Characterization and Outcomes in Patients with SARS-CoV-2 Infection According to Body Mass Index
Received: 05-May-2022 / Manuscript No. JOMB-22-62826 / Editor assigned: 09-May-2022 / PreQC No. JOMB-22-62826(PQ) / Reviewed: 23-May-2022 / QC No. JOMB-22-62826 / Revised: 04-Jul-2022 / Manuscript No. JOMB-22-62826(R) / Published Date: 11-Jul-2022
Abstract
Objective: SARS-CoV-2 was declared a pandemic in 2020. Several factors related to severity and mortality have been described, including obesity. In Colombia, 56% of the population is over-weight/obese. We sought to investigate the characterization and outcomes of patients according to body mass index in a highly complex center in Colombia.
Methods: This is a prospective longitudinal observational descriptive study in patients older than 18 years treated at the Fundación Valle del Lili University Hospital in Cali, Colombia in 2020-2021, with a diagnosis of SARS-CoV-2 infection. Socio demographic characteristics, medical history, clinical presentation, paraclinical characteristics and outcomes were described.
Results: The average age was 48 years, 53% were men. 66.5% of the patients were overweight or obese. The severity of the disease according to the Body Mass Index (BMI) (p=0.011), days of stay in the ICU (p=0.0093), overall stay (p=0.0031), and duration of mechanical ventilation (p=0.0069) had relationships directly proportional to the increase in BMI. Nonetheless, our report does not allow to determine the association between mortality and obesity in patients with SARS-CoV-2 infection, since the differences found were not statistically significant (P=0.493).
Conclusion: Obesity is a risk factor for adverse outcomes and morbidity in SARS-CoV-2 infection. However, more studies are needed to explore additional factors according to the stage of the pandemic and advances in vaccination. We did not find an association with an increase in mortality in overweight and obese patients.
Introduction
The new SARS-CoV-2 coronavirus originated in Wuhan, China, in the second half of 2019 and expanded rapidly until it was declared a global pandemic in March 2020 [1]. Six months later, 68 million people were infected, and it has been responsible for more than 1.5 million deaths worldwide [2]. In Colombia, by March 2021, the number of infections amounted to more than 2 million infected and approximately 60 thousand deaths.
Coronavirus disease 2019 (COVID 2019), is characterized by the wide variety of its clinical presentation, from asymptomatic to fatal, and while most (80%) will present mild symptoms, 15% have severe forms of the disease, and the remaining 5% will be critical patients, with a mortality of 49% between these two latter groups [2,3].
The early detection of risk factors for the severe-critical presentation of SARS-CoV-2 infection is of vital importance to achieve prevention strategies, risk stratification and treatment. Several risk factors for development, severity and mortality from COVID-19 have been described, such as advanced age, chronic lung disease, cardiovascular diseases, diabetes, and obesity [4].
Obesity is a disease with a wide worldwide distribution, and it is understood to be a predisposing factor for chronic non-communicable diseases such as cardiovascular disease, diabetes and hypertension [2].
It has been shown to increase susceptibility to severe respiratory infections [5] and worsen Acute Respiratory Distress Syndrome (ARDS) outcomes [6]. The Centers for Disease Control and Prevention (CDC) describes severe obesity (Body Mass Index (BMI) ≥40 kg/m2) as a risk factor for severe COVID-19 disease [7]. In Colombia, a country with approximately 50 million inhabitants, it has been estimated that 56% of the population is overweight or obese.
Overweight and obese populations are at higher risk of severe COVID-19 [8–10] and related complications. Here, we present a longitudinal descriptive study that aims to reveal the characterization and outcomes of patients according to body mass index and SARSCoV- 2 infection in a highly complex institution, Fundación Valle del Lili, Cali, Colombia.
Materials and Methods
A prospective longitudinal observational descriptive study was carried out in patients treated at the Fundación Valle del Lili University Hospital (FVL) in Cali, Colombia in the years 2020-2021, with a diagnosis of SARS-CoV-2 infection. Male and female patients over 18 years of age who were admitted to FVL in whom a molecular diagnosis of COVID 19 was made were eligible. The information on patients over 18 years of age registered in the institutional database in the REDCAP system (institutional system in which a registry of patients who undergo PCR or antigen test for SARS-CoV-2 is maintained) was obtained sequentially. Later, positive patients were selected sequentially, by admission and diagnosis, and information was collected through a review of the medical history, their in-hospital treatment was followed up, and the records of control visits after the event were reviewed to determine outcomes. We excluded pregnant patients.
Socio demographic identification was included: age, sex, type of affiliation to the health system, medical history such as hypertension, diabetes, Human Immunodeficiency Virus (HIV), heart, lung, rheumatological and neurological diseases, history of transplantation, oncological disease, use of drugs with inhibitors of the Angiotensin Converting Enzyme (ACE), Angiotensin 2 Receptor Blockers (ARB), and steroids. Additionally, variables of the physical examination such as weight, height, body mass index, Pao2 / Fio2 (PAFI), oxygen saturation, and respiratory rate were obtained, and clinical presentation such as presence of cough, dyspnea, diarrhea, headache, fever, odynophagia, abdominal pain, dysgeusia, and loss of smell were recorded. Laboratory variables such as levels of ferritin, D-dimer, and fibrinogen were also considered. Variables related to management, need for Invasive Mechanical Ventilation (IMV), necessity of management with inotropics and vasopressors, the duration of Mechanical Ventilation (MV), days of management in the Intensive Care Unit (ICU) and days of hospitalization were calculated. Complications such as pulmonary thromboembolism, myocarditis, arrhythmias, coronary event, kidney failure requiring dialysis and infectious processes were evaluated; severity on admission (Mild: outpatient management neither asymptomatic nor altered inflammatory response biomarkers. Moderate: moderate hypoxemic respiratory failure with altered inflammatory response biomarkers and general hospital stay. Severe: critically ill patients with acute severe hypoxemic respiratory failure with stay in ICU) and mortality were determined. The protocol was approved by the institutional ethics committee; all methods were performed in accordance with the relevant guidelines and regulations. At the end of the data recording, the information was transferred to Excel16, and data analysis was performed in version 14.
For statistical analysis, categorical variables are presented as absolute values and percentages. Continuous variables are reported as means or medians and measures of dispersion, standard deviation or interquartile range depending on the distribution of the data, followed by evaluation with the Shapiro Wilk test and non-parametric tests (Mann Whitney or Wilcoxon test) or parametric tests (t-student or ANOVA), depending on the case. Statistically significant differences were considered if they had a p value<0.05. The length of hospitalization in the ICU and length of stay in the hospital in days, both in intensive care and in general wards, was analyzed in a complementary way, according to BMI and severity of infection.
Results
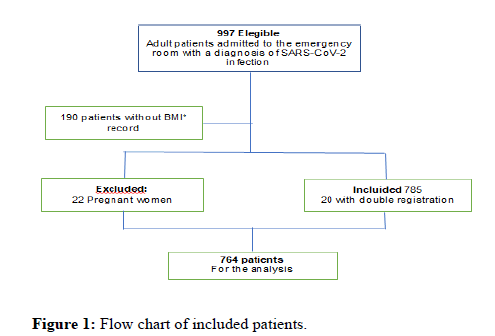
Of the initial 997 patients with SARS-CoV-2 infection that were admitted to the institution since 01.03.2021 to 31.01.2021, we took out 190 patients because BMI could not be calculated, 22 pregnant women and 20 patients with double chart, that required a second admision for clinical deterioration, were excluded. We kept the episode were clinical status was more compromised. Finally, 764 patients were analysed (Figure 1).
The average age of these patients was 48 years, with a range between 18 and 97 years. Overall, 53% were male patients (406/764). Our cohort had arterial hypertension (26.4%), diabetes mellitus (12.2%), chronic renal disease (6.2%) and any type of oncologic disease (5.9%). Of the mentioned comorbidities, arterial hypertension (P=0.003) and smoking (P=0.018) had a significantly higher prevalence in obese patients. The frequency and distribution of the other comorbidities and their relationship with BMI are shown in Table 1.
| Characteristics | General, n =764 | BMI | P value | ||
|---|---|---|---|---|---|
| Normal weight <25, n=256 | Overweight 25-29.9, n=312 | Obese >30, n=196 | |||
| Age (Years) | |||||
| Median (IQR) | 47.5 (33.5 -60) | 41 (28-60) | 52 (36-61) | 47 (35.5-57.5) | 0.0032 |
| Average ± SD | 48 ± 17.4 | 45.9 ± 20.2 | 49.8 ± 16.1 | 47.9 ± 15.04 | |
| Rank (Min-Max) | 18-97 | 18-97 | 20-94 | 18-87 | |
| Sex: Men, n (%) | 406 (53.1%) | 111 (43.3%) | 187 (59.9%) | 108 (55.1%) | 0 |
| Comorbidities, n (%) | |||||
| Hypertension | 202 (26.4%) | 52 (20.4%) | 82 (26.3%) | 68 (34.7%) | 0.003 |
| Diabetes | 93 (12.2%) | 23 (9%) | 38 (12.2%) | 32 (16.3%) | 0.061 |
| Cerebral vascular disease | 13 (1.7%) | 4 (1.6%) | 6 (1.9%) | 3 (1.5%) | 1 |
| Chronic obstructive pulmonary disease | 9 (1.2) | 2 (0.8%) | 3 (1%) | 4 (2%) | 1 |
| Asthma | 15 (2%) | 2 (0.8%) | 7 (2.2%) | 6 (3.1%) | 0.896 |
| Coronary heart disease | 20 (2.6%) | 7 (2.7%) | 9 (2.9%) | 4 (2%) | 0.672 |
| Atrial Fibrillation | 6 (0.8%) | 2 (0.8%) | 3 (1%) | 1 (0.5%) | 1 |
| Chronic kidney disease | 47 (6.2%) | 21 (8.2%) | 20 (6.4%) | 6 (3.1%) | 0.064 |
| Oncologic disease | 45 (5.9%) | 18 (7%) | 16 (5.1%) | 11 (5.6%) | 0.62 |
| Systemic Lupus Erythematosus | 5 (0.7%) | 3 (1.2%) | 1 (0.3%) | 1 (0.5%) | 1 |
| Rheumatoid arthritis | 1 (0.1%) | 0 (0%) | 1 (0.3%) | 0 (0%) | 0.143 |
| Vasculitis | 3 (0.4%) | 3 (1.2%) | 0 (0%) | 0 (0%) | 0.703 |
| Smoking | 18 (2.4%) | 3 (1.2%) | 9 (2.9%) | 6 (3.1%) | 0.018 |
| 2. Smoking cessation (>1 year) | 23 (3%) | 1 (0.4%) | 13 (4.2%) | 9 (4.6%) | |
| Organ transplant recipient | 36 (4.7%) | 14 (5.5%) | 15 (4.8%) | 7 (3.6%) | 0.039 |
| HIV infection | 4 (0.5%) | 1 (0.4%) | 0 (0%) | 3 (1.5%) | 0.085 |
| Home oxygen user | 7 (0.9%) | 5 (2%) | 2 (0.6%) | 0 (0%) | 0.393 |
| Previous medication | |||||
| Steroids | 46 (6%) | 18 (7%) | 18 (5.8%) | 10 (5.1%) | 0.415 |
| ACEIs | 13 (1.7%) | 5 (2%) | 4 (1.3%) | 4 (2%) | 0.777 |
| ARBs | 133 (17.4%) | 31 (12.1%) | 57 (18.3%) | 45 (23%) | 0.008 |
Values reported as absolute number (percentage). * Reported as median, IQR: Interquartile Range; ** Reported as mean (standard deviation). BMI: Body Mass Index reported in Kg/m2. HIV: Acquired Immunodeficiency Virus; ACEIs: Angiotensin Converting Enzyme Inhibitors; ARBs: Angiotensin Receptor Blockers
Table 1: General characteristics of the population with SARS-CoV-2 infection according to BMI.
In relation to the clinical presentation of COVID-19, the most frequent symptoms were cough (56.4%), fever (53.5%), fatigue (45.7%) and dyspnea (40.4%). The latter being the statistically significant manifestation in patients with higher significant BMI (P=0.001). Oxygen saturation at the admission was significantly less in obese patients (P=0.0001) as PAFI<200 (P=0.0002) (Table 2).
| Characteristics | General, n=764 | BMI | P value | ||
|---|---|---|---|---|---|
| Normal weight <25, n=256 | Overweight 25-29.9, n=312 | Obese >30, n =196 | |||
| Clinical manifestations, n (%) | |||||
| Dyspnea | 309 (40.4%) | 85 (33.2%) | 125 (40.1%) | 99 (50.5%) | 0.001 |
| Cough | 431 (56.4%) | 131 (51.2%) | 174 (55.8%) | 126 (64.3%) | 0.019 |
| Rhinorrhoea | 167 (21.9%) | 63 (24.6%) | 67 (21.5%) | 37 (18.9%) | 0.347 |
| Odinofagia | 23 (30.2%) | 76 (29.7%) | 98 (31.4%) | 57 (29.1%) | 0.841 |
| Asthenia | 349 (45.7%) | 104 (40.6%) | 143 (45.8%) | 102 (52%) | 0.055 |
| Arthralgia | 141 (18.5%) | 48 (18.8%) | 55 (17.6%) | 38 (19.4%) | 0.863 |
| Anorexia | 42 (5.5%) | 13 (5.1%) | 18 (5.8%) | 11 (5.6%) | 0.939 |
| Abdominal pain | 43 (5.6%) | 15 (5.9%) | 17 (5.4%) | 11 (5.6%) | 0.98 |
| Diarrhoea | 135 (17.7%) | 46 (18%) | 53 (17%) | 36 (18.4%) | 0.913 |
| Nausea | 29 (3.8%) | 9 (3.5%) | 15 (4.8%) | 5 (2.6%) | 0.444 |
| Vomiting | 30 (3.9%) | 11 (4.3%) | 11 (3.5%) | 8 (4.1%) | 0.891 |
| Taste dysfunction | 86 (11.3%) | 34 (13.3%) | 35 (11.2%) | 17 (8.7%) | 0.319 |
| Cephalea | 214 (28%) | 79% (30.9%) | 90 (28.8%) | 45 (23%) | 0.161 |
| Respiratory frequency | |||||
| >30 bpm | 122 (16%) | 29 (11.3%) | 44 (14.1%) | 49 (25%) | 0.001 |
| Oxigen saturation (%) | 96 (91-98) | 97 (94-98.5) | 96 (91-98) | 95 (87-98) | 0.0001 |
| PAFI | 271.43 (114-352.38) | 308.51 (124.955-390.475) | 289.29 (142.35-357.14) | 180.6 (91.5-02.6) | 0.0002 |
| D-Dimer | 0.79 (0.47-1.43) | 0.903 (0.46-1.42) | 0.79 (0.5-1.55) | 0.76 (0.44-1.3) | 0.3995 |
| Ferritin | 982 (487-1723) | 1083.5 (502-2467) | 892 (483-1623) | 1052 (474-1521) | 0.3791 |
| Fibrinogen | 598 (450-734) | 690 (451.5-752) | 567 (433-690) | 634.5 (489-805) | 0.3034 |
| SOFA score | 3 (2-5) | 2 (1-4) | 3 (2-4.5) | 3 (2-5) | 0.063 |
| APACHE II score | 10 (6-16) | 11 (7-17) | 9 (6-16) | 10 (7-14) | 0.5589 |
| Vasopressor | 112 (14.7%) | 27 (10.5%) | 39 (12.5%) | 46 (23.5%) | 0 |
| Inotropes | 32 (14.7%) | 11 (4.3%) | 9 (2.9%) | 12 (6.1%) | 0.18 |
Table 2: Clinical and laboratory characteristics of patients with SARS CoV 2 infection according to BMI at admission.
There was no statistically significant difference between the biomarkers (D-dimer, ferritin or fibrinogen) discriminated by BMI (P 0.2182).
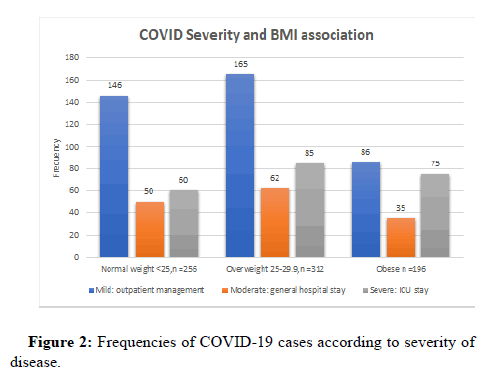
52% of the patients had a mild manifestation of the disease, while 19% and 29% had moderate and severe involvement, respectively. The proportion of patients with severe disease increased as BMI increases, being 27.2% (n=60) in the normal weight group, 27.2% (n=85) in the over-weight group and 38.3% (n=75), in the obese one (p 0.011), (Figure 2).
The presence of sepsis was observed with higher frequency and significantly in obese patients compared to over-weight and normal weight patients (P=0.022). Duration of MV was higher (P=0.0069) as length of general hospital (P=0.0031) and ICU (P=0.0093) stay (Table 3).
| Characteristics | General, n=764 | BMI | P value | ||
|---|---|---|---|---|---|
| Normal weight <25, n=256 | Overweight 25-29.9, n=312 | Obese >30, n=196 | |||
| Died in hospital, n (%) | 65 (8.5%) | 25 (8.7%) | 27 (8.7%) | 13 (6.6%) | 0.493 |
| Mechanical ventilation, n (%) | 128 (16,8%) | 31 (12.1%) | 45 (14.4%) | 52 (26.5%) | 0 |
| Duration in mechanical ventilation (days) | |||||
| Median (IQR) | 11 (6-20.5) | 6 (2-14) | 14 (8-25) | 12 (7-17) | 0.0069 |
| PE, n (%) | 10 (71.45) | 3 (75%) | 2 (66.7%) | 5 (71.4%) | 0 |
| Sepsis n (%) | 168 (22%) | 43 (10,8%) | 68 (21.8%) | 57 (29.1%) | 0.022 |
| Renal injury with RRT, n (%) | 56 (7.3%) | 12 (4.7%) | 23 (7.4%) | 21 (10.7%) | 0.256 |
| Myocarditis n (%) | 4 (0,5%) | 0 (0%) | 1 (0.3%) | 3 (1.5%) | 0.066 |
| Cardiac arrhythmias, n (%) | 25 (3.3%) | 6 (2.3%) | 11 (3.5%) | 8 (4.1%) | 0.577 |
| ICU stay, n (%) | 220 (28.8%) | 60 (23.4%) | 85 (27.2%) | 75 (38.3%) | 0.002 |
| Duration of ICU stay (days) | |||||
| Median (IQR) | 9 (5-19) | 6 (2-11) | 10 (5-19) | 10 (6-23) | 0.0093 |
| Global stay (days) | |||||
| Median (IQR) | 1 (0-8) | 0 (0-6) | 1 (0-8) | 2 (0-11) | 0.0031 |
Values reported as absolute number (percentage). * Reported as median (IQR: interquartile range) BMI: body mass index reported in Kg/m2. PE: Pulmonary Embolism. RRT: Renal Replacement Therapy
Table 3: Outcomes of patients with SARS-CoV-2 infection according to BMI at admission.
Complications as pulmonary embolism, myocarditis and cardiac arrhythmias had low frequency. Therefore, it was difficult to establish an association (P=0).
Discussion
COVID-19 disease is caused by coronavirus SARS-CoV-2. Which is characterized by a wide and variable clinical spectrum; ranging from asymptomatic to fatal infection [2]? The identification of risk factors at the time of diagnosis has allowed risk stratification and the establishment of appropriate management. Several risk factors have been described for the development of severe disease and mortality from COVID-19 among them being obesity, which is identified as an independent risk condition [3,11,12].
Obesity is a public health problem worldwide, and the global prevalence has been estimated at 1.9 billion adults, of which 39% are overweight and 13% are obese, with an annual increase in people with this diagnosis, constituting an epidemic worldwide [3]. In Colombia, according to the National Health Survey, 55.9% of the population is overweight and obese [8]. This is a metabolic disease characterized by increased fat tissue, insulin resistance, elevated glucose, alteration of adipokines and chronic inflammation [4]; alterations can predispose individuals to infections, and SARS-CoV-2 is no exception. A study using data from the UK Biobank (n=285, 817) showed that being overweight increases the risk of infection by this virus by 44% (RR=1.44; CI 1.09-1.92; p=0.01), and for obesity it almost doubles this value (RR=1.97; 95% CI 1.46-2.65) [5].
In our study, the average age of patients with SARS-CoV-2 infection was 48 years, similar to that reported in Wuhan [6], Germany and Switzerland [7] cohorts and lower than that reported in a New York series [6]. Regarding gender, a greater proportion was observed in men, 53%, which was lower than in the New York [9] and Wuhan [6] areas. A total of 25.65% of the cohort had a lower percentage of obesity than the reference series [10] similar to that in Switzerland [13]. The prevalence of arterial hypertension was found to be significantly associated with an increase in BMI [7].
In terms of clinical presentation, dyspnea was the most significant, occurring in a higher proportion in the obese population [3], while in previous series, cough and fever have been the predominant symptoms [14]. However, this observation could be related to lower oxygen saturation and PAFI on admission in these patients, which are parameters associated with greater severity, a situation that has been described in previous studies by an OR of 2.31 (95% CI of 1.3-4.12) [7].
Obesity has been documented to increase the risk of being hospitalized. In our study, 220 (29%) patients were admitted to the ICU. 27.2% (n=85) were overweight and 38.3% were obese. This result is slightly lower than that reported in the New York City cohort, in whom 42.7% of those hospitalized had obesity. In this regard, different publications have found an OR of 2.13 (95 CI, 1.74-2.60; p<0.0001) for hospitalization in obese patients [15]. Other analyses published to date describe that this pathology increases the risk of being admitted to the ICU by 68% (OR=1.68%; 95% CI, 1.46-2.08; p<0.0001) [14], in our case the higher the BMI, the greater the need for hospitalization in the ICU and overall stay in hospitalization. Gao et al, found a significant positive linear association between increased BMI and admission to the ICU for COVID-19. This could indicate an underlying biological association between weight gain and the risk of severe COVID-19 disease [16].
At the beginning of this pandemic, the documentation of acute kidney injury secondary to SARS-CoV-2 infection was widely variable [17]. However, it began to consolidate as a common outcome in several case series with high dialysis requirements, predominantly in populations outside of China and in patients hospitalized in the ICU [18]. However, there is insufficient information on the development of this BMI-guided outcome. In our study, no statistically significant association was found between a higher prevalence of kidney failure requiring replacement therapy and a higher BMI (P 0.256).
Reports of small samples have associated obesity with an increased risk of requiring IMV. One of the first reports in this regard was a study carried out in the city of Seattle in critical patients, which reported that 85% of obese patients required MV. Other studies have shown an increased risk for MV of 66% (OR=1.66%; 95% CI, 1.38-1.99;p<0.0001) and in our study, 75% of the patients on MV were overweight or obese, with a higher proportion having higher BMI; thus, 12.1% of the participants with normal weight required MV versus 43.8% of those with over-weight and obesity [19-21].
In relation to prognosis, the association described between SARSCoV- 2 infection and obesity is complex, and some reports have documented an increase in hospital mortality by up to 48% (OR=1.48%;95% CI, 1.22-1.80; p<0.0001). However, Pouwels et al. found no significant differences in 28-day mortality between patients with and without obesity. As well as Rao et al. who did not found that being overweight was a significant risk factor for mortality, either as a function of the duration from the onset of symptoms to the time of death or from hospital admission to the time of death. Our report does not allow us to determine such the association between mortality and obesity in patients with SARS-CoV-2 infection, since the differences found were not statistically significant (P=0.493).
The mechanisms underlying the association between obesity and severe COVID-19 outcomes remain elusive [16]. In most studies the association between mortality and obesity is determined in patients with SARS-CoV-2 infection, however, the subject should be expanded with clinical studies in this regard.
Conclusion
The COVID-19 disease quickly established itself as a global pandemic with a high morbidity and mortality rate. What forced to take hasty conducts for the treatment of these patients. Obesity is a risk factor for adverse outcomes in SARS VOC 2 infection. However, more studies are needed to explore factors that could be involved in mortality and morbidity results according to BMI, age, stage of the pandemic, and progress in vaccination.
References
- Baloch S, Baloch MA, Zheng T, Pei X (2020) The coronavirus disease 2019 (COVID-19) pandemic. Tohoku J Experimental Med 271–278.
[Crossref]
- Dong E, Du H, Gardner L (2020) An interactive web-based dashboard to track COVID-19 in real time. The Lancet Infect Dis 20:533–534.
[Crossref]
- Hales CM, Carroll MD, Fryar CD, Ogden CL (2015) Prevalence of Obesity Among Adults and Youth: United States, 2015-2016 Key findings Data from the National Health and Nutrition Examination Survey. 1-8. [Crossref]
- Rasouli N, Kern P (2008) Adipocytokines and the metabolic complications of obesity. J Clin Endocrinol Metabol 93: s64–s73.
[Crossref] [Google Scholar] [Indexed]
- Ho FK, Celis-Morales CA, Gray SR, Katikireddi SV, Niedzwiedz CL, et al. (2021) Modifiable and non-modifiable risk factors for COVID-19, and comparison to risk factors for influenza and pneumonia: results from a UK Biobank prospective cohort study. BMJ Open 10:40402.
[Crossref] [Google Scholar] [Indexed]
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395:497–506.
- Soeroto A, Soetedjo N, Purwiga A, Santoso P, Kulsum I, et al. (2021) Effect of increased BMI and obesity on the outcome of COVID-19 adult patients: A systematic review and meta-analysis. Diabetes Metab Syndr; 14:1897–904.
[Crossref] [Google Scholar] [Indexed]
- Goyal P, Choi J, Pinheiro L, Schenck E, Chen R, et al. (2020) Clinical Characteristics of COVID-19 in New York City. The New Engl J Med 382:2372–2374.
[Crossref] [Google Scholar] [Indexed]
- Yang J, Hu J, Zhu C (2021) Obesity aggravates COVID-19: A systematic review and meta-analysis. J Med Virol 93:257–61.
[Crossref] [Google Scholar] [Indexed]
- Romero-Corral A, Montori VM, Somers VK, Korinek J, Thomas RJ, et al. (2006) Association of bodyweight with total mortality and with cardiovascular events in coronary artery disease: a systematic review of cohort studies. The Lancet 368:666–678.
[Crossref] [Google Scholar] [Indexed]
- Kalligeros M, Shehadeh F, Mylona EK, Benitez G, Beckwith CG, et al. Association of Obesity with Disease Severity Among Patients with Coronavirus Disease 2019. Obesity (Silver Spring, Md) 28:1200–1204.
[Crossref] [Google Scholar] [Indexed]
- Gregoriano C, Koch D, Haubitz S, Conen A, Fux C, et al. (2020) Characteristics, predictors and outcomes among 99 patients hospitalised with COVID-19 in a tertiary care centre in Switzerland: an observational analysis. Swiss Medical Weekly 150:29–30.
- Popkin BM, Du S, Green WD, Beck MA, Algaith T, et al. (2020) Individuals with obesity and COVID‐19: A global perspective on the epidemiology and biological relationships. Obesity Reviews 21.
- Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, et al. (2020) Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA 323:2052–2059.
- Gao M, Piernas C, Astbury NM, Hippisley-Cox J, O’Rahilly S, et al. (2021) Associations between body-mass index and COVID-19 severity in 6.9 million people in England: a prospective, community-based, cohort study. The Lancet Diabetes Endocrinol 9:350–359.
- Yang S, Guo B, Ao L, Yang C, Zhang L, et al. (2020) Obesity and activity patterns before and during COVID‐19 lockdown among youths in China. Clin Obesity 10.
- Bruchfeld A. (2020) The COVID-19 pandemic: consequences for nephrology. Nature Rev Nephrol 17:81–82.
- Cai Q, Chen F, Wang T, Luo F, Liu X, et al. (2022) Obesity and COVID-19 Severity in a Designated Hospital in Shenzhen, China. Diabetes care 43: 1392–1398.
- Ebinger JE, Achamallah N, Ji H, Claggett BL, Sun N, Botting P, et al. (2020) Pre-existing traits associated with Covid-19 illness severity. PLOS ONE 15: e0236240.
- Merzon E, Tworowski D, Gorohovski A, Vinker S, Golan Cohen A, et al. (2020) Low plasma 25(OH) vitamin D level is associated with increased risk of COVID-19 infection: an Israeli population-based study. The FEBS J 287:3693–3702.
- Rao X, Wu C, Wang S, Tong S, Wang G, Wu G, et al. (2020) The importance of overweight in COVID-19. Medicine 99:e22766.
Citation: Ballen LJ, Urbano MA, Guzman GE, Feriz KM, Martínez V (2022) Characterization and Outcomes in Patients With SARS-Cov-2 Infection According to Body Mass Index, Colombia. J Obes Metab 5:131.
Copyright: © 2022 Ballen LJ, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Usage
- Total views: 1147
- [From(publication date): 0-2022 - Apr 07, 2025]
- Breakdown by view type
- HTML page views: 834
- PDF downloads: 313