Characteristics of Nutritional Status and the Effect of Pre-transplant Branched-chain Amino Acid Administration in Patients Undergoing Living Donor Liver Transplantation
Received: 15-Feb-2016 / Accepted Date: 15-Apr-2016 / Published Date: 22-Apr-2016 DOI: 10.4172/2475-7640.1000101
Abstract
Background: Protein-energy malnutrition is common in patients with end-stage liver disease undergoing liver transplantation. We examined the characteristics of nutritional status and impact of pre-admission branched-chainamino- acids treatment on skeletal muscle mass, nutritional/metabolic parameters and on post-transplant outcomes.
Methodology: Preoperative skeletal muscle mass and nutritional/metabolic parameter levels were compared in 129 patients undergoing adult-to-adult living donor liver transplantation whether received branched-chain-aminoacids treatment before admission or not. We examined relationships among these parameters, and risk factors for post-transplant bacteremia and early mortality after LT focusing on nutritional parameters.
Results: Pre-albumin and branched-chain-amino-acids-to-tyrosine ratio were significantly higher while tyrosine was lower in branched-chain-amino-acids-pre-supplemented than non-pre-supplemented group, while skeletal muscle mass, total lymphocyte count, zinc, branched-chain-amino-acids and ammonia levels were not significantly different. Skeletal muscle mass positively correlated with tyrosine (r=0.437, P<0.001) and branched-chain-aminoacids (r=0.282, P=0.001) and negatively with branched-chain-amino-acids-to-tyrosine-ratio (r=-0.259, P=0.003). Multivariate predictors of post-transplant bacteremia were: Child-Pugh class C (P=0.012), low preoperative total lymphocyte count (P=0.027), operative blood loss ≥ 10 L (P=0.039) and absence of pre-admission branched-chainamino- acids treatment (P=0.040). Nutritional/metabolic parameters and pre-admission branched-chain-amino-acids treatment were not crucial for post-transplant early mortality.
Conclusion: Pre-admission branched-chain-amino-acid therapy could ameliorate preoperative amino acid imbalance and the incidence of post-transplant bacteremia.
Keywords: Branched-chain amino acids; Bioelectrical impedance analysis; Skeletal muscle mass; Sarcopenia; Zinc
7374Introduction
Derangements of various serum biochemical nutritional parameters including zinc, pre-albumin, branched-chain amino acids (BCAA), tyrosine, total lymphocyte count and the related metabolic parameters as the molar ratio of BCAA-to-tyrosine (BTR) and ammonia, are not uncommon in patients with end-stage liver disease undergoing liver transplantation (LT) due to hepatic debilitating pathology and its medical management [1-5].
Patients with decompensated liver cirrhosis frequently receive nutritional therapy with a nutrient mixture enriched with BCAA or BCAA nutrients. BCAA supplementation was reported to delay reduction of hepatic reserve in patients with end-stage liver disease [6]. However, the effect on patients undergoing living donor LT (LDLT) is unclear. Moreover, the nutritional status and relationships between nutritional parameters including skeletal muscle mass and zinc in such patients are not well understood.
We recently reported that skeletal muscle mass had a significant negative correlation with BTR and low preoperative skeletal muscle mass to be closely involved with post-transplant mortality [7]. However, neither the impact of both pre-admission BCAA treatment and preoperative levels of nutritional/metabolic parameters on posttransplant outcomes nor the reason of the negative relationship is clear.
The aim of the present study was therefore to examine the characteristics of nutritional status and the impact of pre-admission BCAA treatment on both skeletal muscle mass and nutritional/ metabolic parameter levels in LDLT candidates. Additionally, we assessed relationships among these parameters, especially with skeletal muscle mass and zinc levels, and examined risk factor for bacteremia and early mortality after LT focusing on nutritional factors.
Methodology
Patients
Two hundred and eight adult (age ≥ 18 years) patients underwent primary LDLT at Kyoto University Hospital between February 2008 and August 2012. Excluded from the study were 15 patients with acute liver failure as an indication for LT for the following reasons: First, the pathophysiology and nutritional status is different from patients with other end-stage liver diseases. Second, BCAA supplementation was not suitable for those patients and even regarded as a contraindication [8]. Third, they could not undergo preoperative bioelectrical impedance body composition analysis (BIA) examination due to emergent LT. Next, 64 patients who could not undergo BIA per the dietitians, limitations of dietitians’ manpower or the hospital’s circumstances were further excluded. The study thus comprised 129 patients. There were 63 males and 66 females. The median patient age was 53 years (range, 19-65 years). The study was approved by the Ethics Committee of Kyoto University and conducted in accordance with the Declaration of Helsinki of 1996.
The median Model for End-stage Liver Disease (MELD) score was 19 (range, 4-47). Thirty-seven patients were ABO incompatible, and 92 were identical or compatible. The Child-Pugh classifications were C, B, and A for 78, 42, and 9 patients, respectively. The indications for LT were hepatocellular carcinoma (n=36), followed by hepatocellular diseases such as hepatitis B or C virus-associated liver cirrhosis (n=32), progressive intrahepatic cholestatic diseases including primary biliary cirrhosis and primary sclerosing cholangitis (n=24), biliary atresia after the Kasai procedure (n=8), alcoholic liver cirrhosis (n=7), metabolic liver diseases (n=5), nonalcoholic steatohepatitis (n=4), autoimmune hepatitis (n=3) and other causes (n=10).
In February 2008, we introduced body composition measurements on admission for patients undergoing LDLT, using direct segmental multi-frequency BIA with eight tactile electrodes (InBody 720; Biospace, Tokyo, Japan). The BIA device used 6 frequencies and produced 30 impedance values for 5 body segments and takes direct impedance measurements from each, unlike conventional BIA used before [9,10] which takes only partial measurements and relies on formulas to estimate whole body composition. The BIA device was reported as an accurate substitute for the dual-energy X-ray absorptiometry in measurement of total and appendicular body composition [11]. Skeletal muscle mass was measured automatically by the InBody 720 and shown as a percent against standard skeletal muscle mass calculated by sex and height of each patient. The normal skeletal muscle mass ratio obtained by the InBody 720 ranges from 90% to 110% of the standard skeletal muscle mass.
Selection criteria for the recipients as well as surgical techniques for recipient operations have been described in detail elsewhere [12,13]. Immunosuppressive treatment usually consisted of tacrolimus or cyclosporine and low-dose steroids as described elsewhere [14,15]. All patients received intravenous antimicrobial prophylaxis with ampicillin (0.5 g) and cefotaxime (0.5 g) twice daily for 3 days starting 30 min before surgery.
Infections were defined using the criteria proposed by the Centers for Disease Control and Prevention and based on reports of liver transplant patients [16]. The isolation of bacteria other than common skin contaminants from a single blood culture in the presence of clinical symptoms or of an infection was considered bacteremia. When caused by common skin contaminants, bacteremia was considered significant only if an organism was isolated from two blood cultures and clinical signs of infection were evident.
Subgroups assignment
Sixty-six patients out of 129 received BCAA treatment before admission to LDLT for a duration of at least a few months, either in the form of 1 to 3 packets of BCAA-enriched nutrient mixture (Aminoleban EN®; Otsuka Pharmaceutical Co., Tokyo, Japan) per day (50-150 g/day), (n=32) or 3 packets of BCAA granules (Livact®; Ajinomoto Pharma Co., Tokyo, Japan) per day (12.45 g/day), (n=34). The other 63 patients did not receive any BCAA supplements before admission. BCAA were introduced at the discretion of the attending physician before referral of a potential recipient for LDLT. After admission to LDLT, all patients received the same perioperative nutritional therapy as described before [7].
Analyzed parameters
Preoperative laboratory data of nutritional/metabolic parameters on admission were retrospectively reviewed from the clinical charts of the recipients. The standard reference intervals of these parameters at our institute were: zinc (65-110 μg/dL), prealbumin (20-40 mg/ dL), total lymphocyte count (1200-3200/μL), ammonia (20-60 μg/dL), BCAA (344-713 μmol/L), tyrosine (53-98 μmol/L) and the BTR (4.4- 9.3).
Skeletal muscle mass and parameter levels on admission were examined. Correlations of skeletal muscle mass and all parameters and those of zinc with other parameters were examined. Because BCAA treatment before admission may affect BCAA blood level, we separately analyzed relationship between skeletal muscle mass and BCAA in groups with or without BCAA pretreatment. A transversal study was performed where preoperative nutritional/metabolic parameters and skeletal muscle mass, length of postoperative hospital stay, incidence of postoperative bacteremia, and biopsy-proven acute cellular rejection were examined in patients admitted to LDLT according to whether they received BCAA treatment before admission to LDLT (BCAA+, n=66) or not (BCAA-, n=63).
Moreover, we examined risk factors for post-transplant death within 90 days after LT and post-transplant bacteremia focusing on preoperative nutritional/metabolic parameter levels and pre-admission BCAA treatment [18].
Statistical analysis
Data was summarized as mean ± standard deviation (SD) for continuous variables. Categorical variables were compared using the χ2 test or Fisher’s exact test where appropriate. Continuous variables were non-parametrically analyzed using the Mann-Whitney U test with the sequential step-down Holm-Bonferroni method applied to adjust for multiple testing [17]. Correlation between two variables was analyzed using Spearman’s rank correlation coefficient. Any variable identified as significant (P<0.05) in univariate analysis using the above tests was considered a candidate for multivariate analysis using multiple logistic regression models. Survival rate was calculated using Kaplan-Meier methods with differences evaluated using log-rank testing. Two tailed P<0.05 was considered significant. All statistical data were generated using JMP 5.0.1 (SAS Institute, Cary, NC) and Prism 6.02 (GraphPad Software, Inc., La Jolla, CA, USA).
Results
Pretransplant nutritional status
Marked hyperammonemia (94.1 ± 16.8 μg/dL), hypertyrosinemia (138.6 ± 12.1 μmol/L), hypozincemia (44.4 ± 12.6 μg/dL), hypoprealbuminemia (6.8 ± 2.5 mg/dL) and a decreased total lymphocyte count (855.3 ± 207.3/μL), were seen on admission before LDLT. Preoperative BCAA level (397.2 ± 56 μmol/L) was low, yet still within reference range. Consequently, the BTR (3.1 ± 0.5) was subnormal. The median ratio of preoperative skeletal muscle mass was 92% (range 65%-32%) of the standard muscle mass.
Correlations between parameters
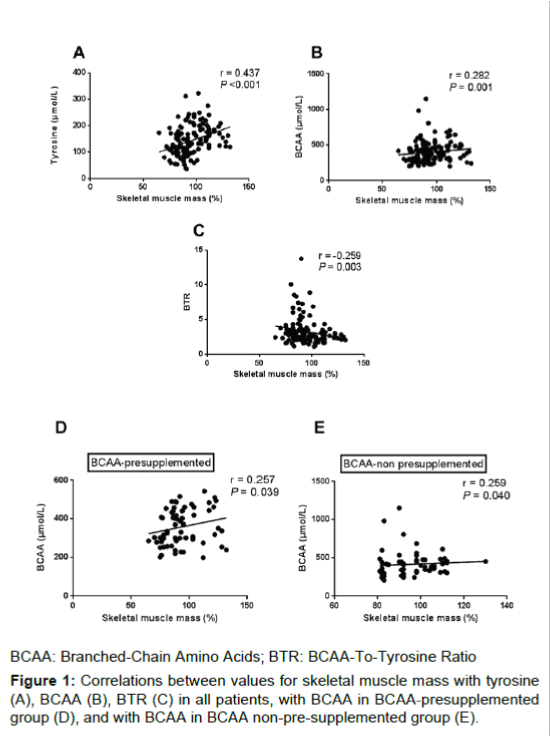
Correlations of preoperative skeletal muscle mass with both tyrosine (r=0.437, P<0.001; Figure 1A) and BCAA levels (r=0.282, P=0.001; Figure 1B) were significantly positive. Correlation of skeletal muscle mass with the BTR was significantly negative (r=-0.259, P=0.003; Figure 1C). Subgroup analysis showed that skeletal muscle mass remained positively correlated with BCAA in both BCAA-presupplemented group (r=0.257, P=0.039; Figure 1D) and non-presupplemented group (r=0.259, P=0.040; Figure 1E), although the correlations between these parameters were weak. No significant correlations were identified between skeletal muscle mass and zinc (P=0.219), pre-albumin (P=0.143), total lymphocyte count (P=0.716) or ammonia (P=0.061).
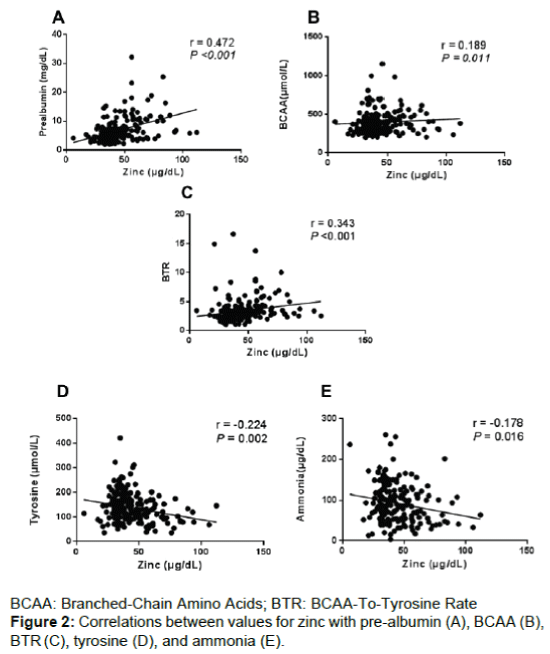
Correlations of preoperative zinc with pre-albumin (r=0.472, P<0.001; Figure 2A), BCAA (r=0.189, P=0.011; Figure 2B) and the BTR (r=0.343, P<0.001; Figure 2C) were significantly positive. While, correlations of zinc with tyrosine (r=-0.224, P=0.002, Figure 2D) and ammonia levels (r=-0.178, P=0.016; Figure 2E) were significantly negative, although the correlations between these parameters were weak. Significant correlations were not identified between zinc and the total lymphocyte count (P=0.750).
The effect of BCAA treatment before admission to LDLT
The clinical characteristics and surgical variables of the patients in BCAA+ and BCAA- groups on admission were compared in Table 1. There were no significant differences in age, sex, body mass index, Child-Pugh classification, MELD score, etiology of disease, number of ABO-incompatible grafts, donor age, operative blood loss and transfusion units (erythrocyte concentrates).
| Variable | BCAA+ (n=66) | BCAA- (n=63) | P |
|---|---|---|---|
| Donor age (years) | 43.7 ± 10.6 | 43.7 ± 10.6 | 0.465 |
| Recipient age at transplantation (years) | 52.2 ± 11.1 | 47.0 ± 14.4 | 0.148 |
| Gender (male/female) | 33/33 | 30/33 | 0.861 |
| Body mass index on admission (kg/m2) | 23.0 ± 4.3 | 21.6 ± 4.4 | 0.153 |
| Underlying disease | |||
| HCC with viral hepatitis | 22 | 14 | 0.168 |
| Viral hepatitis B/C related cirrhosis | 15 | 17 | 0.684 |
| PBC/PSC | 12 | 12 | 0.899 |
| Biliary atresis post Kasai | 3 | 5 | 0.486 |
| Alcoholic cirrhosis | 4 | 3 | 0.745 |
| Metabolic disease | 2 | 3 | 0.675 |
| NASH | 2 | 2 | 0.962 |
| Autoimmune hepatitis | 2 | 1 | 0.587 |
| Others | 4 | 6 | 0.534 |
| ABO compatibility | 0.057 | ||
| identical/compatible | 42 | 50 | |
| incompatible | 24 | 13 | |
| Child-Pugh classification (A, B/C) | 20/46 | 31/32 | 0.366 |
| MELD score | 18.3 ± 6.9 | 20.5 ± 8.2 | 0.754 |
| Graft type | 0.259 | ||
| Left lobe | 27 | 32 | |
| Right lobe | 39 | 31 | |
| Graft weight (g) | 541.1 ± 153.5 | 493.5 ± 113.2 | 0.754 |
| Surgical duration (min) | 914 ± 127 | 957 ± 167 | 0.544 |
| Intra-operative blood loss (ml) | 9556 ± 3544 | 9256 ± 3334 | 0.964 |
| Intra-operative erythrocyte transfusion (U) | 20.9 ± 10.6 | 21.3 ± 10.5 | 0.132 |
†BCAA: Branched-Chain Amino Acids; BCAA+: Received pre-admission BCAA treatment; BCAA-: Did not receive pre-admission BCAA treatment; HCC: Hepatocellular
Carcinoma; PBC: Primary Biliary Cirrhosis; PSC: Primary Sclerosing Cholangitis; NASH: Non-Alcoholic Steatohepatitis; MELD: Model for End-Stage Liver Disease
Table 1: Patient characteristics according to branched chain amino acids treatment given before admission.
Pre-albumin level on admission was significantly higher, while tyrosine level was significantly lower in the BCAA+ than in BCAAgroup (P=0.023 and P<0.001), respectively (Table 2). Zinc, BCAA, total lymphocyte count and ammonia levels did not significantly differ between both groups (P=0.834, P=0.421, P=0.781 and P=0.560), respectively. Consequently, the BTR was significantly higher in BCAA+ than in BCAA- group (P=0.046). Preoperative skeletal muscle mass did not significantly differ between both groups (P=0.143).
| Variable | BCAA+ (n=66) | BCAA- (n=63) | P | P* |
|---|---|---|---|---|
| Zinc (µg/dL) | 40.5 ± 10.2 | 47.2 ± 11.2 | 0.834 | |
| Prealbumin (mg/dL) | 7.9 ± 2.1 | 5.2 ± 2.1 | 0.0038 | 0.023 |
| BCAA (µmol/L) | 401.3 ± 48.2 | 392.7 ± 49.5 | 0.421 | |
| Tyrosine (µmol/L) | 124.7 ± 12.4 | 149.2 ± 13.1 | <0.001 | <0.001 |
| BTR | 3.7 ± 0.3 | 3.0 ± 0.3 | 0.0066 | 0.046 |
| Total lymphocyte count | 893.3 ± 213.1 | 841.9 ± 215.6 | 0.781 | |
| Ammonia (µg/dL) | 96.1 ± 17.3 | 93.5 ± 15.3 | 0.56 | |
| Skeletal muscle mass (%) | 96.8 ± 13.4 | 92.7 ± 12.5 | 0.143 |
Table 2: Levels of nutritional/metabolic parameters and skeletal muscle mass on admission according to pre-admission BCAA supplementation.
As for post-transplant outcomes, incidence of post-transplant bacteremia was significantly lower in BCAA+ than BCAA- group (30% versus 52%, P=0.011). However, the incidence of acute cellular rejection and the mean length of postoperative hospital stay were similar in the two groups (P=0.279, P=0.576, respectively).
Risk factor analysis for early posttransplant mortality
Univariate analysis revealed that neither pre-admission BCAA supplementation nor preoperative levels of nutritional/metabolic parameters on admission were significant risk factors for early posttransplant mortality (Table 3).
| Variable | 90-days OS | P | |
|---|---|---|---|
| Recipient Age (y) | <60 (n=88) | 86% | 0.283 |
| ≥ 60 (n=41) | 91% | ||
| Donor Age (y) | <50 (n=93) | 91% | 0.335 |
| ≥ 50 (n=36) | 85% | ||
| Sex | Male (n=63) | 91% | 0.143 |
| Female (n=66) | 83% | ||
| Original disease | HCC (n=36) | 81% | 0.144 |
| Non-HCC (n=93) | 90% | ||
| ABO blood type | Compatible (n=92) | 90% | 0.087 |
| Incompatible (n=37) | 81% | ||
| Child-Pugh classification | A, B (n=51) | 91% | 0.227 |
| C (n=78) | 84% | ||
| MELD score | <20 (n=69) | 92% | 0.069 |
| ≥ 20 (n=50) | 81% | ||
| GRWR | <0.8% (n=36) | ||
| ≥ 0.8% (n=93) | 91% | ||
| Graft | Right lobe (n=70) | 88% | 0.535 |
| Left lobe (n=59) | 86% | ||
| Operative time (h) | <12 (n=30) | 89% | 0.09 |
| ≥ 12 (n=99) | 83% | ||
| Operative blood loss (L) | <10 (n=88) | 92% | 0.079 |
| ≥ 10 (n=41) | 81% | ||
| Pre-transplant zinc level (µg/dL) | <39 (n=58) | 84% | 0.634 |
| ≥ 39 (n=71) | 88% | ||
| Pre-transplant prealbumin level (mg/dL) | <5.4 (n=64) | 83% | 0.343 |
| ≥ 5.4 (n=65) | 90% | ||
| Pre-transplant BCAA level (µmol/L) | <375.2 (n=62) | 81% | 0.476 |
| ≥ 375.2 (n=67) | 85% | ||
| Pre-transplant BTR | <2.92 (n=60) | 85% | 0.786 |
| ≥ 2.92 (n=69) | 87% | ||
| Pre-transplant tyrosine (µmol/L) | <131.7 (n=63) | 89% | 0.1 |
| ≥ 131.7 (n=66) | 85% | ||
| Pre-transplant total lymphocyte count (/µL) | <700 (n=61) | 85% | 0.698 |
| ≥ 700 (n=68) | 87% | ||
| Pre-transplant ammonia level (µg/dL) | <87 (n=61) | 90% | 0.5 |
| ≥ 87 (n=68) | 85% | ||
| BCAA supplementation before admission | with (n=66) | 91% | 0.329 |
| absent (n=63) | 86% |
Table 3: Univariate analysis of factors affecting post-transplant patient survival.
Risk factor analysis for post-transplant bacteremia
Fifty-three patients of 129 developed post-transplant bacteremia. Univariate analysis showed that incidence of post-transplant bacteremia was significantly higher in patients with Child-Pugh classification C (P=0.001), operative blood loss ≥ 10 L (P=0.010), preoperative total lymphocyte count <700/μL (P=0.004), and absence of preadmission BCAA treatment (P=0.011) (Table 4). Multivariate analysis demonstrated four independent adverse risk factors for posttransplant bacteremia: Child-Pugh classification C (P=0.012), preoperative total lymphocyte count <700/μL (P=0.027), operative blood loss of ≥ 10 L (P=0.039) and absence of pre-admission BCAA treatment (P=0.040) (Table 5).
| Variable | Incidence of post-transplant bacteremia | P | |
|---|---|---|---|
| Recipient Age (y) | <60 (n=88) | 47% | 0.063 |
| ≥ 60 (n=41) | 29% | ||
| Donor Age (y) | <50 (n=93) | 38% | 0.2 |
| ≥ 50 (n=36) | 50% | ||
| Sex | Male (n=63) | 41% | 0.967 |
| Female (n=66) | 41% | ||
| Original disease | HCC (n=36) | 42% | 0.933 |
| Non-HCC (n=93) | 41% | ||
| ABO blood type | Compatible (n=92) | 38% | 0.268 |
| Incompatible (n=37) | 49% | ||
| Child-Pugh | A, B (n=51) | 24% | 0.001 |
| C (n=78) | 53% | ||
| MELD score | <20 (n=69) | 48% | 0.397 |
| ≥ 20 (n=50) | 40% | ||
| GRWR | <0.8% (n=36) | 42% | 0.933 |
| ≥ 0.8% (n=93) | 41% | ||
| Graft | Right (n=70) | 47% | 0.128 |
| Left (n=59) | 34% | ||
| Operative time (h) | <12 (n=30) | 43% | 0.775 |
| ≥ 12 (n=99) | 40% | ||
| Operative blood loss (L) | <10 (n=87) | 33% | 0.01 |
| ≥ 10 (n=42) | 57% | ||
| Pre-transplant zinc level (µg/dL) | <39 (n=58) | 41% | 0.951 |
| ≥ 39 (n=71) | 41% | ||
| Pre-transplant pre-albumin level (mg/dL) | <5.4 (n=64) | 41% | 0.916 |
| ≥ 5.4 (n=65) | 42% | ||
| Pre-transplant BCAA level (µmol/L) | <375.2 (n=62) | 42% | 0.85 |
| ≥ 375.2 (n=67) | 40% | ||
| Pre-transplant BTR | <2.92 (n=60) | 42% | 0.9 |
| ≥ 2.92 (n=69) | 41% | ||
| Pre-transplant tyrosine (µmol/L) | <131.7 (n=63) | 37% | 0.229 |
| ≥ 131.7 (n=66) | 47% | ||
| Pre-transplant total lymphocyte count (/µL) | <700 (n=61) | 54% | 0.004 |
| ≥ 700 (n=68) | 29% | ||
| Pre-transplant ammonia level (µg/dL) | <87 (n=61) | 33% | 0.07 |
| ≥ 87 (n=68) | 49% | ||
| BCAA supplementation before admission | with (n=66) | 30% | 0.011 |
| absent (n=63) | 52% |
Table 4: Univariate analysis of factors affecting post-transplant bacteremia.
| Variable | Odds ratio | 95% CI | P |
|---|---|---|---|
| Child-Pugh classification C | 7.322 | 1.600-29.996 | 0.012 |
| Preoperative low total lymphocyte count (<700/µL) |
5.434 | 1.313-20.133 | 0.027 |
| Operative blood loss ≥ 10 L | 4.230 | 1.373-16.763 | 0.039 |
| Absence of pre-admission BCAA treatment | 2.942 | 1.644-8.643 | 0.040 |
Table 5: Multivariate analysis of factors affecting post-transplant bacteremia.
Discussion
This retrospective study is the first to collectively examine pretransplant characteristics of nutritional/metabolic parameters and skeletal muscle mass among patients with end-stage liver disease undergoing LDLT and investigate the impact of preoperative BCAA treatment before admission. Moreover, BCAA treatment before admission improved nutritional status in LDLT candidates. BCAA treatment before admission and preoperative TLC level has potential impacts on the incidence of post-transplant bacteremia. Selberg et al. [18] conducted a prospective cohort study of nutritional and metabolic parameters in 150 patients with end-stage liver disease undergoing LT. They showed that a poor nutritional state as well as hypermetabolism was not only an important prognostic factor in the evaluation of the risks of patients but also adversely affected survival after LT, the concept of which is in line with our recent report showing that low preoperative skeletal muscle mass was closely involved with post-transplant mortality [7]. In the present study, we revealed that preoperative BCAA treatment before admission could ameliorate the incidence of post-transplant bacteremia and improve post-transplant mortality.
We previously uncovered a significantly negative correlation between skeletal muscle mass and the BTR [7]. At that time, we speculated that since BCAA are mainly metabolized in the skeletal muscle of patients with cirrhosis, more skeletal muscle mass and more BCAA consumption would lead to a decrease in the BTR. However, the present study identified a significantly positive correlation between skeletal muscle mass and BCAA. This finding can be explained by the fact that BCAA is also released from skeletal muscle protein due to endogenous breakdown under hypercatabolic conditions in liver cirrhosis [19]. This represents another source of plasma BCAA beside dietary intake in patients with cirrhosis. Therefore, when skeletal muscle mass decreases in such patients, BCAA also decreases due to skeletal muscle mass depletion. Moreover, the correlation between skeletal muscle mass and tyrosine was notably significant and positive. Tyrosine was similarly reported to be released from muscle protein breakdown [20], however, it is solely metabolized by the liver without being metabolized nor consumed by the muscle, probably explaining the stronger correlation of pre-transplant skeletal muscle mass with tyrosine than with BCAA. Thus, the negative correlation between skeletal muscle mass and BTR would be mainly due to positive correlation between skeletal muscle mass and tyrosine.
We recently reported that Child-Pugh classification C and massive operative blood loss were independent risk factors for post-transplant bacteremia [21]. In the present study, in addition to these variables, low pre-operative total lymphocyte count and absence of pre-admission BCAA therapy were newly revealed to be independent risk factors for post-transplant bacteremia. This finding strongly suggests that pre-transplant nutritional status is closely involved with the onset of post-transplant bacteremia. We are now investigating immunological mechanism why pre-transplant nutritional treatment is beneficial to prevent post-transplant severe infection.
Some limitations must be borne in mind when considering this study. First, BCAA were introduced at the discretion of the attending physician before referral of a potential recipient for LDLT. In the present study, however, clinical background including Child-Pugh and MELD scores between both groups were comparable on admission. Therefore, general condition and selection bias might have been at minimum. Second, because of retrospective nature of this study, nutritional status before BCAA administration was unclear and the exact duration and amount of BCAA given within the preoperative nutritional therapy protocol were different patient by patient. Prospective randomized study to examine the effect of pre-admission BCAA treatment is thus needed to adjust these biases. Third, as this was a cross-sectional study, we have not completely verified a causal relationship between BCAA pre-supplementation and parameter levels on admission. Therefore, it will be necessary to perform a future cohort study. Lastly, a limitation was reported for BIA in assessment of body water components of cirrhotic patients with ascites [22]. Fürstenberg et al reported that BIA was highly correlated with other methods in measuring lean body mass even in over-hydrated subjects [23]. Moreover, we recently reported that the effect of over hydration on overestimation of BIA might be, if any, minimum in using this device [24]. At present, therefore, segmental multi-frequency BIA used in this study would be best modality to assess skeletal muscle mass.
In conclusion, BCAA therapy before admission could ameliorate the incidence of post-transplant bacteremia and could enhance improve disease-induced amino acid imbalance or protein status in LDLT candidates. Further randomized clinical trials are warranted to confirm our hypothesis.
References
- Marchesini G, Fabbri A, Bianchi G, Brisi M, Zoli M (1996) Zinc supplementation and amino acid-nitrogen metabolism in patients with advanced cirrhosis. Hepatology 23: 1084-1092.
- Shenkin A (2006) Serum prealbumin: Is it a marker of nutritional status or of risk of malnutrition? Clin Chem 52: 2177-2179.
- Nagai S, Yoshida A, Kohno K, Altshuler D, Nakamura M, et al. (2014) Peri-transplant absolute lymphocyte count as predictive factor for advanced recurrence of hepatitis c after liver transplantation. Hepatology 59: 35-45.
- Goldbecker A, Buchert R, Berding G, Bokemeyer M, Lichtinghagen R, et al. (2010) Blood-brain barrier permeability for ammonia in patients with different grades of liver fibrosis is not different from healthy controls. J Cereb Blood Flow Metab 30: 1384-1393.
- Michitaka K, Hiraoka A, Kume M, Uehara T, Hidaka S, et al. (2010) Amino acid imbalance in patients with chronic liver disease. Hepatol Res 40: 393-398.
- Stickel F, Inderbitzin D, Candinas D (2008) Role of nutrition in livertransplantation for end-stage chronic liver disease. Nutr Rev 66: 47-54.
- Kaido T, Ogawa K, Fujimoto Y, Ogura Y, Hata K, et al. (2013) Impact of sarcopenia on survival in patients undergoing living donor liver transplantation. Am J Transplant 13: 1549-1556.
- Shimomura Y, Murakami T, Nagasaki M, Honda T, Goto H, et al. (2004) Regulation of branched-chain amino acid metabolism and pharmacological effects of branched-chain amino acids. Hepatol Res 30: 3-8.
- Vulcano DS, Carvalhaes MA, Bakonyi N (2013) Evaluation of nutritional indicators and body composition in patients with advanced liver disease enrolled for liver transplantation. Acta Cir Bras 28: 733-739.
- Ward LC. (2012) Segmental bioelectrical impedance analysis: An update. Curr Opin Clin Nutr Metab Care 15: 424-429.
- Malavolti M, Mussi C, Poli M, Fantuzzi AL, Salvioli G, et al. (2003) Cross-calibration of eight-polar bioelectrical impedance analysis versus dual-energy X-ray absorptiometry for the assessment of total and appendicular body composition in healthy subjects aged 21-82 years. Ann Hum Biol 30: 380-391.
- Morioka D, Egawa H, Kasahara M, Ito T, Haga H, et al. (2007) Outcomes of adult-to adult living donor liver transplantation: a single institution’s experience with 335 consecutive cases. Ann Surg 245: 315-325.
- Inomata Y, Uemoto S, Asonuma K, Egawa H (2000) Right lobe graft in living donor liver transplantation. Transplantation 69: 258-264.
- Inomata Y, Tanaka K, Egawa H, Uemoto S, Ozaki N, et al. (1996) The evolution of immunosuppression with FK 506 in pediatric living related liver transplantation. Transplantation 61: 247-252.
- Egawa H, Ohdan H, Haga H, Tsuruyama T, Oike F, et al. (2008) Current status of liver transplantation across ABO blood-type barrier. J Hepatobiliary Pancreat Surg 15: 131-138.
- Garner J, Jarvis W, Emori T, Horan TC, Hughes JM (1988) CDC definitions for nosocomial infections, 1988. Am J Infect Control 16: 128-140.
- Holm S (1979) A simple sequentially rejective multiple test procedure. Scand J Stat 6: 65-70.
- Selberg O, Bottcher J, Tusch G, Pichlmayr R, Henkel E, et al. (1997) Identification of high- and low-risk patients before liver transplantation: a prospective cohort study of nutritional and metabolic parameters in 150 patients. Hepatology 25: 652-657.
- Mager DR, Wykes LJ, Roberts EA, Ball RO, Pencharz PB (2006) Mild-to-moderate chronic cholestatic liver disease increases leucine oxidation in children. J Nutr 136: 965-970.
- Hasselgren PO, Hall-Angeras M, Angerås U, Benson D, James JH, et al. (1990) Regulation of total and myofibrillar protein breakdown in rat extensor digitorum longus and soleus muscle incubated flaccid or at resting length. Biochem J 267: 37-44.
- Kaido T, Mori A, Ogura Y, Ogawa K, Hata K, et al. (2012) Pre- and perioperative factors affecting infection after living donor liver transplantation. Nutrition 28: 1104-1108.
- Hara N, Iwasa M, Iwata K, Miyachi H, Tanaka H, et al. (2009) Value of the extracellular water ratio for assessment of cirrhotic patients with and without ascites. Hepatol Res 39: 1072-1079
- Fürstenberg A, Davenport A (2011) Assessment of body composition in peritoneal dialysis patients using bioelectrical impedance and dual-energy X-ray absorptiometry. Am J Nephrol 33: 150-156.
- Kaido T, Uemoto S (2013) Direct segmental multi-frequency bioelectrical impedance analysis is useful to evaluate sarcopenia. Am J Transplant 13: 2506-2507.
Citation: Hammad A, Kaido T, Yagi S, Okajima H, Uemoto S (2016) Characteristics of Nutritional Status and the Effect of Pre-Transplant Branched-Chain Amino Acid Administration in Patients Undergoing Living Donor Liver Transplantation. J Clin Exp Transplant 1: 101. DOI: 10.4172/2475-7640.1000101
Copyright: © 2016 Hammad A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 12397
- [From(publication date): 7-2016 - Apr 05, 2025]
- Breakdown by view type
- HTML page views: 11538
- PDF downloads: 859