Characteristics of Radiological Features and Laboratory Parameters in Patients with COVID-19 Pneumonia during Second Phase of Pandemic in Nepal: A Retroprospective Study
Received: 24-Feb-2022 / Manuscript No. JIDT-22-55417 / Editor assigned: 28-Feb-2022 / PreQC No. JIDT-22-55417 (PQ) / Reviewed: 14-Mar-2022 / QC No. JIDT-22-55417 / Revised: 21-Mar-2022 / Manuscript No. JIDT-22-55417 (R) / Published Date: 28-Mar-2022 DOI: 10.4172/2332-0877.1000498
Abstract
Objectives: The country Nepal was badly hit in second phase of epidemic due to highly transmitted Delta and Delta plus variant. The aim of this study was to investigate the different characteristics of chest HRCT, X-Ray and abnormalities in laboratory parameters of COVID-19 patients during second phase of epidemic.
Methods: COVID-19 Patients were admitted to COVID ward, Bharatpur Cancer Hospital. Patients underwent for Chest X-Ray or HRCT and laboratory tests for further evaluation were retrospectively analyzed. Patient without pneumonia were excluded from study. Statistical analysis was performed to evaluate the characteristics of Group-A (laboratory parameters with HRCT) and Group-B (laboratory parameters with X-Ray).
Results: A total number of 116 patients (72 males and 44 females, age range 3-90 years) were admitted to COVID ward. A total number of 67 patients in which 55 patients (Group A) and 12 patients (Group B) were included in the study. Different laboratory test were evaluated for all those 67 patients. In our study, typical and atypical appearances of HRCT was Ground-Glass Opacities (GGO), Crazy Paving, Consolidation, Bronchiectasis, Multifocal GGO, Bronchiectasis, Collapse and Fibrotic. In group A, CT severity of 11, 18 and 26 were mild, moderate and severe respectively. In Group B, 6 patients were mild and 6 were moderate. There were alteration in laboratory tests i.e., platelets, WBC, neutrophil, lymphocytes, eosinophil, monocyte, CRP, Glucose, Bilirubin Total, Bilirubin Direct, ALT, AST, and LDH were 43%, 40%, 85%, 89%, 42%, 74%, 95%, 61%, 10.9%, 14.5%, 85%, 63%, and 100% respectively.
Conclusion: There were mostly typical Ground-Glass-Opacities (GGO) appearances in HRCT chest with some atypical appearances. There was elevation in Neutrophil, CRP, Glucose, ALT, AST, and LDH whereas low counts in platelets, WBC, lymphocyte, eosinophil, and monocyte. Statistical correlation was found between laboratory analyses and amount of damaged lung. We concluded that symptomatic patients even with negative RT-PCR should be considered as COVID-19 patients if CT and biochemical tests are positive.
Keywords: COVID-19; SARS-CoV-2; RT-PCR; Biochemical Laboratory test
Introduction
First identified case of COVID-19 (named by WHO of Corona Virus Disease 2019) was found in Wuhan China which is an infectious disease caused by Severe Acute Respiratory Syndrome Corona Virus (SARS-CoV-2). Worldwide total diagnosed case of COVID-19 was 456,908,767 patients, global total death due to this disease was reported 6,041,077 till date March 14, 2022 [1]. It is increasing day by day worldwide. Some countries maintain the disciplined life style according to COVID-19 protocol and it seems that there is control of infection rate and death rate due to COVID-19 in those countries. Some countries are still badly affected and both the infection rate and death rate are too high.
The most common symptoms of COVID-19 are fever, dry cough, fatigue and other symptoms that are less common and may affect some patients include loss of smell or taste, nasal, headache, conjunctivitis, congestion, muscle or joint pain, sore throat, nausea or vomiting, different types of skin, rash, diarrhea and chills or dizziness [2]. Research study already reported that symptoms of patient with severe COVID-19 disease include loss of appetite, shortness of breath, confusion, persistent pain, high temperature (above 38°C) and other less common symptoms are irritability, reduced consciousness (sometimes associated with seizures), sleep disorder, depression, anxiety, more severe and rare neurological complications such as brain inflammation, strokes, nerve damage, and delirium [2]. These are not only all the possible symptoms. Center for Disease Control and Prevention (CDC) is continuing updating more and more about COVID-19. There are several studies reported that older age population, heart disease patient, lung disease patient and diabetic patient seems to be at higher risk for developing more serious complications due to COVID-19 illness [3]. It is concluded by many literatures that there are majority of asymptomatic COVID-19 patients. One literature concluded that about 15.6% of confirmed COVID-19 patients are asymptomatic [4]. It was also reported that nearly 50% of the patients with no symptoms at detection time will develop symptoms later and asymptomatic COVID-19 patients could have laboratory inaccuracy and swab collection techniques, storage of collected swab may be used as screening strategies to identify asymptomatic infection [4].
National government data showed that country Nepal is also badly hit by COVID-19. According to data updated by government of Nepal, total diagnosed case of COVID-19 was 977937 patients, total death due to this disease was reported 11950 and total 961070 patient were recovered in Nepal till date March 14, 2022 [5].
According to the different world official guidelines, patients infected with SARS-COV-2 virus must be went for nasopharyngeal or oropharyngeal RT-PCR swab test and Chest X-ray imaging for first step and for the further detail evaluation, HRCT chest and biochemical laboratory test are recommended in case of discrepancy between clinical and radiographic characteristics [6].
There are several studies which have reported that the sensitivity of nasopharyngeal or oropharyngeal Real-Time Reverse Transcriptase Polymerase Chain Reaction (RT-PCR) swab test applied to respiratory tract specimens are only 60% to 70% due to different technical reasons (reagents, sample transport conditions, etc.), intrinsic limitations like viral load in different anatomic sites and sampling procedures [7-9].
Some patients whose double-swab RT-PCR test was negative but had all the other symptoms positive for COVID-19, went for HRCT chest and diagnosed as an interstitial COVID pneumonia. This condition may complicate the management of patients suspected of COVID-19 infection so it is mandatory to evaluate other supporting diagnostic tool which help to differentiate COVD-19 infected and not infected patients.
There are several studies reported an outline of the most typical laboratory abnormalities parameters found in patients infected with SARS-COV-2 virus [10,11]. One retrospective study concluded that there is strong correlation between radiological characteristics (HRCT chest findings) and altered laboratory test results in COVID-19 patients [12]. One another study reported that biochemical laboratory parameters like serum ferritin and D-dimer levels found high in the CTpositive COVID-19 patients and it was moderate positive correlation with CT severity. The author suggested that D-dimer and ferritin levels measured were consideration to predict radiological severity [13].
Recently, several studied suggested that there is strong impact of types of SARS-COV-2 virus variant on all testing tools. One article reported on SARS-CoV-2 variant and its impact on diagnostic testing and author suggested that the ability of some molecular diagnostic assays to diagnose the Variant of Concern (VOC) may be affected by the mutations [14]. According to the press report on June 21, 2021 of Ministry of Health and Population of Nepal (MOHP), a total number of 48 double swab method confirmed COVID-19 positive samples were collected from different national laboratory of various region of country with various age group from May 9, 2021 to June 3, 2021. All the samples sent to WHO identified Center for Excellence in Geonomics, The Institute of Genomics and Integrative Biology (IGIB) for genomic sequencing of circulation of SARS-COV-2 variants in Nepal. Final result of testing concluded that among the 48 sample, one sample (2.08%) was diagnosed as an Alpha Variant (B.1.1.7) and 47 samples (97.91%) were diagnosed as a Delta Variant (B.1.617.2). Among all 47 samples, which were diagnosed as a Delta Variant, 9 samples (18.75%) were diagnosed as addition K417N mutation named as AY.1 Variant of Concern (VOC) [15]. According to second press release on July 27, 2021 of MOHP regarding circulation of different SARS-COV-2 variants in Nepal, total number of 47 double swab method confirmed COVID-19 positive samples were collected from different national laboratory of various region of country with various age group from May 29, 2021 to July 16, 2021 and sent for genomic sequencing to same center. Final result of testing concluded that all the 47 samples (100%) were diagnosed as a Delta Variant (B.1.617.2) and some samples were diagnosed as additional K417N mutation named as AY.1 Variant of Concern (VOC). Ministry of Health and Population Nepal additionally suggested that these prominent variants which are found in Nepal, are highly infectious and may infected all age groups people [15].
The aim of our study to evaluate the characteristics of Chest X-Ray, characteristics of HRCT Chest and abnormalities in laboratory biochemical parameters and their correlation during second phase of COVID-19 pandemic when there is circulation of Delta variant (B.1.617.2) and additionally mutated Delta variant (K417N) variant was prominent. Our aim of study was also to evaluate impacts of variants on the characteristics of Chest X-Ray, characteristics of HRCT Chest and abnormalities in laboratory biochemical parameters.
Methods
Study population: This retrospective and observational study was performed at the Department of Radio-diagnosis, Imaging and Nuclear Medicine of BP Koirala Memorial Cancer Hospital Bharatpur Nepal.
The study included consecutive symptomatic patients with suspected COVID-19 interstitial pneumonia who underwent chest HRCT and Chest X-Ray at Department of Radio-diagnosis, Imaging and Nuclear Medicine from May 16th to June 21st, 2021. Laboratory findings of each patient were collected from Hospital Information System (HIS) of hospital. HRCT Chest and Chest X-Ray were performed for the clinical evaluation and correlation.
In order to select chest HRCT scans for analysis, our exclusion criteria were:
a. Patient with negative chest HRCT for COVID-19 interstitial pneumonia;
b. Patient with negative chest X-Ray interstitial pneumonia;
c. Patient with any other laboratory test positive for other viral or bacterial infection.
d. Lack of complete reports (Missing of Laboratory parameters etc.)
CT protocol: Non-enhanced chest HRCT scan was performed in supine position, during inspiratory breath-hold, from the apex to the lung bases with multidetector scanner 64 slices (NeoSoft 64i Model, NeoSoft Company, China). Low-dose HRCT acquisition was executed as follows: tube voltage, 120 kV; automatic tube current control (40-90 mAs) was used; pitch, 1; collimation, 0.1 mm. Image data sets were reconstructed with 1 mm slice thickness.
HRCT protocol was used for image acquisition 1 mm collimation at 2 cm intervals in full inspiration, Measure field of view, High spatial frequency reconstruction algorithm, Full inspiration, Mediastinum Window 440 width, level 40, Lung Window 1400 width, level -700, lung filter 10/20 (NeoSoft 64i) and less than 1 second gantry rotation (depends on MAS selected).
Analysis of CT images: Two highly skilled and experience radiologists reviewed the CT images independently and resolved discrepancies by consensus. All images were viewed on both lung (width, 1400 Hounsfield Unit [HU]; level, -700 HU) and mediastinal (width, 440 HU; level, 40 HU) settings. The presence or absence of following features was recorded: Ground-Glass Opacities (GGO), Crazy Paving, Consolidation, Bronchiotasis, Multifocal GGO, Consolidation, Collapse, Fibrotic and multiple of above in one.
The images were analyzed according to CT severity score tool. The numbers of involved lung lobes were registered. The lobes of lung division were identified as the upper lobe (above the level of the carina), middle lobe (between carina and infra-pulmonary vein), and lower lobe (below the level of the infra-pulmonary vein). The axial allocation, were classified as peripheral (prevalent in the outer third of the lung) or central (predominant in the inner two-third). The allocation patterns were classified as diffuse when a clear predominant cranio-caudal or axial distribution was absent.
Chest X-Ray PA view protocol and analysis: Chest X-Ray PA view was conducted by positioned the x-ray source so that the X-Ray enters through the posterior portion (back) of the body and exits out of the anterior portion (front) of the body, where the beam is detected with detector. To obtain chest PA view, the patient should stand facing to an x-ray detector. A standard distance from radiation source to the body part should be 6 feet or 2 m.
The exposure should be made at full inspiration and should show 2 inch above the shoulder joint, both costophrenic angles and the lower parts of the diaphragm should be visible. The both lungs and vertebra should be visible behind the heart shadow. Two highly skilled and experience radiologists reviewed the X-Ray images independently and resolved discrepancies by consensus. For the analysis of chest X-Ray, we didn’t use any specific tool. We just used visual infected percentages of lung area and categorized as a) less than 25% infected lung as Mild b) less than 50% infected lung as moderate and c) more than 50% as severe.
Analysis of laboratory findings: There is COVID-19 RT-PCR laboratory in our hospital laboratory with highly specialized and modern equipment i.e., Quantum 3 and 5 Model RT-PCR laboratory equipment with 96-well of Thermo-Scientific Company. The protocol of RT-PCR assays targeting the RNA-dependent RNA polymerase (RdRp), Nucleocapsid (N) and Envelope (E) genes of SARS-CoV-2. All three RdRp, N and E genes were considered according to COVID-19 Prevention and Control Guideline from National Public Health Laboratory Nepal [16]. The cut off value of RT-PCR Ct value is 34 to distinguish positive and negative amplifications. Patients with all three positive RdRp, Positive N gene and Positive E genes were reported as SARSCoV- 2 infected patients. If patients tested RT-PCR negative but have all the related symptoms of COVID-19, were sent for further investigation like CT scan and other laboratory blood investigations to confirm it.
RT-PCR results of all patients were retrospectively evaluated and the patient with only 1 positive nasopharyngeal swab was identified as “positive” and patients with 2 negative swabs as “negative.”
Complete blood counts, biochemical parameters, and variables reflecting hepatic and renal functions on admission and data of follow-up laboratory tests during hospital stay were collected for each patient, including hemoglobin, leukocytes, platelets, neutrophil, lymphocytes, monocyte, eosinophil, basophil, C-Reactive Protein (CRP), Alanine Aminotransferase (ALT), Aspartate Aminotransferase (AST), blood urea, blood glucose random (R), sodium, potassium, Lactate Dehydrogenase (LDH) and serum Creatinine.
The considerations of normal and abnormal laboratory parameters of blood tests at this hospital are as follows (Table 1):
| SN | Laboratory parameters | Normal reference value |
|---|---|---|
| 1 | Hemoglobin | 13-17 g/dl |
| 2 | Platelets | 1,50,000-4,00,000/cumm |
| 3 | WBC | 4,000-10,000/cumm |
| 4 | Neutrophil | 40%-80% |
| 5 | Lymphocyte | 20%-40% |
| 6 | Eosinophil | 01%-06% |
| 7 | Monocyte | 02%–10% |
| 8 | CRP | Positive/Negative |
| 9 | Glucose Random | 50–130 mg/dl |
| 10 | Creatinine | 0.6-1.3 mg/dl |
| 11 | Urea | 15–40 mg/dl |
| 12 | Sodium | 136–145 mmol/L |
| 13 | Potassium | 3.5–5.1 mmol/L |
| 14 | Bilirubin Total | 0.2-1.0 mg/dl |
| 15 | Bilirubin Direct | 0.0-0.2 mg/dl |
| 16 | SGOT AST | 15-37 U/L |
| 17 | SGPT ALT | 14-63 U/L |
| 18 | LDH | 81-234 U/L |
Table 1: Showing normal reference value of laboratory parameters.
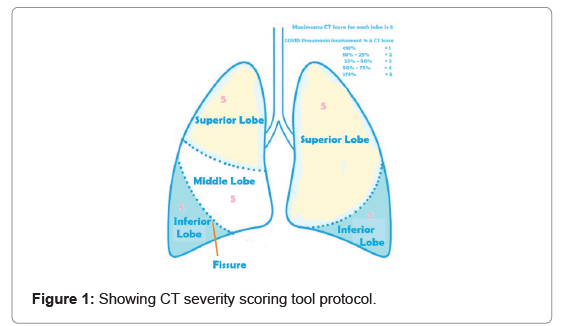
Statistical analysis: We followed chest CT severity score tool for assessing severe COVID-19 using the classification of European Society of Radiology- European Society of Thoracic Imaging (ESR-ESTI) [17]. The damage to lung lobe was measure in percentage. Each lobe of lung was given maximum CT severity score 5 according to percentage of lung lobe damage due to COVID pneumonia. The percentage of lung lobe damage and CT severity score was given as <10% for 1, 10% to 25% for 2, 25% to 50% for 3, 50% to 75% for 4 and >75% for 5 (Figure 1).
The Chest CT severity score calculated according to above tool. The severity of COVID patients were evaluated according to final CT score. If the total calculated CT score was less than 8, severity was diagnosed as mild. Similarly, if the total calculated score is in between 8 to 15 and above 15, severity was moderate and severe respectively (Table 2).
| CT Score | Severity |
|---|---|
| <8 | Mild |
| 8-15 | Moderate |
| >15 | Severe |
Table 2: Chest CT score verses severity
Descriptive statistics of all included patients variables are summarized as counts and percentages. We performed one sample t-test to find out significant of altered laboratory parameters and independent t-test compare the HRCT severity (Mild, Severe) with various performed laboratory parameters. We also performed Pearson correlation of CT severity score (1-25) with various altered laboratory parameters to evaluate significance of correlation between them.
We analyzed the percentage of abnormalities in laboratory parameters count of both groups (Group A and Group B). All statistical analyses were conducted using IBM SPSS Statistics 25 version.
Results
Patient’s populations
A total number of 116 patients (72 males and 44 females, age range 3-90 years, mean age 51.34 years) with RT-PCR swab test positive for COVID-19 were admitted to COVID ward at BP Koirala Memorial Cancer Hospital Bharatpur, Nepal from May 16th to June 21st, 2021, during second phase of COVID-19 pandemic. All the patients were evaluated either by chest X-Ray or chest HRCT at Department of Radio-diagnosis, Imaging and Nuclear Medicine and different laboratory parameters at Department of Pathology. Total numbers of 142 isolation beds are available at COVID Isolation Ward (General Beds-106, HDU Beds-26, ICU Beds-8 and Ventilator Beds-2) at our hospital. During the study period, total 22 patients died, 87 patients recovered from COVID-19 and discharged from COVID ward till date June 21st, 2021. Remaining 7 patients also recovered and discharged later. The outflows of SARS-COV-2 infected patients to COVID ward on daily basis from May 16th to June 21st, 2021 are summarized in Table 3.
| SN | Date | New admission | Pts. in ICU | Pts. in ventilator and HFNC | Pts. in HDU | Pts. in general bed | Total Pts. | Total death | Discharge |
|---|---|---|---|---|---|---|---|---|---|
| 1 | May 16, 2021 | 20 | 0 | 0 | 0 | 19 | 19 | 1 | 0 |
| 2 | May 17, 2021 | 14 | 3 | 0 | 0 | 28 | 31 | 2 | 0 |
| 3 | May 18, 2021 | 12 | 4 | 2 | 23 | 12 | 41 | 2 | 0 |
| 4 | May 19, 2021 | 8 | 6 | 2 | 25 | 12 | 43 | 3 | 3 |
| 5 | May 20, 2021 | 4 | 6 | 2 | 25 | 12 | 43 | 2 | 2 |
| 6 | May 21, 2021 | 9 | 6 | 0 | 19 | 23 | 48 | 0 | 4 |
| 7 | May 22, 2021 | 10 | 7 | 0 | 10 | 36 | 53 | 1 | 4 |
| 8 | May 23, 2021 | 6 | 7 | 0 | 18 | 34 | 59 | 0 | 0 |
| 9 | May 24, 2021 | 2 | 8 | 0 | 26 | 22 | 56 | 1 | 4 |
| 10 | May 25, 2021 | 0 | 8 | 0 | 24 | 18 | 51 | 0 | 5 |
| 11 | May 26, 2021 | 1 | 8 | 0 | 2 | 35 | 45 | 2 | 5 |
| 12 | May 27, 2021 | 4 | 8 | 0 | 2 | 26 | 44 | 2 | 3 |
| 13 | May 28, 2021 | 2 | 7 | 0 | 4 | 32 | 43 | 1 | 2 |
| 14 | May 29, 2021 | 4 | 6 | 0 | 4 | 29 | 43 | 1 | 3 |
| 15 | May 30, 2021 | 0 | 7 | 0 | 6 | 25 | 38 | 0 | 5 |
| 16 | May 31, 2021 | 0 | 5 | 0 | 5 | 23 | 30 | 0 | 8 |
| 17 | June 01, 2021 | 6 | 8 | 0 | 5 | 16 | 29 | 0 | 7 |
| 18 | June 02, 2022 | 0 | 5 | 0 | 5 | 18 | 28 | 0 | 1 |
| 19 | June 03, 2023 | 1 | 7 | 0 | 5 | 17 | 29 | 0 | 0 |
| 20 | June 04, 2024 | 2 | 4 | 0 | 5 | 17 | 26 | 1 | 4 |
| 21 | June 05, 2025 | 0 | 3 | 0 | 6 | 12 | 21 | 1 | 4 |
| 22 | June 06, 2026 | 0 | 3 | 0 | 6 | 10 | 19 | 1 | 1 |
| 23 | June 07, 2027 | 0 | 0 | 0 | 6 | 9 | 15 | 1 | 3 |
| 24 | June 08, 2028 | 2 | 1 | 0 | 5 | 10 | 16 | 0 | 1 |
| 25 | June 09, 2029 | 0 | 1 | 0 | 5 | 9 | 15 | 0 | 1 |
| 26 | June 10, 2030 | 0 | 1 | 0 | 4 | 7 | 12 | 0 | 3 |
| 27 | June 11, 2031 | 3 | 1 | 0 | 4 | 9 | 14 | 0 | 1 |
| 28 | June 12, 2032 | 0 | 1 | 0 | 4 | 9 | 14 | 0 | 0 |
| 29 | June 13, 2033 | 1 | 2 | 0 | 4 | 9 | 15 | 0 | 0 |
| 30 | June 14, 2034 | 0 | 0 | 0 | 0 | 11 | 11 | 0 | 4 |
| 31 | June 15, 2035 | 0 | 2 | 0 | 3 | 6 | 11 | 0 | 0 |
| 32 | June 16, 2036 | 1 | 1 | 0 | 2 | 6 | 9 | 0 | 3 |
| 33 | June 17, 2037 | 1 | 1 | 0 | 2 | 5 | 8 | 0 | 2 |
| 34 | June 18, 2038 | 3 | 1 | 0 | 4 | 4 | 9 | 0 | 2 |
| 35 | June 19, 2039 | 0 | 1 | 0 | 4 | 4 | 9 | 0 | 0 |
| 36 | June 20, 2040 | 0 | 0 | 0 | 4 | 3 | 7 | 0 | 2 |
| 37 | June 21, 2041 | 0 | 0 | 0 | 2 | 5 | 7 | 0 | 0 |
| Total | 116 | 7 | 22 | 87 |
Table 3: Showing new admission, total number of patients in ward, total death, and total discharge at COVID ward during the study. Note: Pts.: Patients; ICU: Intensive Care Unit; HFNC: High-Flow Nasal Cannula Oxygenation; HDU: Highly Dedicated Unit.
The common symptoms of patients were headache, body ache, muscle pain, stomach upset, nausea, fever and red eyes, etc. The major symptoms were pneumonia especially in adults. There were some rare symptoms like rashes, bluish discoloration of the fingers and toes, bleeding from the nose, confusion state and brain fog. We observed a lot more drastic drop in oxygen levels including silent hypoxia and the second wave is seeing a lot more symptomatic cases. The main cause of death was reported due to silent hypoxia (happy hypoxia) in COVID-19 patients in this hospital. The descriptive statistics of gender and age of total patients are summarized in Table 4 and frequency of number of COVID-19 patients with age group summarized in Table 5. Age group analysis of total COVID-19 patients showed that all age group of populations are infected with SARS-CoV-2 virus during second phase of pandemic in Nepal. The highest infected age groups were 30-40 years (16.38%), 40-50 years (12.93%), 50-60 years (27.58%) and 60-70 years (18.10) whereas the lowest age group was 00-10 years (1.72).
| Total patients number | 116 | ||
|---|---|---|---|
| Frequency | Percent% | ||
| Male | 72 | 62.1 | |
| Female | 44 | 37.9 | |
| Statistics of Age | |||
| Mean | 51.34 | ||
| Median | 53.5 | ||
| Std. Deviation | 17.588 | ||
| Variance | 309.321 | ||
| Range | 87 | ||
| Minimum | 3 | ||
| Maximum | 90 | ||
| Percentiles | 25 | 39 | |
| 50 | 53.5 | ||
| 75 | 62 | ||
Table 4: Statistics total patients.
| Age group (Years) | Total number of patients | Percentages (%) |
|---|---|---|
| 00-10 | 2 | 1.72 |
| Oct-20 | 4 | 3.45 |
| 20-30 | 9 | 7.76 |
| 30-40 | 19 | 16.38 |
| 40-50 | 15 | 12.93 |
| 50-60 | 32 | 27.58 |
| 60-70 | 21 | 18.1 |
| 70-80 | 9 | 7.76 |
| 80-90 | 5 | 4.31 |
| Total | 116 | 100 |
Table 5: Number of patients in age groups.
Only 67 patients out of total 116 were included in study due to incomplete or missing different data availability. A total number of 67 patients in which 55 patients (Group A) evaluated with High Resolution Computed Tomography (HRCT) scans and 12 patients (Group B) evaluated with Chest X-Ray for interstitial pneumonia, were included in the study. The reason behind making group B was some clinically stable patients were evaluated with chest X-Ray. Clinically unstable and sever patients were evaluated with HRCT chest. Different laboratory parameters including biochemistry and hematology blood tests reports were evaluated for all those 67 patients. Statistical characteristics of age and gender of both groups are summarized (Tables 6 and 7).
| Age | ||
|---|---|---|
| Total Patients | 55 | |
| Mean | 52.76 | |
| Median | 53.00 | |
| Mode | 58 | |
| Std. Deviation | 16.190 | |
| Variance | 262.110 | |
| Range | 70 | |
| Minimum | 20 | |
| Maximum | 90 | |
| Percentiles | 25 | 41.00 |
| 50 | 53.00 | |
| 75 | 62.00 | |
| Gender | ||
| Frequency | Percentage (%) | |
| Male | 32 | 58.2 |
| Female | 23 | 41.8 |
Table 6: Age and gender statistics (Group A).
| Age | ||
|---|---|---|
| Total Patients | 12 | |
| Mean | 50.42 | |
| Median | 55.50 | |
| Mode | 55a | |
| Std. Deviation | 18.058 | |
| Variance | 326.083 | |
| Range | 71 | |
| Minimum | 3 | |
| Maximum | 74 | |
| Percentiles | 25 | 41.00 |
| 50 | 55.50 | |
| 75 | 58.75 | |
| Gender | ||
| Frequency | Percentage (%) | |
| Male | 6 | 50.0 |
| Female | 6 | 50.0 |
Table 7: Age and gender statistics (Group B).
HRCT chest features and analysis
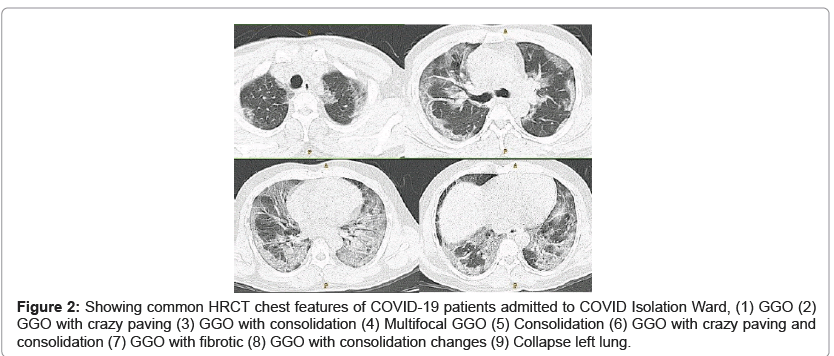
HRCT chest of SARS-CoV-2 infected patients were reported as following features Ground-Glass Opacities (GGO), Crazy Paving, Consolidation, Bronchiotasis, Multifocal GGO, Consolidation, Collapse, Fibrotic and multiple of above in one. In this study, we found all typical and some atypical appearances of COVID-19 interstitial pneumonia in HRCT chest i.e., Ground-Glass Opacities (GGO) (27.27%), GGO with Crazy Paving (29.09%), GGO with Consolidation (12.72%), GGO with crazy paving with consolidation (3.63%), Crazy paving with Bronchiotasis (1.81%), Multifocal GGO (5.45%), Consolidation (5.45%), GGO with Bronchiotasis (1.81%), Collapse (1.81%), GGO with Fibrotic (7.27%) and GGO with Fibrotic with crazy paving (3.63%). Among the 55 patients HRCT chest appearances, Highest number of 16 patients HRCT chest appearances were Ground-glass opacities with crazy paving (29.09%) and 15 HRCT chest appearances were only Ground-glass opacities (27.27). HRCT chest scan features of all 55 COVID patients are summarized in Table 8 and some typical and some atypical appearances HRCT chests of COVID-19 patients are shown in Figure 2.
| SN | HRCT appearances | Total patients | Percentages (%) |
|---|---|---|---|
| 1 | GGO | 15 | 27.27 |
| 2 | GGO+Crazy Paving | 16 | 29.09 |
| 3 | GGO+Consolidation | 7 | 12.72 |
| 4 | Crazy Paving+Bronchiectasis | 1 | 1.81 |
| 5 | Multifocal GGO | 3 | 5.45 |
| 6 | Consolidation | 3 | 5.45 |
| 7 | GGO+Crazy Paving +Consolidation | 2 | 3.63 |
| 8 | GGO+Bronchiectasis | 1 | 1.81 |
| 9 | Collapse | 1 | 1.81 |
| 10 | GGO+Fibrotic | 4 | 7.27 |
| 11 | GGO+Fibrotic+Crazy Paving | 2 | 3.63 |
| Total | 55 | 100 |
Table 8: HRCT appearances of COVID patients.
Figure 2: Showing common HRCT chest features of COVID-19 patients admitted to COVID Isolation Ward, (1) GGO (2) GGO with crazy paving (3) GGO with consolidation (4) Multifocal GGO (5) Consolidation (6) GGO with crazy paving and consolidation (7) GGO with fibrotic (8) GGO with consolidation changes (9) Collapse left lung.
Descriptive statistics of CT severity scores of all 55 patients (Group A) are summarized in Table 9. Maximum CT severity score 25 was also found in one patient and in 15 patients have CT severity score more than 20. Among total 55 patients HRCT, CT severity score of 11 (20%) patients had less than 8, 18 (32.7%) patients had in between 8 to 15 and 26 (47.3%) patients had more than 15. CT severity score and CT severity are summarized in Table 10.
| Total Patients | Range | Minimum | Maximum | Mean | Std. Deviation | Variance | ||
|---|---|---|---|---|---|---|---|---|
| Statistic | Statistic | Statistic | Statistic | Statistic | Std. Error | Statistic | Statistic | |
| HRCT Score | 55 | 24 | 1 | 25 | 13.65 | 0.994 | 7.369 | 54.304 |
Table 9: Descriptive statistics CT severity score of all 55 patient (Group A).
| Severity | CT Score group | Frequency | Percent | Valid Percent | Cumulative Percent |
|---|---|---|---|---|---|
| Mild | <8 | 11 | 20.0 | 20.0 | 20.0 |
| Moderate | 8-15 | 18 | 32.7 | 32.7 | 52.7 |
| Sever | >15 | 26 | 47.3 | 47.3 | 100.0 |
| Total | 55 | 100.0 | 100.0 |
Table 10: HRCT severity score and CT severity.
Cases
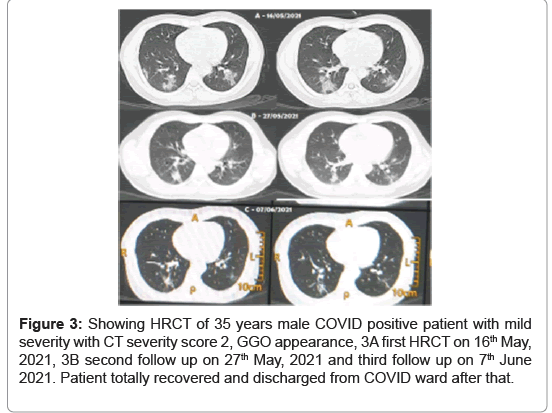
Some examples of mild and severe COVID-19 cases encountered to department of radio-diagnosis, imaging and nuclear medicine are shown in Figures 3-6. First case (Figures 3A-3C), 35 years male COVID positive patient (RT-PCR positive with interstitial pneumonia) admitted to COVID ward on 16th May, 2021. On the same day, HRCT and laboratory blood tests were done. HRCT chest was positive interstitial pneumonia with mild severity with CT severity score 2, GGO appearance diagnosed on 16th May, 2021 (Figure 3A). Second follow up HRCT was done on 27th May, 2021 which shows reduction in chest infection (Figure 3B) and third follow up on 7th June 2021 (Figure 3C) which shows huge reduction in chest infection. Patient totally recovered COVID-19 and discharged from COVID ward after that.
Figure 3: Showing HRCT of 35 years male COVID positive patient with mild severity with CT severity score 2, GGO appearance, 3A first HRCT on 16th May, 2021, 3B second follow up on 27th May, 2021 and third follow up on 7th June 2021. Patient totally recovered and discharged from COVID ward after that.
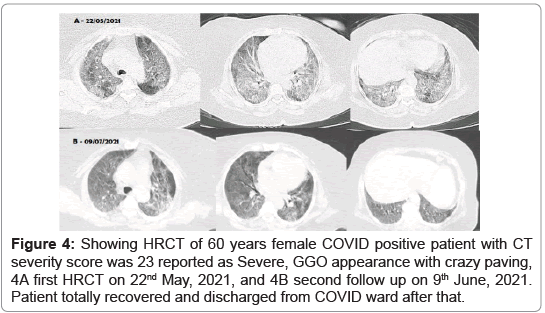
Second case (Figure 4), 60 years female COVID positive patient (RTPCR positive with interstitial pneumonia) admitted to COVID ward on 22nd May, 2021. On the same day, HRCT and laboratory blood tests were done. HRCT chest was positive interstitial pneumonia with severe severity with CT severity score 23, GGO with crazy paving appearance diagnosed on 22nd May, 2021 (Figure 4A). Second follow up HRCT was done on 9th June, 2021 which shows huge reduction in chest infection (Figure 4B). Patient totally recovered COVID-19 and discharged from COVID ward after that.
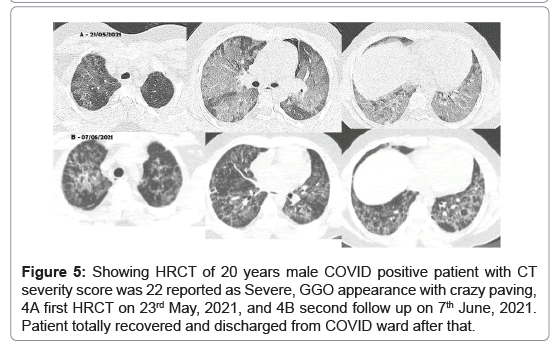
Third case (Figure 5), 20 years male COVID positive patient (RT-PCR positive with interstitial pneumonia) admitted to COVID ward on 23rd May, 2021. On the same day, HRCT and laboratory blood tests were done. HRCT chest was positive interstitial pneumonia with worse severity with CT severity score 22, GGO with crazy paving appearance diagnosed on 23rd May, 2021 (Figure 5A). Second follow up HRCT was done on 7th June, 2021 which shows magnificent reduction in chest infection (Figure 5B). Patient totally recovered COVID-19 and discharged from COVID ward after that.
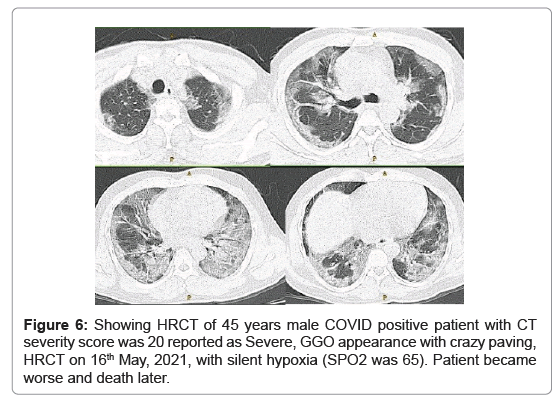
Fourth case (Figure 6), 45 years male COVID positive patient (RT-PCR positive with interstitial pneumonia) admitted to COVID ward on 16th May, 2021. On the same day, HRCT and laboratory blood tests were done. HRCT chest was positive interstitial pneumonia with worse severity with CT severity score 20, GGO with crazy paving appearance diagnosed on 16th May, 2021. Second follow up HRCT was done on 7th June, 2021 which shows magnificent reduction in chest infection. Patient had silent hypoxia (SPO2 was 65). Patient became worse and death later.
Chest X-Ray analysis
A total number of 12 clinically stable COVID-19 patients were evaluated with chest X-Ray. There was no death reported in this group (Group B). Only rough analysis of chest X-Ray was done, we didn’t use any specific tool. We just used visual infected percentages of lung area and categorized as a) less than 25% infected lung as Mild b) less than 50% infected lung as moderate and c) more than 50% as Severe. The Group B is very small in sample size and 6 (50%) patients’ chest X-Ray infection severity were mild and remaining 6 patients' chest X-Ray infection severity were moderate. Statistical analyses of severity seen in chest X-Ray of all patients are summarized in Table 11.
| Frequency | Percent | Valid Percent | Cumulative Percent | |
|---|---|---|---|---|
| Mild | 6 | 50.0 | 50.0 | 50.0 |
| Moderate | 6 | 50.0 | 50.0 | 100.0 |
| Total | 12 | 100.0 | 100.0 |
Table 11: Percentage severity of Group B patients.
Laboratory parameters
Laboratory parameters of all COVID-19 patients admitted to COVID ward like complete blood counts, biochemical parameters, and variables reflecting hepatic and renal functions on admission and data of followup laboratory tests during hospital stay were done for each patient. Main laboratory parameters which were included in our study were hemoglobin, leukocytes, platelets, neutrophil, lymphocytes, monocyte, eosinophil, basophil, C-Reactive Protein (CRP), Alanine Aminotransferase (ALT), Aspartate Aminotransferase (AST), blood urea, blood glucose Random (R), sodium, potassium, Lactate Dehydrogenase (LDH) and serum Creatinine.
The detailed of laboratory parameters results (Group A) are tabulated in Table 12. The abnormalities found in laboratory tests i.e., hemoglobin, platelets, leukocytes (WBC), neutrophil, lymphocyte, eosinophil, monocyte, C-Reactive Protein (CRP), Glucose Random (R), Urea, Creatinine, Sodium, Potassium, Bilirubin Total, Bilirubin Direct, Alanine Aminotransferase (ALT), Aspartate Aminotransferase (AST), and Lactate Dehydrogenase (LDH) were 20%, 43%, 40%, 85%, 89%, 42%, 74%, 95%, 61%, 29%, 10%, 1.8%, 1.8%, 10.9%, 14.5%, 85%, 63%, and 100% respectively. There was decline in following laboratory parameters counts i.e., hemoglobin, platelets, leukocytes (WBC), lymphocytes, eosinophil, monocyte. Among the total 55 patients, 95% patients with positive C-Reactive Protein (CRP) were found. There was elevation in following laboratory parameters counts found i.e., Neutrophil, C-Reactive Protein (CRP), Glucose Random (R), Urea, Creatinine, Bilirubin Total, Bilirubin Direct, Alanine Aminotransferase (ALT), Aspartate Aminotransferase (AST), and Lactate Dehydrogenase (LDH). We thought alteration in following laboratory tests i.e., hemoglobin, urea, Creatinine, sodium and potassium may be acceptable.
| Laboratory Parameters | Total Patients | Normal | High or Low | Abnormal (%) |
|---|---|---|---|---|
| Hemoglobin | 55 | 44 | Low-11 | 20% |
| Platelets | 55 | 31 | Low-24 | 43% |
| WBC | 55 | 33 | Low-22 | 40% |
| Neutrophil | 55 | 8 | High-47 | 85% |
| Lymphocyte | 55 | 6 | Low-49 | 89% |
| Eosinophil | 55 | 13 | Low-42 | 42% |
| Monocyte | 55 | 14 | Low-41 | 74% |
| CRP | 55 | 3 | High-52 | 95% |
| Glucose | 55 | 21 | High-34 | 61% |
| Urea | 55 | 39 | High-16 | 29% |
| Creatinine | 55 | 49 | High-06 | 10.90% |
| Sodium | 55 | 54 | High-01 | 1.80% |
| Potassium | 55 | 54 | High-01 | 1.80% |
| Bilirubin Total | 55 | 49 | High-06 | 10.90% |
| Bilirubin Direct | 55 | 47 | High-08 | 14.50% |
| SGOT AST | 55 | 8 | High-47 | 85.50% |
| SGPT ALT | 55 | 20 | High-35 | 63.60% |
| LDH | 55 | 0 | High-55 | 100% |
Table 12: Abnormalities in laboratory parameters (Group A).
One Sample t-test of all laboratory parameters (Group A) with average reference test value was conducted and the results are summarized in Table 13. There were significant differences found in most laboratory parameters.
| Laboratory Parameters | Total Patients | Sample Test Mean | Reference Test Value | t-Value | Degree of Freedom (df) | Sig. (2-tailed) (p-Value) |
95% Confidence Interval of the Difference |
|---|---|---|---|---|---|---|---|
| Hemoglobin | 55 | 12.502 | 15 | -9.671 | 54 | 0.000 | -3.0160 to -1.980 |
| Platelets | 55 | 213408.33 | 275000 | -3.802 | 54 | 0.000 | -94073.73 to -29109.62 |
| WBC | 55 | 13201.82 | 5500 | 2.106 | 54 | 0.040 | 369.50 to 15034.13 |
| Neutrophil | 55 | 81.24 | 60 | 12.116 | 54 | 0.000 | 17.72 to 24.75 |
| Lymphocyte | 55 | 15.16 | 30 | -15.126 | 54 | 0.000 | -16.80 to -12.87 |
| Eosinophil | 55 | 0.47 | 03 | -18.130 | 54 | 0.000 | -2.81 to -2.25 |
| Monocyte | 55 | 1.91 | 06 | -24.841 | 54 | 0.000 | -4.42 to -3.76 |
| CRP | 55 | 1.95 | 1 | 30.594 | 54 | 0.000 | 0.88 to 1.01 |
| Glucose | 55 | 188.62 | 90 | 6.089 | 54 | 0.000 | 66.15 to 131.09 |
| Urea | 55 | 37.96 | 27 | 3.260 | 54 | 0.002 | 4.22 to 17.71 |
| Creatinine | 55 | 0.8296 | 0.70 | 3.059 | 54 | 0.003 | 0.0447 to 0.2146 |
| Sodium | 55 | 134.34 | 140 | -6.904 | 54 | 0.000 | -7.2956 to -4.0120 |
| Potassium | 55 | 4.1844 | 3.8 | 3.900 | 54 | 0.000 | 0.1868 to 0.5820 |
| Bilirubin Total | 55 | 1.1955 | 0.2 | 2.020 | 54 | 0.048 | 0.0077 to 1.9832 |
| Bilirubin Direct | 55 | 0.4076 | 0.11 | 1.208 | 54 | 0.232 | -0.1964 to 0.7916 |
| SGOT AST | 55 | 107.49 | 26 | 2.179 | 54 | 0.034 | 6.51 to 156.47 |
| SGPT ALT | 55 | 106.22 | 35 | 3.230 | 54 | 0.002 | 27.01 to 115.43 |
| LDH | 55 | 597 | 157 | 11.375 | 54 | 0.000 | 362.63 to 517.81 |
Table 13: One sample t-test of all laboratory parameters with average test value (Group A). Note: 2-tailed significant value was conducted at the 0.05.
The laboratory test results of Group B patients are summarized in Table 14. The abnormalities found in laboratory tests i.e., hemoglobin, platelets, leukocytes (WBC), neutrophil, lymphocyte, monocyte, Glucose Random (R), Urea, Creatinine, Sodium, Potassium, Bilirubin Total, Bilirubin Direct, Alanine Aminotransferase (ALT), Aspartate Aminotransferase (AST), and Lactate Dehydrogenase (LDH) were 58.3%, 50.0%, 50.0%, 33.3%, 25.0%, 66.7%, 41.7%, 33.0%, 25.0%, 0%, 0%, 8.3%, 8.3%, 91.7%, 25.5%, and 100% respectively. There was significant decline in following laboratory parameters counts i.e., hemoglobin, platelets, leukocytes (WBC), lymphocytes, monocyte. There was elevation found in following laboratory parameters counts i.e., Glucose random (R), Urea, Creatinine, Bilirubin Total, Bilirubin Direct, Alanine Aminotransferase (ALT), Aspartate Aminotransferase (AST), and Lactate Dehydrogenase (LDH).
| Laboratory Parameters | Total Patients | Normal | High or Low | Abnormal (%) |
|---|---|---|---|---|
| Hemoglobin | 12 | 5 | Low-7 | 58.3% |
| Platelets | 12 | 6 | Low-6 | 50.0% |
| WBC | 12 | 6 | Low-5 High-1 |
50% |
| Neutrophil | 12 | 8 | High-2 Low-2 |
33.3% |
| Lymphocyte | 12 | 9 | Low-3 | 25% |
| Monocyte | 12 | 4 | Low-8 | 66.7% |
| Glucose | 12 | 7 | High-5 | 41.7% |
| Urea | 12 | 8 | High-4 | 33% |
| Creatinine | 12 | 9 | High-3 | 25% |
| Sodium | 12 | 12 | High-0 | 0% |
| Potassium | 12 | 12 | High-0 | 0% |
| Bilirubin Total | 12 | 11 | High-1 | 8.3% |
| Bilirubin Direct | 12 | 11 | High-1 | 8.3% |
| SGOT AST | 12 | 1 | High-11 | 91.7% |
| SGPT ALT | 12 | 9 | High-3 | 25% |
| LDH | 12 | 0 | High-12 | 100% |
Table 14: Abnormalities in laboratory parameters (Group B).
One Sample t-test of all laboratory parameters (Group B) with average reference test value were conducted with the help of IMB SPSS 25 version and the results are summarized in Table 15. There were significant differences found in most of laboratory parameters. Besides that, due to the small sample sizes in group B, significant may not present great impact.
| Laboratory Parameters | Total Patients | Sample Test Mean | Reference Test Value | t-Value | Degree of Freedom (df) | Sig. (2-tailed) (p-Value) |
95% Confidence Interval of the Difference |
|---|---|---|---|---|---|---|---|
| Hemoglobin | 12 | 11.808 | 15 | -4.342 | 11 | 0.001 | -4.809 to -1.574 |
| Platelets | 12 | 138416.67 | 275000 | -7.629 | 11 | 0.000 | -175986.00 to –97180.67 |
| WBC | 12 | 18033.33 | 5500 | 0.956 | 11 | 0.360 | -16325.24 to 41391.90 |
| Neutrophil | 12 | 65.92 | 60 | 0.758 | 11 | 0.465 | -11.27 to 23.11 |
| Lymphocyte | 12 | 21.83 | 30 | -3.781 | 11 | 0.003 | -12.92 to -3.41 |
| Monocyte | 12 | 1.33 | 06 | -32.833 | 11 | 0.000 | -4.98 to -4.35 |
| Glucose | 12 | 113.33 | 90 | 1.699 | 11 | 0.117 | -6.89 to –53.56 |
| Urea | 12 | 35.75 | 27 | 1.256 | 11 | 0.235 | -6.58 to 24.08 |
| Creatinine | 12 | 2.9583 | 0.70 | 1.176 | 11 | 0.264 | -1.9681 to 6.4848 |
| Sodium | 12 | 134.86 | 140 | -3.785 | 11 | 0.003 | -8.1148 to –2.1469 |
| Potassium | 12 | 3.93 | 3.8 | 1.082 | 11 | 0.302 | -0.1344 to 0.3944 |
| Bilirubin Total | 6 | 0.7233 | 0.2 | 3.835 | 5 | 0.012 | 0.2023 to 1.0244 |
| Bilirubin Direct | 5 | 0.22 | 0.11 | 1.117 | 4 | 0.327 | -0.1754 to 0.4114 |
| SGOT AST | 12 | 57.75 | 26 | 4.822 | 11 | 0.001 | 17.2585 to 46.2415 |
| SGPT ALT | 12 | 58.66 | 35 | 2.399 | 11 | 0.035 | 1.9500 to 45.3833 |
| LDH | 12 | 616 | 157 | 8.411 | 11 | 0.000 | 339.56 to 580.27 |
Table 15: One sample t-test of all laboratory parameters with average test value (Group B). Note: 2-tailed significant value was conducted at the 0.05.
Correlations and regression
There was two different correlation technique conducted for Chest CT severity with all laboratory parameters (Group A). a) Compare mean Independent Samples t-test for Chest CT interstitial pneumonia severity (correlation of nominal variables with scale variables) with all laboratory parameters, b) Pearson Correlation t-test for Chest CT severity score (correlation of scale variables with scale variables) with all laboratory parameters. For the correlation of Chest X-Ray pneumonia severity, compare mean Independent Samples t-test with all laboratory parameters (correlation of nominal variables with scale variables) was conducted.
Chest CT severity (Mild, Severe) was compared with all laboratory parameters by using compare mean Independent Samples t-test which is summarized in Tables 16,17. The result showed overall a good correlation of chest CT interstitial pneumonia severity with most of laboratory parameters. There was significant correlation of Chest CT interstitial pneumonia severity with following laboratory parameters i.e., platelets, leukocytes (WBC), neutrophil, lymphocytes, monocyte, glucose Random (R), Bilirubin total, Bilirubin direct, Alanine Aminotransferase (ALT), Aspartate Aminotransferase (AST), and Lactate Dehydrogenase (LDH) in Group A patients.
| HRCT Severity | N | Mean | Std. Deviation | Std. Error Mean | |
|---|---|---|---|---|---|
| Hemoglobin | Mild | 11 | 11.427 | 1.6493 | 0.4973 |
| Severe | 26 | 12.465 | 1.9964 | 0.3915 | |
| Platelets | Mild | 11 | 266909.82 | 150760.360 | 45455.959 |
| Severe | 26 | 214648.08 | 120476.587 | 23627.403 | |
| WBC | Mild | 11 | 7763.64 | 4269.256 | 1287.229 |
| Severe | 26 | 19673.08 | 38560.794 | 7562.394 | |
| Neutrophil | Mild | 11 | 77.27 | 9.941 | 2.997 |
| Severe | 26 | 80.42 | 16.843 | 3.303 | |
| Lymphocyte | Mild | 11 | 19.91 | 6.978 | 2.104 |
| Severe | 26 | 14.08 | 7.929 | 1.555 | |
| Eosinophil | Mild | 11 | 1.00 | 1.549 | 0.467 |
| Severe | 26 | 0.58 | 1.027 | 0.201 | |
| Monocyte | Mild | 11 | 2.27 | 1.737 | 0.524 |
| Severe | 26 | 1.54 | 0.706 | 0.138 | |
| Glucose | Mild | 11 | 169.82 | 74.853 | 22.569 |
| Severe | 26 | 190.85 | 116.540 | 22.855 | |
| Urea | Mild | 11 | 43.82 | 45.963 | 13.858 |
| Severe | 26 | 37.08 | 14.824 | 2.907 | |
| Creatinine | Mild | 11 | 0.9536 | 0.55929 | 0.16863 |
| Severe | 26 | 0.7523 | 0.20806 | 0.04080 | |
| Sodium | Mild | 11 | 133.2000 | 10.66637 | 3.21603 |
| Severe | 26 | 134.1508 | 4.68992 | .91977 | |
| Potassium | Mild | 11 | 4.3073 | 0.49713 | 0.14989 |
| Severe | 26 | 4.2358 | 0.72571 | 0.14232 | |
| Bilirubin Total | Mild | 11 | 3.3464 | 8.08281 | 2.43706 |
| Severe | 26 | .6038 | 0.19785 | 0.03880 | |
| Bilirubin Direct | Mild | 11 | 1.5391 | 4.02996 | 1.21508 |
| Severe | 26 | .1158 | 0.05300 | 0.01039 | |
| SGOT AST | Mild | 11 | 241.82 | 616.678 | 185.935 |
| Severe | 26 | 80.50 | 59.805 | 11.729 | |
| SGPT ALT | Mild | 11 | 149.09 | 311.308 | 93.863 |
| Severe | 26 | 104.96 | 127.465 | 24.998 | |
| LDH | Mild | 11 | 586.45 | 342.731 | 103.337 |
| Severe | 26 | 636.62 | 303.932 | 59.606 |
Table 16: Correlation of CT severity with laboratory parameters (Group A).
| Levene's Test for Equality of Variances | t-test for Equality of Means | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| F | Sig. | t | df | Sig. (2-tailed) | Mean Difference | Std. Error Difference | 95% Confidence Interval of the Difference | |||
| Lower | Upper | |||||||||
| Hemoglobin | EVA | .055 | .816 | -1.516 | 35 | 0.138 | -1.0381 | .6847 | -2.4282 | .3519 |
| EVNA | -1.640 | 22.744 | 0.115 | -1.0381 | .6329 | -2.3482 | .2720 | |||
| Platelets | EVA | .894 | .351 | 1.119 | 35 | 0.271 | 52261.741 | 46705.259 | -42554.976 | 147078.459 |
| EVNA | 1.020 | 15.676 | 0.323 | 52261.741 | 51229.858 | -56523.527 | 161047.010 | |||
| EVA | 2.690 | .110 | -1.014 | 35 | 0.318 | -11909.441 | 11750.650 | -35764.528 | 11945.647 | |
| EVNA | -1.552 | 26.414 | 0.132 | -11909.441 | 7671.164 | -27665.719 | 3846.838 | |||
| Neutrophil | EVA | 0.714 | 0.404 | -0.576 | 35 | 0.568 | -3.150 | 5.465 | -14.245 | 7.945 |
| EVNA | -0.706 | 30.844 | 0.485 | -3.150 | 4.460 | -12.249 | 5.948 | |||
| Lymphocyte | EVA | 0.539 | 0.468 | 2.114 | 35 | 0.042 | 5.832 | 2.759 | 0.232 | 11.432 |
| EVNA | 2.229 | 21.361 | 0.037 | 5.832 | 2.616 | 0.397 | 11.267 | |||
| Eosinophil | EVA | 1.117 | 0.298 | 0.981 | 35 | 0.333 | 0.423 | 0.431 | -0.453 | 1.299 |
| EVNA | 0.832 | 13.869 | 0.420 | 0.423 | 0.509 | -0.669 | 1.515 | |||
| Monocyte | EVA | 3.653 | 0.064 | 1.849 | 35 | 0.073 | 0.734 | 0.397 | -0.072 | 1.540 |
| EVNA | 1.355 | 11.424 | 0.202 | 0.734 | 0.542 | -0.453 | 1.921 | |||
| Glucose | EVA | 1.884 | 0.179 | -0.550 | 35 | 0.586 | -21.028 | 38.238 | -98.655 | 56.599 |
| EVNA | -0.655 | 28.879 | 0.518 | -21.028 | 32.121 | -86.734 | 44.678 | |||
| Urea | EVA | 4.998 | 0.032 | 0.680 | 35 | 0.501 | 6.741 | 9.919 | -13.396 | 26.879 |
| EVNA | 0.476 | 10.891 | 0.643 | 6.741 | 14.160 | -24.463 | 37.945 | |||
| Creatinine | EVA | 2.040 | 0.162 | 1.614 | 35 | 0.116 | 0.20133 | 0.12475 | -0.05193 | 0.45458 |
| EVNA | 1.160 | 11.190 | 0.270 | 0.20133 | 0.17350 | -0.17975 | 0.58241 | |||
| Sodium | EVA | 5.733 | 0.022 | -0.381 | 35 | 0.706 | -0.95077 | 2.49757 | -6.02111 | 4.11958 |
| EVNA | -0.284 | 11.672 | 0.781 | -0.95077 | 3.34497 | -8.26165 | 6.36011 | |||
| Potassium | EVA | 0.052 | 0.820 | 0.297 | 35 | 0.768 | 0.07150 | 0.24042 | -0.41658 | 0.55958 |
| EVNA | 0.346 | 27.288 | 0.732 | 0.07150 | 0.20670 | -0.35239 | 0.49540 | |||
| Bilirubin Total | EVA | 11.203 | 0.002 | 1.764 | 35 | 0.087 | 2.74252 | 1.55515 | -0.41460 | 5.89963 |
| EVNA | 1.125 | 10.005 | 0.287 | 2.74252 | 2.43737 | -2.68790 | 8.17294 | |||
| Bilirubin Direct | EVA | 11.504 | 0.002 | 1.837 | 35 | 0.075 | 1.42332 | 0.77496 | -0.14993 | 2.99657 |
| EVNA | 1.171 | 10.001 | 0.269 | 1.42332 | 1.21512 | -1.28409 | 4.13073 | |||
| SGOT AST | EVA | 9.188 | 0.005 | 1.345 | 35 | 0.187 | 161.318 | 119.947 | -82.187 | 404.823 |
| EVNA | 0.866 | 10.080 | 0.407 | 161.318 | 186.305 | -253.351 | 575.987 | |||
| SGPT ALT | EVA | 3.189 | 0.083 | 0.619 | 35 | 0.540 | 44.129 | 71.299 | -100.616 | 188.874 |
| EVNA | 0.454 | 11.446 | 0.658 | 44.129 | 97.135 | -168.651 | 256.910 | |||
| LDH | EVA | 0.224 | 0.639 | -0.442 | 35 | 0.661 | -50.161 | 113.481 | -280.540 | 180.218 |
| EVNA | -0.420 | 17.008 | 0.679 | -50.161 | 119.296 | -301.844 | 201.522 | |||
Table 17: Correlation of CT severity with laboratory parameters of group A (Independent Samples t-Test).
Chest CT interstitial pneumonia severity score (1-25) was compared with all laboratory parameters by Pearson Correlation test which is summarized in Table 18. The result showed overall a greater correlation of Chest CT interstitial pneumonia severity with most of laboratory parameters. There was significant correlation of Chest CT interstitial pneumonia severity with following laboratory parameters i.e., platelets, leukocytes (WBC), neutrophil, lymphocyte, monocyte, glucose Random (R), Bilirubin Total, Bilirubin Direct, Alanine Aminotransferase (ALT), Aspartate Aminotransferase (AST), and Lactate Dehydrogenase (LDH) in Group A patients.
| Laboratory Parameters | Total Patients | Pearson Correlation | Sig. (2-tailed) |
|---|---|---|---|
| Hemoglobin | 55 | 0.038 | 0.781 |
| Platelets | 55 | 0.053 | 0.703 |
| WBC | 55 | 0.2.1 | 0.140 |
| Neutrophil | 55 | 0.044 | 0.750 |
| Lymphocyte | 55 | 0.261 | 0.054 |
| Eosinophil | 55 | 0.061 | 0.659 |
| Monocyte | 55 | 0.316 | 0.016 |
| Glucose | 55 | 0.006 | 0.963 |
| Urea | 55 | 0.019 | 0.893 |
| Creatinine | 55 | 0.241 | 0.077 |
| Sodium | 55 | 0.108 | 0.433 |
| Potassium | 55 | 0.053 | 0.699 |
| Bilirubin Total | 55 | 0.261 | 0.054 |
| Bilirubin Direct | 55 | 0.264 | 0.051 |
| SGOT AST | 55 | 0.093 | 0.499 |
| SGPT ALT | 55 | 0.008 | 0.956 |
| LDH | 55 | 0.100 | 0.466 |
Note: Correlation is significant at the 0.05 level (2-tailed).
Table 18: Pearson Correlation of CT severity score with Laboratory Parameters (Group A).
The logistic linear regression of chest CT severity score of interstitial pneumonia with all parameters were conducted and the results are summarized in Table 19 (Regression Collinearity Diagnostics of all laboratory parameters with CT Severity Score), Table 20 (Regression Coefficients of all laboratory parameters with CT Severity Score), Table 21 (Regression Collinearity Correlations of all laboratory parameters with CT Severity Score) and Table 22 (Regression Collinearity Covariances of all laboratory parameters with CT Severity Score). Overall results present that there is correlation between chest CT severity of interstitial pneumonia and altered laboratory test results i.e., platelets, leukocytes (WBC), neutrophil, lymphocytes, monocyte, glucose Random (R), Bilirubin Total, Bilirubin Direct, Alanine Aminotransferase (ALT), Aspartate Aminotransferase (AST), and Lactate Dehydrogenase (LDH) in Group A patients.
| Model | DM | EV | CI | Variance Proportions | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cn | Hb | Pts | WBC | Nt | Lph | Es | Mc | Gl | Ur | Ct | Sd | Pt | BT | BD | AST | ALT | LDH | ||||
| 1 | 1 | 11.586 | 1.000 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 2 | 1.866 | 2.492 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| 3 | 1.628 | 2.668 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.01 | 0.00 | |
| 4 | 0.920 | 3.549 | 0.00 | 0.00 | 0.00 | 0.09 | 0.00 | 0.00 | 0.13 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| 5 | 0.869 | 3.652 | 0.00 | 0.00 | 0.01 | 0.10 | 0.00 | 0.00 | .09 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| 6 | 0.320 | 6.016 | 0.00 | 0.00 | 0.02 | 0.01 | 0.00 | 0.00 | 0.05 | 0.26 | 0.14 | 0.02 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.01 | |
| 7 | 0.228 | 7.126 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.02 | 0.02 | 0.34 | 0.17 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.03 | |
| 8 | 0.206 | 7.507 | 0.00 | 0.00 | 0.35 | 0.00 | 0.00 | 0.00 | 0.02 | 0.01 | 0.20 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.03 | |
| 9 | 0.151 | 8.747 | 0.00 | 0.00 | 0.09 | 0.00 | 0.00 | 0.02 | 0.04 | 0.00 | 0.12 | 0.11 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.07 | 0.02 | |
| 10 | 0.073 | 12.621 | 0.00 | 0.01 | 0.26 | 0.29 | .00 | 0.03 | 0.13 | 0.00 | 0.00 | 0.02 | 0.04 | 0.00 | 0.00 | 0.00 | 0.00 | 0.06 | 0.04 | 0.27 | |
| 11 | 0.049 | 15.372 | 0.00 | 0.01 | 0.04 | 0.01 | 0.00 | 0.11 | 0.11 | 0.00 | 0.01 | 0.27 | 0.04 | 0.00 | 0.01 | 0.00 | 0.00 | 0.24 | 0.06 | 0.03 | |
| 12 | 0.041 | 16.830 | 0.00 | 0.05 | 0.02 | 0.00 | 0.01 | 0.02 | 0.01 | 0.18 | 0.19 | 0.05 | 0.12 | 0.00 | 0.01 | 0.00 | 0.00 | 0.03 | 0.12 | 0.25 | |
| 13 | 0.030 | 19.521 | 0.00 | 0.03 | 0.01 | 0.03 | 0.00 | 0.19 | 0.27 | 0.12 | 0.00 | 0.11 | 0.48 | 0.00 | 0.00 | 0.00 | 0.00 | 0.11 | 0.08 | 0.02 | |
| 14 | 0.021 | 23.412 | 0.00 | 0.04 | 0.10 | 0.00 | 0.00 | 0.05 | 0.01 | 0.01 | 0.05 | 0.03 | 0.01 | 0.00 | 0.82 | 0.00 | .00 | 0.01 | 0.02 | 0.19 | |
| 15 | 0.008 | 37.477 | 0.01 | 0.82 | 0.01 | 0.00 | 0.04 | 0.06 | 0.08 | 0.01 | 0.00 | 0.23 | 0.13 | 0.02 | 0.11 | 0.00 | .00 | 0.01 | 0.01 | 0.02 | |
| 16 | 0.002 | 79.706 | 0.02 | 0.03 | 0.07 | 0.39 | 0.71 | 0.42 | 0.01 | 0.05 | 0.05 | 0.00 | 0.03 | 0.24 | 0.02 | 0.03 | 0.03 | 0.22 | 0.36 | 0.03 | |
| 17 | 0.001 | 103.331 | 0.01 | 0.00 | 0.00 | 0.00 | 0.11 | 0.01 | 0.03 | 0.02 | 0.05 | 0.01 | 0.02 | 0.00 | 0.01 | 0.95 | 0.95 | 0.21 | 0.17 | 0.04 | |
| 18 | 0.001 | 147.518 | 0.96 | 0.00 | 0.00 | 0.06 | 0.12 | 0.06 | 0.00 | 0.00 | 0.00 | 0.14 | 0.12 | 0.73 | 0.01 | 0.01 | 0.01 | 0.07 | 0.04 | 0.04 | |
Table 19: Regression collinearity diagnosticsa of all laboratory parameters with CT severity score.
| Model | Unstandardized Coefficients | Standardized Coefficients | t | Sig. | 95.0% Confidence Interval for B | Correlations | Collinearity Statistics | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | Std. Error | Beta | Lower Bound | Upper Bound | Zero-order | Partial | Part | Tolerance | VIF | ||||
| 1 | (Constant) | -11.368 | 30.967 | -0.367 | 0.716 | -74.113 | 51.378 | ||||||
| Hemoglobin | 0.331 | 0.588 | 0.086 | 0.562 | 0.577 | -0.861 | 1.522 | 0.038 | 0.092 | 0.067 | 0.606 | 1.650 | |
| Platelets | -7.008E-6 | 0.000 | -0.114 | -0.730 | 0.470 | 0.000 | 0.000 | -0.053 | -0.119 | -0.087 | 0.578 | 1.730 | |
| WBC | 6.006E-6 | 0.000 | 0.022 | 0.094 | 0.926 | 0.000 | 0.000 | 0.201 | 0.015 | .011 | 0.254 | 3.932 | |
| Neutrophil | 0.198 | 0.203 | 0.350 | 0.979 | 0.334 | -0.212 | 0.609 | 0.044 | 0.159 | 0.116 | 0.111 | 9.037 | |
| Lymphocyte | -0.414 | 0.300 | -0.409 | -1.378 | 0.177 | -1.022 | 0.195 | -0.261 | -0.221 | -0.164 | 0.161 | 6.213 | |
| Eosinophil | 2.398 | 1.461 | 0.336 | 1.641 | 0.109 | -0.563 | 5.359 | -0.061 | 0.261 | 0.195 | 0.337 | 2.969 | |
| Monocyte | -3.222 | 0.864 | -0.534 | -3.727 | 0.001 | -4.973 | -1.470 | -0.316 | -0.522 | -0.443 | 0.690 | 1.450 | |
| Glucose | -0.016 | 0.009 | -0.259 | -1.719 | 0.094 | -0.035 | 0.003 | 0.006 | -0.272 | -0.205 | 0.622 | 1.609 | |
| Urea | 0.008 | 0.082 | 0.027 | 0.099 | 0.922 | -0.159 | 0.175 | -0.019 | 0.016 | 0.012 | 0.183 | 5.479 | |
| Creatinine | -5.863 | 5.524 | -0.250 | -1.061 | 0.295 | -17.056 | 5.331 | -0.241 | -0.172 | -0.126 | 0.255 | 3.923 | |
| Sodium | 0.184 | 0.183 | 0.151 | 1.002 | 0.323 | -.188 | 0.555 | 0.108 | 0.162 | 0.119 | 0.620 | 1.612 | |
| Potassium | 0.906 | 1.367 | 0.090 | 0.663 | 0.512 | -1.864 | 3.677 | -0.053 | 0.108 | 0.079 | 0.769 | 1.300 | |
| Bilirubin Total | -8.224 | 4.885 | -4.078 | -1.683 | 0.101 | -18.123 | 1.675 | -0.261 | -0.267 | -0.200 | 0.002 | 414.607 | |
| Bilirubin Direct | 15.645 | 9.816 | 3.879 | 1.594 | 0.119 | -4.244 | 35.533 | -0.264 | 0.253 | 0.190 | 0.002 | 418.580 | |
| SGOT AST | -0.025 | 0.013 | -0.941 | -1.902 | 0.065 | -0.052 | 0.002 | -0.093 | -0.298 | -0.226 | 0.058 | 17.279 | |
| SGPT ALT | 0.044 | 0.021 | 0.984 | 2.080 | 0.044 | 0.001 | 0.088 | 0.008 | 0.324 | 0.247 | 0.063 | 15.811 | |
| LDH | -0.003 | 0.005 | -0.129 | -0.637 | 0.528 | -0.014 | 0.007 | 0.100 | -0.104 | -0.076 | 0.343 | 2.917 | |
Note: a. dependent variable: HRCT score.
Table 20: Regression coefficients of all laboratory parameters with CT severity score.
| Model | LDH | Gl | BD | Pt | Sd | Mc | WBC | Hb | Ct | Es | Pt | Nt | ALT | Ur | Lph | AST | BT | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Correlations | LDH | 1.000 | 0.152 | 0.147 | -0.282 | 0.037 | 0.210 | -0.047 | 0.052 | 0.005 | 0.119 | 0.323 | 0.238 | 0.000 | -0.160 | 0.313 | -0.105 | -0.147 |
| Gl | 0.152 | 1.000 | -0.170 | -0.106 | 0.160 | 0.169 | -0.087 | -0.043 | 0.091 | -0.029 | 0.072 | -0.265 | -0.346 | -0.284 | -0.049 | 0.326 | 0.171 | ||
| BD | 0.147 | -0.170 | 1.000 | -0.078 | -0.072 | -0.158 | -0.131 | 0.041 | -0.204 | 0.150 | 0.107 | 0.137 | 0.273 | 0.126 | -0.045 | -0.332 | -0.999 | ||
| Pt | -0.282 | -0.106 | -0.078 | 1.000 | 0.102 | -0.045 | -0.088 | 0.123 | -0.156 | -0.042 | -0.187 | -0.183 | -0.095 | 0.200 | -0.205 | 0.080 | 0.074 | ||
| Sd | 0.037 | 0.160 | -0.072 | 0.102 | 1.000 | -0.150 | -0.101 | -0.043 | 0.246 | -0.002 | 0.149 | -0.097 | -0.114 | -0.348 | -0.139 | 0.011 | 0.071 | ||
| Mc | 0.210 | 0.169 | -0.158 | -0.045 | -0.150 | 1.000 | 0.259 | -0.110 | -0.064 | -0.294 | -0.057 | 0.083 | -0.153 | 0.072 | 0.319 | 0.146 | 0.157 | ||
| WBC | -0.047 | -0.087 | -0.131 | -0.088 | -0.101 | 0.259 | 1.000 | -0.058 | 0.161 | -0.277 | -0.453 | 0.605 | 0.279 | -0.074 | 0.684 | -0.217 | 0.130 | ||
| Hb | 0.052 | -0.043 | 0.041 | 0.123 | -0.043 | -0.110 | -0.058 | 1.000 | -0.301 | -0.173 | 0.162 | -0.318 | -0.162 | 0.329 | 0.004 | 0.162 | -0.037 | ||
| Ct | 0.005 | 0.091 | -0.204 | -0.156 | 0.246 | -0.064 | 0.161 | -0.301 | 1.000 | 0.284 | -0.063 | 0.234 | 0.206 | -0.534 | -0.043 | -0.335 | 0.200 | ||
| Es | 0.119 | -0.029 | 0.150 | -0.042 | -0.002 | -0.294 | -0.277 | -0.173 | 0.284 | 1.000 | 0.101 | 0.208 | 0.293 | -0.301 | -0.439 | -0.264 | -0.150 | ||
| Pts | 0.323 | 0.072 | 0.107 | -0.187 | 0.149 | -0.057 | -0.453 | 0.162 | -0.063 | 0.101 | 1.000 | -0.214 | -0.096 | -.146 | -0.196 | 0.130 | -0.104 | ||
| Nt | 0.238 | -0.265 | 0.137 | -0.183 | -0.097 | 0.083 | 0.605 | -0.318 | 0.234 | 0.208 | -0.214 | 1.000 | 0.737 | -0.256 | 0.597 | -0.631 | -0.136 | ||
| ALT | 0.000 | -0.346 | 0.273 | -0.095 | -0.114 | -0.153 | 0.279 | -0.162 | 0.206 | 0.293 | -0.096 | 0.737 | 1.000 | -0.071 | 0.324 | -0.886 | -0.269 | ||
| Ur | -0.160 | -0.284 | 0.126 | 0.200 | -0.348 | 0.072 | -0.074 | 0.329 | -0.534 | -0.301 | -0.146 | -0.256 | -0.071 | 1.000 | 0.139 | -0.082 | -0.125 | ||
| Lph | 0.313 | -0.049 | -0.045 | -0.205 | -0.139 | 0.319 | 0.684 | 0.004 | -0.043 | -0.439 | -0.196 | 0.597 | 0.324 | 0.139 | 1.000 | -0.314 | 0.045 | ||
| AST | -0.105 | 0.326 | -0.332 | 0.080 | 0.011 | 0.146 | -0.217 | 0.162 | -0.335 | -0.264 | 0.130 | -0.631 | -0.886 | -0.082 | -0.314 | 1.000 | 0.330 | ||
| BT | -0.147 | 0.171 | -0.999 | 0.074 | 0.071 | 0.157 | 0.130 | -0.037 | 0.200 | -0.150 | -0.104 | -0.136 | -0.269 | -0.125 | 0.045 | 0.330 | 1.000 | ||
Note: Hb: Hemoglobin; Pts: Platelets; Nt: Neutrophil; Lph: Lymphocytes; Es: Eosinophil; Mc: Monocyte; Gl: Glucose; Ur: Urea; Ct: Creatinine; Sd: Sodium; Pt: Potassium; BT: Bilirubin Total; BD: Bilirubin Direct.
Table 21: Regression collinearity correlations of all laboratory parameters with CT severity score. Dependent variable: HRCT score.
| Covariances | LDH | 2.721E-5 | 7.325E-6 | .008 | -.002 | 3.523E-5 | .001 | -1.584E-8 | .000 | .000 | .001 | 1.619E-8 | .000 | 5.431E-8 | -6.853E-5 | .000 | -7.205E-6 | -.004 |
| Gl | 7.325E-6 | 8.571E-5 | -.015 | -.001 | .000 | .001 | -5.152E-8 | .000 | .005 | .000 | 6.419E-9 | .000 | -6.823E-5 | .000 | .000 | 3.966E-5 | .008 | |
| BD | .008 | -.015 | 96.347 | -1.045 | -.129 | -1.337 | -8.219E-5 | .234 | -11.067 | 2.147 | 1.010E-5 | .273 | .057 | .102 | -.133 | -.043 | -47.893 | |
| Pt | -.002 | -.001 | -1.045 | 1.870 | .026 | -.054 | -7.691E-6 | .099 | -1.179 | -.084 | -2.458E-6 | -.051 | -.003 | .022 | -.084 | .001 | .493 | |
| Sd | 3.523E-5 | .000 | -.129 | .026 | .034 | -.024 | -1.188E-6 | -.005 | .249 | -.001 | 2.612E-7 | -.004 | .000 | -.005 | -.008 | 2.711E-5 | .064 | |
| Mc | .001 | .001 | -1.337 | -.054 | -.024 | .747 | 1.435E-5 | -.056 | -.306 | -.371 | -4.755E-7 | .014 | -.003 | .005 | .083 | .002 | .664 | |
| WBC | -1.584E-8 | -5.152E-8 | -8.219E-5 | -7.691E-6 | -1.188E-6 | 1.435E-5 | 4.108E-9 | -2.172E-6 | 5.700E-5 | -2.595E-5 | -2.789E-10 | 7.865E-6 | 3.813E-7 | -3.916E-7 | 1.316E-5 | -1.828E-7 | 4.060E-5 | |
| Hb | .000 | .000 | .234 | .099 | -.005 | -.056 | -2.172E-6 | .346 | -.979 | -.148 | 9.167E-7 | -.038 | -.002 | .016 | .001 | .001 | -.107 | |
| Ct | .000 | .005 | -11.067 | -1.179 | .249 | -.306 | 5.700E-5 | -.979 | 30.519 | 2.295 | -3.358E-6 | .263 | .024 | -.243 | -.071 | -.024 | 5.395 | |
| Es | .001 | .000 | 2.147 | -.084 | -.001 | -.371 | -2.595E-5 | -.148 | 2.295 | 2.135 | 1.412E-6 | .062 | .009 | -.036 | -.193 | -.005 | -1.073 | |
| Pts | 1.619E-8 | 6.419E-9 | 1.010E-5 | -2.458E-6 | 2.612E-7 | -4.755E-7 | -2.789E-10 | 9.167E-7 | -3.358E-6 | 1.412E-6 | 9.212E-11 | -4.161E-7 | -1.957E-8 | -1.157E-7 | -5.647E-7 | 1.639E-8 | -4.875E-6 | |
| Nt | .000 | .000 | .273 | -.051 | -.004 | .014 | 7.865E-6 | -.038 | .263 | .062 | -4.161E-7 | .041 | .003 | -.004 | .036 | -.002 | -.135 | |
| ALT | 5.431E-8 | -6.823E-5 | .057 | -.003 | .000 | -.003 | 3.813E-7 | -.002 | .024 | .009 | -1.957E-8 | .003 | .000 | .000 | .002 | .000 | -.028 | |
| Ur | -6.853E-5 | .000 | .102 | .022 | -.005 | .005 | -3.916E-7 | .016 | -.243 | -.036 | -1.157E-7 | -.004 | .000 | .007 | .003 | -8.831E-5 | -.050 | |
| Lph | .000 | .000 | -.133 | -.084 | -.008 | .083 | 1.316E-5 | .001 | -.071 | -.193 | -5.647E-7 | .036 | .002 | .003 | .090 | -.001 | .066 | |
| AST | -7.205E-6 | 3.966E-5 | -.043 | .001 | 2.711E-5 | .002 | -1.828E-7 | .001 | -.024 | -.005 | 1.639E-8 | -.002 | .000 | -8.831E-5 | -.001 | .000 | .021 | |
| BT | -.004 | .008 | -47.893 | .493 | .064 | .664 | 4.060E-5 | -.107 | 5.395 | -1.073 | -4.875E-6 | -.135 | -.028 | -.050 | .066 | .021 | 23.867 |
Table 22: Regression Collinearity Covariancesa of all laboratory parameters with CT Severity Score. Dependent Variable: HRCT Score. Note: Hb-Hemoglobin; Pts-Platelets; Nt-Neutrophil; Lph-Lymphocytes; Es-Eosinophil; Mc- Monocyte; Gl-Glucose; Ur-Urea; Ct-Creatinine; Sd-Sodium; Pt-Potassium; BT-Bilirubin Total; BD-Bilirubin Direct.
The graphical presentation of normal P-P Plot of regression Standardized Residual with dependent variable CT severity Score is shown in Figure 7A and graph of Scatterplot of regression with Dependent Variable CT Score is shown in Figure 7B. We realized that study sample size is not big but somehow results shown that there is correlation between chest CT severity of interstitial pneumonia and altered laboratory test results.
For the correlation of Chest X-Ray pneumonia severity, compare mean Independent Samples t-test with all laboratory parameters was conducted and results are summarized in Tables 23,24. In our study, we only included mild and moderate COVID-19 patients with clinically stable in group B. Although sample size was also very small only 12, that is the reason, there is little bit weak correlation seen between Chest X-Ray pneumonia severities, compare mean Independent Samples t-test with all laboratory parameters. But we strongly suggest that if sample size will big, there will be strong correlation between lung volume pneumatic severity and altered parameters.
| X-ray Severity | N | Mean | Std. Deviation | Std. Error Mean | |
|---|---|---|---|---|---|
| Hemoglobin | Mild | 6 | 10.067 | 2.2491 | .9182 |
| Moderate | 6 | 13.550 | 1.3867 | .5661 | |
| Platelets | Mild | 6 | 140000.00 | 50521.283 | 20625.227 |
| Moderate | 6 | 136833.33 | 76828.163 | 31364.966 | |
| WBC | Mild | 6 | 30516.67 | 64479.746 | 26323.746 |
| Moderate | 6 | 5550.00 | 2633.439 | 1075.097 | |
| Neutrophil | Mild | 6 | 54.33 | 35.212 | 14.375 |
| Moderate | 6 | 77.50 | 6.950 | 2.837 | |
| Lymphocyte | Mild | 6 | 22.50 | 8.289 | 3.384 |
| Moderate | 6 | 21.17 | 7.305 | 2.982 | |
| Monocyte | Mild | 6 | 1.33 | .516 | .211 |
| Moderate | 6 | 1.33 | .516 | .211 | |
| Glucose | Mild | 6 | 108.17 | 54.068 | 22.073 |
| Moderate | 6 | 118.50 | 44.626 | 18.219 | |
| Urea | Mild | 6 | 22.33 | 4.844 | 1.978 |
| Moderate | 6 | 49.17 | 28.722 | 11.726 | |
| Creatinine | Mild | 6 | 4.5383 | 9.53746 | 3.89365 |
| Moderate | 6 | 1.3783 | .62637 | .25571 | |
| Sodium | Mild | 6 | 135.4933 | 4.00343 | 1.63439 |
| Moderate | 6 | 134.2450 | 5.61784 | 2.29347 | |
| Potassium | Mild | 6 | 3.7383 | .51289 | .20939 |
| Moderate | 6 | 4.1217 | .17233 | .07035 | |
| Bilirubin Total | Mild | 2 | .5700 | .05657 | .04000 |
| Moderate | 4 | .8000 | .48076 | .24038 | |
| Bilirubin Direct | Mild | 2 | .1350 | .00707 | .00500 |
| Moderate | 3 | .2900 | .31177 | .18000 | |
| SGOT AST | Mild | 6 | 51.1667 | 14.51092 | 5.92406 |
| Moderate | 6 | 64.3333 | 28.80741 | 11.76057 | |
| SGPT ALT | Mild | 6 | 68.1667 | 32.96918 | 13.45961 |
| Moderate | 6 | 49.1667 | 35.58886 | 14.52909 | |
| LDH | Mild | 6 | 658.33 | 170.472 | 69.595 |
| Moderate | 6 | 575.50 | 213.918 | 87.332 |
Table 23: Correlation of Chest X-Ray severity with Laboratory Parameters (Group B).
| Levene's Test for Equality of Variances | t-test for Equality of Means | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| F | Sig. | t | df | Sig. (2-tailed) | Mean Difference | Std. Error Difference | 95% Confidence Interval of the Difference | |||
| Lower | Upper | |||||||||
| Hemoglobin | EVA | .065 | .804 | -3.229 | 10 | .009 | -3.4833 | 1.0787 | -5.8868 | -1.0798 |
| EVNA | -3.229 | 8.321 | .011 | -3.4833 | 1.0787 | -5.9542 | -1.0125 | |||
| Platelets | EVA | 3.461 | .092 | .084 | 10 | .934 | 3166.667 | 37538.795 | -80474.980 | 86808.314 |
| EVNA | .084 | 8.643 | .935 | 3166.667 | 37538.795 | -82289.658 | 88622.992 | |||
| WBC | EVA | 5.677 | .038 | .948 | 10 | .366 | 24966.667 | 26345.691 | -33735.191 | 83668.525 |
| EVNA | .948 | 5.017 | .387 | 24966.667 | 26345.691 | -42689.417 | 92622.750 | |||
| Neutrophil | EVA | 20.159 | .001 | -1.581 | 10 | .145 | -23.167 | 14.652 | -55.814 | 9.481 |
| EVNA | -1.581 | 5.389 | .170 | -23.167 | 14.652 | -60.029 | 13.695 | |||
| Lymphocyte | EVA | .757 | .405 | .296 | 10 | .774 | 1.333 | 4.510 | -8.717 | 11.383 |
| EVNA | .296 | 9.845 | .774 | 1.333 | 4.510 | -8.738 | 11.405 | |||
| Monocyte | EVA | .000 | 1.000 | .000 | 10 | 1.000 | .000 | .298 | -.664 | .664 |
| EVNA | .000 | 10.000 | 1.000 | .000 | .298 | -.664 | .664 | |||
| Glucose | EVA | .121 | .735 | -.361 | 10 | .726 | -10.333 | 28.621 | -74.104 | 53.438 |
| EVNA | -.361 | 9.653 | .726 | -10.333 | 28.621 | -74.416 | 53.750 | |||
| Urea | EVA | 3.046 | .112 | -2.257 | 10 | .048 | -26.833 | 11.891 | -53.329 | -.338 |
| EVNA | -2.257 | 5.284 | .071 | -26.833 | 11.891 | -56.913 | 3.247 | |||
| Creatinine | EVA | 5.302 | .044 | .810 | 10 | .437 | 3.16000 | 3.90204 | -5.53428 | 11.85428 |
| EVNA | .810 | 5.043 | .455 | 3.16000 | 3.90204 | -6.84476 | 13.16476 | |||
| Sodium | EVA | .029 | .868 | .443 | 10 | .667 | 1.24833 | 2.81625 | -5.02666 | 7.52333 |
| EVNA | .443 | 9.037 | .668 | 1.24833 | 2.81625 | -5.11847 | 7.61514 | |||
| Potassium | EVA | 5.259 | .045 | -1.735 | 10 | .113 | -.38333 | .22089 | -.87551 | .10884 |
| EVNA | -1.735 | 6.115 | .132 | -.38333 | .22089 | -.92138 | .15472 | |||
| Bilirubin Total | EVA | 3.984 | .117 | -.636 | 4 | .559 | -.23000 | .36140 | -1.23342 | .77342 |
| EVNA | -.944 | 3.161 | .412 | -.23000 | .24369 | -.98363 | .52363 | |||
| Bilirubin Direct | EVA | 9.204 | .056 | -.667 | 3 | .553 | -.15500 | .23241 | -.89463 | .58463 |
| EVNA | -.861 | 2.003 | .480 | -.15500 | .18007 | -.92863 | .61863 | |||
| SGOT AST | EVA | .773 | .400 | -1.000 | 10 | .341 | -13.16667 | 13.16835 | -42.50759 | 16.17426 |
| EVNA | -1.000 | 7.384 | .349 | -13.16667 | 13.16835 | -43.97970 | 17.64636 | |||
| SGPT ALT | EVA | .004 | .952 | .959 | 10 | .360 | 19.00000 | 19.80544 | -25.12928 | 63.12928 |
| EVNA | .959 | 9.942 | .360 | 19.00000 | 19.80544 | -25.16413 | 63.16413 | |||
| LDH | EVA | .171 | .688 | .742 | 10 | .475 | 82.833 | 111.670 | -165.984 | 331.651 |
| EVNA | .742 | 9.525 | .476 | 82.833 | 111.670 | -167.675 | 333.341 | |||
*EVA-Equal variances assumed; *EVNA-Equal variances not assumed
Table 24: Correlation of Chest X-Ray severity with Laboratory Parameters of Group B (Independent Samples t-Test).
Discussion
The fear of COVID-19 of under developed country like Nepal is threatening to general public as well as health workers. There was highest transmission rate of SARS-CoV-2 virus recorded during second phase of pandemic in Nepal. The continually increasing number of suspected SARS CoV-2 virus infection to people is overwhelming medical staffs. As we know, an early diagnosis is the main key for prognosis as well as control the infection rate. In Nepal, highly transmission rated Delta Variant (B.1.617.2) and additional mutated delta variant K417N mutation named as AY.1 Variant of Concern (VOC) was prominent during second phase of COVID-19 pandemic [15].
According to the COVID-19 diagnostic guidelines, double swab RT-PCR test by collecting swab from upper or lower respiratory specimens' method is the gold standard for clinical COVID-19 diagnosis and it is easy to conduct in hospital laboratories [18]. In spite of gold standard test, there are some limitations with a RT-PCR sensitivity and the sensitivity rate of RT-PCT test for SARS-CoV-2 was reported to be around 60% [7,19]. In one research study, the author concluded that there is an inverse correlation between the number of false negatives and the sampling timing i.e., different period of the disease development with a median false negative rate of 39% on the day of symptom onset, evaluating the accuracy of different respiratory specimens in the laboratory diagnosis and monitoring the viral shedding of SARS-CoV-2 infections [20].
The false negative results of RT-PCR test of SARS-CoV-2 virus create a problem to a group of patients without a definitive diagnosis and it create difficult to manage the problem. There was a clinical trial study which reported that 308 among 1014 patients with suspected chest HRCT reports for SARS-CoV-2 viral pneumonia. Among them, 147 patients whose RT-PCR samples were negative considered as highly likely cases, considering the clinical characteristics [21].
There are several studies have shown alterations in some laboratory parameters with greater frequency in patients with SARS-CoV-2 virus infection i.e., LDH, D-dimer, CRP, Lymphocyte and fibrinogen [9,22,23]. In present study, our aim was to find out characteristics of Chest HRCT features, chest X-Ray features and abnormalities in laboratory parameters findings of COVID-19 patients with interstitial pneumonia. Additionally in this retrospective study, we correlate between chest CT alterations severity and laboratory parameters to find additional supportive tool of diagnosis to assist in the diagnosis and management of highly suspicious patients of SARS-CoV-2 infection [15].
Several studies reported that CT has a vital role for diagnosis and follow up management of patients with COVID-19 pneumonia. One study reported that the sensitivity of chest HRCT for COVID-19 pneumonia was 91% (95% CI, 85-96%) [12]. In this study, we found all typical and some atypical appearances of COVID-19 interstitial pneumonia in HRCT chest i.e., Ground-Glass Opacities (GGO) (27.27%), GGO with Crazy Paving (29.09%), GGO with Consolidation (12.72%), GGO with crazy paving with consolidation (3.63%), Crazy paving with Bronchiotasis (1.81%), Multifocal GGO (5.45%), Consolidation (5.45%), GGO with Bronchiotasis (1.81%), Collapse (1.81%), GGO with Fibrotic (7.27%) and GGO with Fibrotic with crazy paving (3.63%). We also evaluate the treatment response of most of the patients with chest CT outcome.
In the 5th edition of the guideline of diagnosis and treatment in Hubei China, HRCT chest is already adopted as a diagnostic tool for COVID-19 pneumonia due to its high sensitivity [24]. It is strongly recommended by Fleischner Society that diagnosis of COVID-19 interstitial pneumonia in COVID-19 patients with moderate to severe lung severity may be presumed based on chest HRCT findings even though RT-PCR is negative for SARS-COV-2 virus [25,26]. There are several research studies reported regarding crucial laboratories parameters for COVID-19 patients' diagnosis and prognosis. One of the retro prospective studies reported that Lymphocytopenia, C-Reactive Protein (CRP), Lactate Dehydrogenase (LDH), D-dimer, and fibrinogen increased levels occurred in most COVID-19 patients [12]. In present study, we also evaluated the laboratory parameters and result for the abnormalities found in laboratory tests i.e., hemoglobin, platelets, leukocytes (WBC), neutrophil, lymphocytes, eosinophil, monocyte, C-Reactive Protein (CRP), Glucose Random (R), Urea, Creatinine, Sodium, Potassium, Bilirubin Total, Bilirubin Direct, Alanine Aminotransferase (ALT), Aspartate Aminotransferase (AST), and Lactate Dehydrogenase (LDH) were 20%, 43%, 40%, 85%, 89%, 42%, 74%, 95%, 61%, 29%, 10%, 1.8%, 1.8%, 10.9%, 14.5%, 85%, 63%, and 100% respectively. There was significant decrease in following laboratory parameters counts i.e., platelets, leukocytes (WBC), lymphocytes, monocyte and elevation in following laboratory parameters test counts i.e., CRP, Glucose Random (R), Urea, Creatinine, Bilirubin Total, Bilirubin Direct, Alanine Aminotransferase (ALT), Aspartate Aminotransferase (AST), and Lactate Dehydrogenase (LDH). Abnormalities in biochemical sodium and potassium were not significant.
In an observational cross-sectional study Simone Canovi reported that lung lesions probably employ a vital role in COVID-19 pathogenesis and clinical orientations [23]. In our study, we also correlated the radiological lung pneumatic severity with different biochemical laboratory parameters, correlation p-Value-0.05. We also found there was strong correlation with some biochemical parameters like CRP, LDH, WBC, Lymphocytes, Neutrophil and ALT. With some parameters, there was average correlation like glucose, platelets, monocyte, AST, Bilirubin Total, Bilirubin Direct, Urea and creatinine. There was poor correlation with sodium and potassium level. Our study suggests that there is correlation between severity of CT lung parenchyma severity and abnormal laboratory parameters.
Limitation of study
A limitation of CT is the possibility of having some false positive cases because the CT imaging features of COVID-19 pneumonia are like those of other viral pneumonia. However, the evaluation of clinical symptoms and laboratory biochemical parameters can reduce the possibility of false positivity. However, this study had some other limitations. It is a retrospective study with very small sample size and conducted at single center. We specially would like to mention about alteration in hemoglobin level. Some of the patient had history of chemotherapy and highly prominent to low hemoglobin level due to chemotherapy. Further large sample study needed to evaluate either blood hemoglobin level affect by SARS-CoV-2 virus or not? There was another concern biochemical test, random blood glucose level in which our record shown 61% SARS-CoV-2 virus infected patients with elevated. The elevation of glucose level was seen after one or two weeks after infection and seen in most of the severe patients. Further large sample study needed to evaluate either blood glucose level affect by SARS-COV-2 virus or not? Some studies also suggest that there may be elevation of blood glucose level occurred due to SARS-COV-2 virus infection [27]. D-dimer and ferritin test records were not included in our study due to lack of complete data.
Conclusion
In conclusion, we think that in case of high clinical suspicion of COVID-19, patients should not be ruled out based on RT-PCR test alone and the clinical and epidemiologic situation should be carefully considered.
In this study, we found all typical and some atypical appearances of COVID-19 interstitial pneumonia in HRCT chest i.e., Ground-Glass Opacities (GGO) with Crazy Paving or Consolidation or Bronchiotasis or Consolidation or Fibrotic and Collapse.
There was decrease in following laboratory parameters counts i.e., hemoglobin, platelets, leukocytes (WBC), lymphocyte, eosinophil, monocyte. Among the total 55 patients, 95 % patients with positive C-Reactive Protein (CRP) were found. There was elevation in following laboratory parameters counts i.e., Neutrophil, C-Reactive Protein (CRP), Glucose random (R), Bilirubin Total, Bilirubin Direct, Alanine aminotransferase (ALT), Aspartate Aminotransferase (AST), and Lactate Dehydrogenase (LDH). Abnormalities in following laboratory tests i.e., urea, creatinine, sodium and potassium may be acceptable.
Statistical correlation was found between laboratory analyses and amount of damaged lung in CT scan i.e., volume of lung damage was strongly associated with altered laboratory test results. These altered laboratory parameters can be used as an adjunctive diagnostic tool in patient with double negative RT-PCR test and highly suspicious clinic and chest CT scan features.
In addition, it is safe to suggest that a symptomatic patient with classic CT of COVID-19 and abnormal Biochemical lab findings should be treated as COVID 19 patients even after two negative RT-PCR tests.
Acknowledgments
It was retroprospective study and there was no need of funding for this study. All the authors contributed equally to the study. We would like to thank department of Radio-diagnosis, Imaging and Nuclear Medicine and Department of pathology for the swift and efficient support to make this work possible.
Funding
No Funding required
Conflicts of interest statement and funding
The authors declared that they have no conflicts of interest in this paper.
Advances in knowledge
COVID-19 may cause elevation of glucose level after few weeks after infection in severely infected patients.
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethics approval and consent to participate
It is a retrospective study so ethics approval and consent of participants are not applicable.
Consent for publication
Not applicable
References
- Coronavirus resource center, Jons Hopkins University of Medicine. 2021.
- COVID-19 Symptoms, Coronavirus Disease, World Health Organization (WHO).
- Symptoms, COVID-19, Centers for Disease Control and Prevention (CDC).
- Jingjing He, Yifei Guo, Richeng Mao, Jiming Zhang (2021) Proportion of asymptomatic coronavirus disease 2019: A systematic review and meta-analysis.J Med Virol . 93: 820-830.
[Crossref] [Google Scholar] [Pubmed]
- Covid-19 update portal, Ministry of Health and population, Government of Nepal. 2021.
- Kooraki S, Hosseiny M, Myers L, Gholamrezanezhad A (2020) Coronavirus (COVID-19) outbreak: what the department of radiology should know. J Am Coll Radiol 17:447–51.
[Crossref] [Google Scholar] [Pubmed]
- Yang Y, Yang M, Shen C, Fuxiang W, Jing Y, et al. (2020) Evaluating the accuracy of different respiratory specimens in the laboratory diagnosis and monitoring the viral shedding of 2019-nCoV infections. medRxiv.
- Wang Y, Kang H, Liu X, Tong Z (2020) Combination of RT-qPCR testing and clinical features for diagnosis of COVID-19 facilitates management of SARS-CoV-2 outbreak. J Med Virol 92:538-539.
[Crossref] [Google Scholar] [Pubmed]
- Corman VM, Landt O, Kaiser M, Richard M, Adam M, et al. (2020) Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill 25:2000045.
[Crossref] [Google Scholar] [Pubmed]
- Lippi G, Plebani M (2020) Laboratory abnormalities in patients with COVID-2019 infection. Clin Chem Lab Med 58:1131–1134.
[Crossref] [Google Scholar] [Pubmed]
- Mardani R, Vasmehjani AA, Zali F, Gholami A, Nasab SDM et al. (2020) Laboratory parameters in detection of COVID-19 patients with positive RT-PCR; a diagnostic accuracy study. Arch Acad Emerg Med;8:e43.
[Google Scholar] [Pubmed]
- Orlacchio A, Gasparrini F, Roma S, Ravà, MS; Salvatori E, et al. (2021) Correlations between chest-CT and laboratory parameters in SARS-CoV-2 pneumonia. Medicine 100 :pe25310
[Crossref] [Google Scholar] [Pubmed]
- Yilmaz, R. Sabirli, M. Seyit, Ozen M, Oskay A, et al (2021) Association between laboratory parameters and CT severity in patients infected with COVID-19: a retrospective, observational study. Am. J. Emerg. Med 42:110-114.
[Crossref] [Google Scholar] [Pubmed]
- Nam K, Miller C, Waldman S (2021) The SARS-CoV-2 Variant and its Impact on Diagnostic Testing on Coronavirus.
- Press release on Covid-19 update portal, Ministry of Health and population, Government of Nepal.
- Guidelines-for-SARS-CoV-2-PCR-laboratories IN National Public Health Laboratory.
- Chen A, Karwoski RA, Gierada DS, Bartholmai BJ, Koo CW, et al. (2020) Quantitative CT analysis of diffuse lung disease. Radiographics.40:28-43.
- East M, Committee I, States M (2020) Laboratory Guidelines for the Detection and Diagnosis of COVID-19 Virus Infection Sample collection and proper shipment Laboratory testing.
- Li J, Ye G, Chen L, Wang J, Li Y. (2020) Analysis of false-negative results for 2019 novel coronavirus nucleic acid test and related countermeasures. J Lab Med 12:E006.
- Kucirka L, Lauer S, Laeyendecker O, Boon D, Lessler J, et al. (2020) Variation in false negative rate of rt-pcr based sars-cov-2 tests by time since exposure. Ann Intern Med 173:262-267.
[Croosref] [Google scholar] [PubMed]
- Liu Y, Yang Y, Zhang C, Huang F, Wang F, et al. (2020) Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci 63:364-374.
[Crossref] [Google scholar] [PubMed]
- Tang N, Li D, Wang X, Sun Z (2020) Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost 18:844-847.
[Crossref] [Google scholar] [PubMed]
- Canovi S, Besutti G, Bonelli E, Lotti V, Ottone M, et al. (2021) The association between clinical laboratory data and chest CT findings explains disease severity in a large Italian cohort of COVID-19 patients. BMC Infect Dis 21: 157.
[Crossref] [Google scholar] [PubMed]
- Diagnosis and Treatment. (2020) Protocol for Novel Coronavirus Pneumonia. Version 7.
- Rubin GD, Ryerson CJ, Haramati LB, Sverzellati N, Kanne JP, et al. (2020) The role of chest imaging in patient management during the COVID-19 pandemic: a multinational consensus statement from the fleischner society. Chest 296:172-180.
[Crossref] [Google scholar] [PubMed]
- Orlacchio A, Roma S, Minieri M, Legramante JM, Grelli S, et al. (2020) Our Experience in COVID-19. Radiology Brief Communications Blog.
- Chen J, Wu C, Wang X, Yu J, Sun Z (2020) The Impact of COVID-19 on Blood Glucose: A Systematic Review and Meta-Analysis". Front Endocrinol 11:732.
[Crossref] [Google scholar] [PubMed]
Citation: Yadav AK, Gnawali S, Mandal S, Thakur S, Shrestha GB, et al. (2022) Characteristics of Radiological Features and Laboratory Parameters in Patients with COVID-19 Pneumonia During Second Phase of Pandemic in Nepal: A Retroprospective Study. J Infect Dis Ther 10:498. DOI: 10.4172/2332-0877.1000498
Copyright: © 2022 Yadav AK, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2345
- [From(publication date): 0-2022 - Apr 26, 2025]
- Breakdown by view type
- HTML page views: 1801
- PDF downloads: 544