: 71-76.
- Morel S (2014) Multiple roles of connexins in atherosclerosis- and restenosis-induced vascular remodelling. J Vasc Res 51: 149-61.
- Alderton WK, Cooper CE, Knowles RG (2001) Nitric oxide synthases: structure, function and inhibition. Biochem J 357: 593-615.
- Dudzinski DM, Igarashi J, Greif D, Michel T (2006) The regulation and pharmacology of endothelial nitric oxide synthase. Annu Rev Pharmacol Toxicol 46: 235-276.
- Hambrecht R, Adams V, Erbs S, Linke A, Krankel N, et al. (2003) Regular physical activity improves endothelial function in patients with coronary artery disease by increasing phosphorylation of endothelial nitric oxide synthase. Circulation 107: 3152-3158.
- Adams V, Linke A, Krankel N, Erbs S, Gielen S, et al. (2005) Impact of regular physical activity on the NAD(P)H oxidase and angiotensin receptor system in patients with coronary artery disease. Circulation 111: 555-562.
- Duranteau J, Chandel NS, Kulisz A, Shao Z, Schumacker PT (1998) Intracellular signaling by reactive oxygen species during hypoxia in cardiomyocytes. J Biol Chem 273: 11619-11624.
- Bagheri F, Khori V, Alizadeh AM, Khalighfard S, Khodayari S, et al. (2016) Reactive oxygen species-mediated cardiac-reperfusion injury: Mechanisms and therapies. Life Sci 165: 43-55.
- Urbich C, Dimmeler S (2004) Endothelial progenitor cells: characterization and role in vascular biology. Circ Res 95: 343-53.
- Hur J, Yoon CH, Kim HS, Choi JH, Kang HJ, et al. (2004) Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler Thromb Vasc Biol 24: 288-293.
- Chintalgattu V, Nair DM, Katwa LC (2003) Cardiac myofibroblasts: a novel source of vascular endothelial growth factor (VEGF) and its receptors Flt-1 and KDR. J Mol Cell Cardiol 35: 277-286.
- Harmey JH, Bouchier-Hayes D (2002) Vascular endothelial growth factor (VEGF), a survival factor for tumour cells: implications for anti-angiogenic therapy. Bioessays 24: 280-283.
- Sanada S, Taniyama Y, Azuma J, Yuka, II, Iwabayashi M, et al. (2014) Endothelial progenitor cells in clinical settings. J Stem Cells 9: 117-125.
- Green DR, Reed JC (1998) Mitochondria and apoptosis. Science 281: 1309-1312.
- Murphy MP (2009) How mitochondria produce reactive oxygen species. Biochem J 417: 1-13.
- Stowe DF, Camara AK (2009) Mitochondrial reactive oxygen species production in excitable cells: modulators of mitochondrial and cell function. Antioxid Redox Signal 11: 1373-1414.
- Hool LC, Arthur PG (2002) Decreasing cellular hydrogen peroxide with catalase mimics the effects of hypoxia on the sensitivity of the L-type Ca2+ channel to beta-adrenergic receptor stimulation in cardiac myocytes. Circ Res 91: 601-609.
- Cook-Mills JM, Marchese ME, Abdala-Valencia H (2011 Vascular cell adhesion molecule-1 expression and signaling during disease: regulation by reactive oxygen species and antioxidants. Antioxid Redox Signal 15: 1607-1638.
- Lu H, Youker K, Ballantyne C, Entman M, Smith CW (2000) Hydrogen peroxide induces LFA-1-dependent neutrophil adherence to cardiac myocytes. Am J Physiol Heart Circ Physiol 278: H835-H842.
- Long X, Boluyt MO, Hipolito ML, Lundberg MS, Zheng JS, et al. (1997) p53 and the hypoxia-induced apoptosis of cultured neonatal rat cardiac myocytes. J Clin Invest 99: 2635-2643.
- Marzullo A, Ambrosi F, Inchingolo M, Manca F, Devito F, et al. (2016) ST2L Transmembrane Receptor Expression: An Immunochemical Study on Endarterectomy Samples. PLoS ONE 11: e0156315.
Relevant Topics
- Acupuncture Therapy
- Advances in Naturopathic Treatment
- African Traditional Medicine
- Australian Traditional Medicine
- Chinese Acupuncture
- Chinese Medicine
- Clinical Naturopathic Medicine
- Clinical Naturopathy
- Herbal Medicines
- Holistic Cancer Treatment
- Holistic health
- Holistic Nutrition
- Homeopathic Medicine
- Homeopathic Remedies
- Japanese Traditional Medicine
- Korean Traditional Medicine
- Natural Remedies
- Naturopathic Medicine
- Naturopathic Practioner Communications
- Naturopathy
- Naturopathy Clinic Management
- Traditional Asian Medicine
- Traditional medicine
- Traditional Plant Medicine
- UK naturopathy
Recommended Journals
Article Tools
Article Usage
- Total views: 4217
- [From(publication date):
February-2017 - Jul 04, 2025] - Breakdown by view type
- HTML page views : 3203
- PDF downloads : 1014