Research Article Open Access
Characteristic Features of an Analytical Column with a Pentafluorophenylpropyl Stationary Phase Applied To a Determination of a Fluorinated Phenyl Alanyl Derivative Compound, Gw823093, In Human Urine Using an Lc-Esi-Ms/Ms Method
Harue Igarashi1*, Shizuko Mogi1, Akira Wakamatsu1, Yoshiyuki Minamide2 and Shinobu Kudoh22Shimadzu Techno-Research, Inc, Japan
- *Corresponding Author:
- Harue Igarashi
4-6-15, Sendagaya, Shibuya-ku, Tokyo, 151-8566, Japan
Tel: +81-3-5786-4509
Fax: +81-3-5786-5227
E-mail: harue.2.igarashi@gsk.com
Received date: November 04, 2011; Accepted date: December 02, 2011; Published date: December 05, 2011
Citation: Igarashi H, Mogi S, Wakamatsu A, Minamide Y, Kudoh S (2011) Characteristic Features of an Analytical Column with a Pentafluorophenylpropyl Stationary Phase Applied To a Determination of a Fluorinated Phenyl Alanyl Derivative Compound, Gw823093, In Human Urine Using an Lc-Esi-Ms/Ms Method. J Anal Bioanal Techniques S5:001. doi: 10.4172/2155-9872.S5-001
Copyright: © 2011 Igarashi H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
Pentafluorophenylpropyl (PFPP) stationary phases allow for dipole-dipole, pi-pi, charge transfer and ion- exchange interactions and have shown unique retention of small, polar analytes. PFPP columns are able to retain protonated bases in the presence of high percentages of an organic modifier, which is a suitable condition for liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS) analysis. Taking advantage of the characteristics of PFPP columns, a liquid chromatography-electrospray ionization coupled with tandem mass spectrometry (LC-ESI-MS/MS) method was developed to quantify the concentration of a fluorinated phenylalanyl derivative compound, GW823093, in human urine. The analyte is retained with great difficulty on an octadecylsilane- bonded silica (ODS) column, but was strongly retained on a PFPP column in 5 mmol/L ammonium formate buffer (pH 2.9) even with an acetonitrile concentration as high as 80%; these conditions protonated the analyte and enabled higher MS signal intensity. However, the analyte peak shape deteriorated after repeated sample injections when urine samples were analyzed under the selected conditions. To solve this problem, an on-line sample clean-up process with an ODS column was employed before the sample was introduced to the PFPP analytical column. A good peak shape without ion suppression was achieved when using acidified buffer containing 10% acetonitrile as a washing solvent for the process, suggesting that components retained by ionic and weakly hydrophobic interactions were removed. This method was optimized and was linear in quantitation in the range of 25-5000 ng/mL, with a mean coefficient of determination over 0.9998. The validated method was successfully applied to a clinical study of GW823093.
Keywords
LC-MS/MS; ESI; PFPP; Polar compound; Basic drug; Quantification; Human urine
Abbreviations
PFPP:Pentafluorophenylpropyl; ODS: Octadecylsilane- bonded silica; HILIC: Hydrophilic interaction chromatography; LC-MS/MS: Liquid chromatography coupled with tandem mass spectrometry; ESI: Electrospray Ionization; IS: Internal Standard; DMF: Dimethylformamide
Introduction
Octadecylsilane-bonded silica (ODS) columns are the most widely used columns for the liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS) analysis of small molecules such as pharmaceuticals, but the retention of polar compounds containing hydrophilic groups, such as amino groups, on this type of column is weak. To achieve adequate retention of polar compounds, ion- pairing reagents are often added to the mobile phase in reversed- phase chromatography. Ion-pairing reagents suppress the ionization of analyte, particularly during electrospray ionization (ESI), due to competitive ionization of the analyte and the reagents. Ion-exchange chromatography, hydrophilic interaction chromatography (HILIC) and normal-phase chromatography all adequately retain polar compounds. However, they are particularly difficult to apply to LC- MS/MS analysis due to the use of non-volatile buffers and to the loss of polar compounds by precipitation.
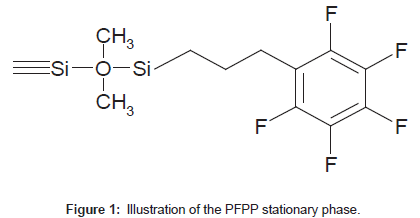
Pentafluorophenylpropyl (PFPP) stationary phases (Figure 1) allow for dipole-dipole, pi-pi, charge transfer and ion-exchange interactions [1-3] and have shown unique retention of small, polar analytes. PFPP columns exhibit both reversed- and normal-phase retention of polar analytes, depending on the mobile phase composition [2]. PFPP columns are able to retain protonated bases in the presence of high percentages of an organic modifier, which are suitable conditions for LC-MS/MS analysis [2-6].
The fluorinated phenylalanyl derivative compound GW823093 is retained with great difficulty on ODS columns. PFPP columns are considered to be suitable for the quantitative analysis of this analyte using LC-MS/MS techniques. This report describes the characteristic features of an analytical column with a PFPP phase applied to the determination of the concentration of a fluorinated phenylalanyl derivative compound, GW823093, in human urine using an LC-ESI-MS/MS method.
Experimental
Chemicals and materials
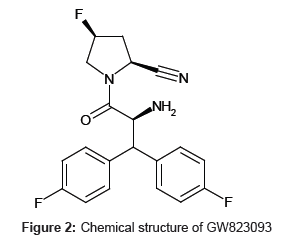
Standard reference materials for GW823093 tosylate (Figure 2) and [13C2, 15N]-GW823093 tosylate, used as an internal standard (IS), were obtained from GlaxoSmithKline (London, UK). Formic acid (HPLC grade) and ammonium formate (analytical grade) were purchased from Wako Pure Chemical Industries (Osaka, Japan). Acetonitrile (HPLC grade) and dimethyl formamide (DMF, analytical grade) were purchased from Kanto Chemical (Tokyo, Japan). Water purified with a Milli-Q gradient-A10 purification system (Millipore, Bedford, MA, USA) was used.
Preparation of standard solutions and samples
Each sock solution of GW823093 (1 mg/mL as a free base) or IS (1 mg/mL as a free base) was prepared by dissolving the compound in DMF. An IS working solution was prepared by diluting the stock solution in 5 mmol/L formic acid/ammonium formate buffer in 10% acetonitrile (100 ng/mL). A stock solution of the analyte was diluted with blank human urine or various mobile phases to meet experimental objectives.
LC-MS/MS system
An alliance HT Separation Module 2795 HPLC system equipped with a 10-port switching valve (Waters, MA, USA) was used. Mass spectrometry detection was performed on a Quattro II mass spectrometer (Micromass, Manchester, UK) utilizing electrospray ionization (ESI) in the positive mode. The instrument was initially tuned to obtain the MS conditions that enabled the highest responses. All data were acquired and analyzed using MassLynx version 4.0 (Micromass).
Quantification procedures
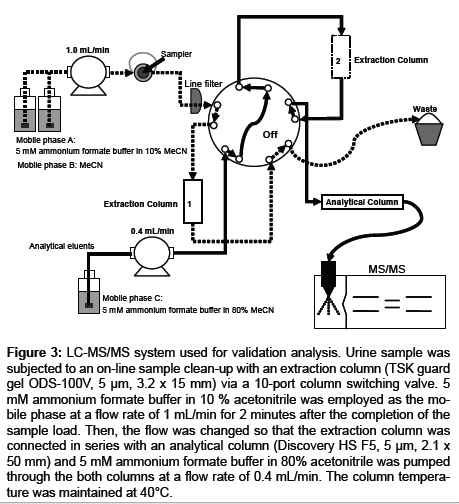
One hundred microliters of urine samples and 400 μL aliquots of IS working solution were mixed, and the mixture was subjected to an on-line sample clean-up with an extraction column (TSK guard gel ODS-100V, 15 mm length x 3.2 mm id, 5 μm, Tosoh, Tokyo, Japan) via a 10-port column switching valve (Waters, MA, USA). Five millimolar formic acid/ammonium formate (9/1, v/v) in 10 % acetonitrile (extraction solvent) was employed as the mobile phase for the clean-up process at a flow rate of 1 mL/min. The mobile phase was introduced for an additional 2 minutes after the completion of the sample load. The eluate was discarded to the waste. Then, the flow was changed so that the extraction column was connected in series with an analytical column (Discovery HS F5, 50 mm length x 2.1 mm id, 5 μm, Supelco, PA, USA), and 5 mmol/L formic acid/ammonium formate (9/1, v/v) in 80% acetonitrile was pumped through the both columns at a flow rate of 0.4 mL/min. The column temperature was maintained at 40°C (Figure 3).
Figure 3: LC-MS/MS system used for validation analysis. Urine sample was subjected to an on-line sample clean-up with an extraction column (TSK guard gel ODS-100V, 5 μm, 3.2 x 15 mm) via a 10-port column switching valve. 5 mM ammonium formate buffer in 10 % acetonitrile was employed as the mobile phase at a flow rate of 1 mL/min for 2 minutes after the completion of the sample load. Then, the flow was changed so that the extraction column was connected in series with an analytical column (Discovery HS F5, 5 μm, 2.1 x 50 mm) and 5 mM ammonium formate buffer in 80% acetonitrile was pumped through the both columns at a flow rate of 0.4 mL/min. The column temperature was maintained at 40°C.
The method validation
The validation was conducted according to the established quantitation method in accordance with the FDA guidance for Industry “Bioanalytical Method Validation” issued and enforced in 2001 [7].
Results and Discussion
Ionization
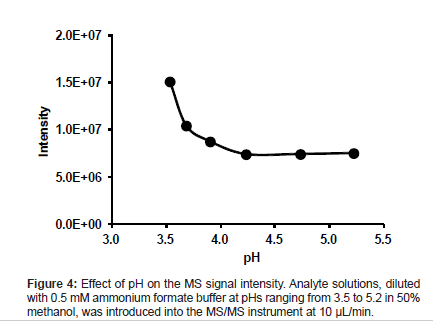
The effects of the pH of the eluent to be used on the MS signal intensity and MS spectra were investigated using a mixture of methanol and buffer (1:1, v/v) at pH values ranging from 3.5 to 5.2. An analyte stock solution was diluted with each eluent prepared (50 ng/mL), and each diluted solution was introduced into the MS/MS instrument through the ESI ionization interface at 10 μL/min.
The MS signal intensity of the analyte increased with acidity at pHs lower than 4.2. The highest MS signal intensity was obtained at pH 3.5 (Figure 4). As the pKa of the analyte is 6.1, the analyte exists mostly in the ion form at all pH values studied. However, the MS signal intensity did not change at pHs higher than 4.2. This result indicates that the ionization of the analyte is influenced by unknown mechanisms in addition to dissociation.
Effect of buffer pH and acetonitrile content
The analyte was retained with great difficulty on an ODS column even though buffer pH was 6.5, which keeps the analyte in the molecular form. Because PFPP columns are able to retain protonated bases even in the presence of high percentages of organic solvent [2-6], we reasoned that a PFPP column was likely to be suitable for the LC- MS/MS analysis of this analyte.
Using 5 mmol/L formic acid, whose pH was close to the one giving the highest ionization abundance, with acetonitrile as the mobile phase, the analyte was strongly retained on the PFPP column and was not eluted with acetonitrile contents as high as 80% (data not shown). To elute an analyte from a PFPP column, counter ions are needed because the retention of protonated bases at high percentages of organic modifier is characterized by strong ion-exchange interactions between the analyte and the ionized surface silanol groups in addition to non- ionic interactions [3].
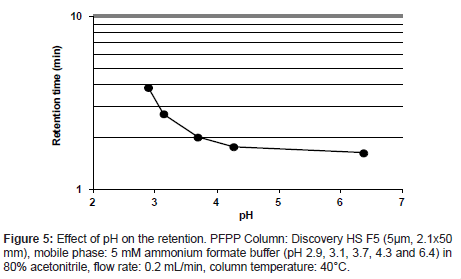
A retention behavior of the analyte on the PFPP column was confirmed by varying the mixing ratio of 5 mmol/L formic acid to 5 mmol/L ammonium formate solution. The rations tested were 9/1, 8/2, 5/5, 2/8 and 0/10 (v/v), yielding solutions with pHs ranging from 2.9 to 6.4. The analyte was eluted at all pH values of buffer prepared (Figure 5). The retention was stronger at lower pHs, and the maximum retention was observed at pH 2.9, which would enable a higher MS signal intensity. Therefore, it was considered that PFPP columns are suitable for the quantitative analysis of the analyte using LC-MS/MS techniques.
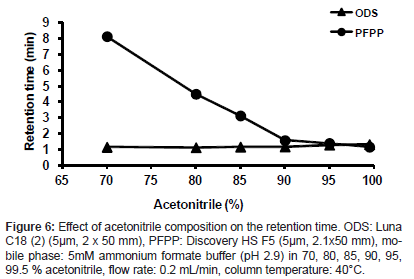
When varying the acetonitrile concentration from 70 to 99.5% in a mobile phase containing 5 mmol/L ammonium formate buffer (pH 2.9), the retention ability of the analyte on the PFPP column was compared with that on an ODS column (Luna C18 (2), 50 mm length x 2 mm, 5 μm, Phenomenex, CA, USA). The analyte was retained on the PFPP column at acetonitrile percentages of 85% or below but was not retained on the ODS column under any conditions studied (Figure 6). At 80% acetonitrile or below, a good peak shape of the analyte was obtained. Therefore, 80% acetonitrile containing 5 mmol/L ammonium formate buffer (pH 2.9) was selected as the mobile phase for the LC- MS/MS analysis. Thus, conditions enabling a high ionization efficiency and an adequate retention of the analyte were achieved with an acidic buffer solution and with high percentages of an organic component, which is highly advantageous for the use of a PFPP column with LC- ESI-MS analysis.
Remove matrix effect
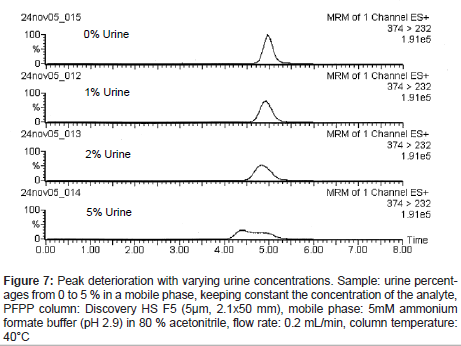
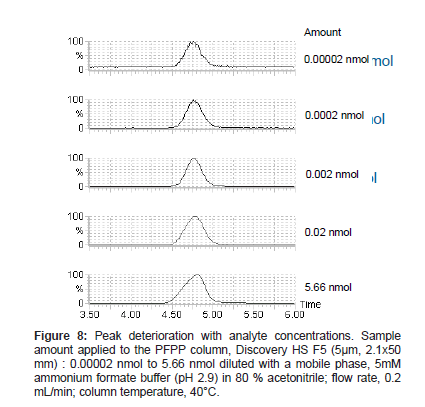
When urine samples were analyzed under the selected conditions, the analyte peak shape deteriorated. It was thought that the peak deterioration might be caused by endogenous substances in the urine. Samples prepared with varying urine percentages from 0 to 5 %, keeping constant the concentration of the analyte, were analyzed, and the analyte peak shape deteriorated more with increasing urine percentages (Figure 7). In addition, peak shape deterioration was also observed in standard samples at high analyte concentrations applied to the column (Figure 8). Based on these observations, we reasoned that the peak shape deterioration was caused by the total amount of ionic substances applied to the column, which max out the possible sites for ionic interaction. Therefore, a process to remove highly ionized substances coexisting with the analyte was considered to be necessary before the sample was subjected to the PFPP column. When acidified aqueous buffer solution was used with a reversed-phase column for the process, the analyte peak shape was improved. However, the peak area was lower than those of the standard solution, suggesting that ion suppression occurred due to the co-elution of endogenous components probably participating in weakly hydrophobic interactions (data not shown).
Figure 7: Peak deterioration with varying urine concentrations. Sample: urine percent- ages from 0 to 5 % in a mobile phase, keeping constant the concentration of the analyte, PFPP column: Discovery HS F5 (5μm, 2.1x50 mm), mobile phase: 5mM ammonium formate buffer (pH 2.9) in 80 % acetonitrile, flow rate: 0.2 mL/min, column temperature: 40°C
The ion suppression could be avoided by adding acetonitrile to the buffer. A good peak shape without ion suppression was achieved with 5 mmol/L ammonium formate buffer (pH 2.9) with 10% acetonitrile content, suggesting that the components retained by the ionic and weakly hydrophobic interactions were removed.
Application of the method
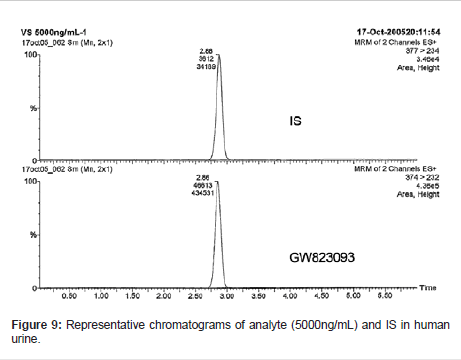
The optimized method was validated for assaying the analyte in human urine in accordance with the FDA guidelines for bioanalytical method validation [7]. The LC-MS/MS system used in the validation study is depicted in Figure 3.
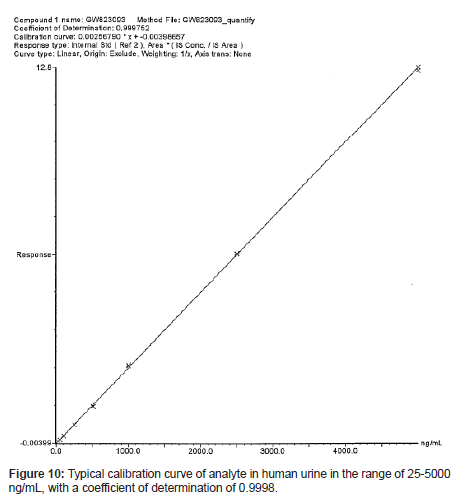
The calibration curves (n=3) were constructed by plotting the analyte/IS peak area ratio versus the spiked drug concentration, and these curves were linear in the range of 25-5000 ng/mL, with a mean coefficient of determination over 0.9998. A typical calibration curve is presented in Figure 10.
The intra-day and inter-day variabilities were assessed by analyzing QC samples at 5 concentration levels with 6 replicates. The CV and Bias of the analyte at all concentration levels were within 4.6% and 5.1%, respectively. The comprehensive results for the intra-day and inter-day variability assessments are presented in Table 1.
| Nominal Conc. (ng/mL) |
Intra-day reproducibility (n=6) | Inter-day reproducibility (n=3) | ||||
| Measured Conc. (ng/mL) | CV (%) | Bias (%) | Measured Conc. (ng/mL) | CV (%) | Bias (%) | |
| 25.3 | 26.3 | 4.6 | 3.8 | 26.6 | 3.4 | 5.1 |
| 101.3 | 101.1 | 1.7 | -0.2 | 99.3 | 2.2 | -2.0 |
| 506.5 | 507.5 | 1.2 | 0.2 | 505.2 | 1.4 | -0.3 |
| 4051.7 | 4008.2 | 0.9 | -1.1 | 4030.0 | 0.8 | -0.5 |
| 5064.7 | 4971.8 | 1.1 | -1.8 | 4972.4 | 1.1 | -1.8 |
Table 1: Intra-day and inter-day variability assesments
The validated method was successfully applied to a Phase I study of GW823093 in healthy Japanese subjects.
Conclusion
An LC-ESI-MS/MS method to quantify the concentration of a fluorinated phenylalanyl derivative compound, GW823093, in human urine was developed using a PFPP analytical column connected in series with an ODS column employed for sample clean-up. The analyte was strongly retained on the PFPP column, and a good peak shape was obtained without ion suppression. The method was validated and successfully applied to a clinical study.
Conflict of Interest Statement
The authors declared no conflict of interest.
Acknowledgements
This study was performed at the Bioanalysis Department, Takasaki Development Laboratories, GlaxoSmithKline K.K., which was closed in 2008.
The authors wish to express their deep gratitude for Dr. Toshiyasu Hirama and Mr. Zene Matsumoto (GlaxoSmithKline K.K. Japan) for allowing the publication of this manuscript. Mr. Koji Teramura’s cooperation with this study is greatly appreciated.
References
- Reta M, Carr PW, Sadek PC, Rutan SC (1999) Comparative Study of Hydrocarbon, Fluorocarbon, and Aromatic Bonded RP-HPLC Stationary Phases by Linear Solvation Energy Relationships. Anal Chem 71: 3484-3496.
- Bell DS, Jones AD (2005) Solute attributes and molecular interactions contributing to“U-shape”retention on a fluorinated high-performance liquid chromatography stationary phase. J Chromatogr A 1073: 99-109.
- Bell DS, Cramer HM, Jones AD (2005) Rational method development strategies on a fluorinated liquid chromatography stationary phase: Mobile phase ion concentration and temperature effects on the separation of ephedrine alkaloids. J Chromatogr A 1095: 113-118.
- Needham SR, Brown PR, Duff K, Bell DS (2000) Optimized stationary phases for the high-performance liquid chromatography–electrospray ionization mass spectrometric analysis of basic pharmaceuticals. J Chromatogr A 869: 159-170.
- Needham SR, Jeabville PM, Brown PR, Estape ES (2000) Performance of a pentafluorophenylpropyl stationary phase for the electrospray ionization high-performance liquid chromatography–mass spectrometry–mass spectrometry assay of cocaine and its metabolite ecgonine methyl ester in human urine. J Chromatogr B Biomed Sci App 748: 77-87.
- Hëctor Gallart-Ayala, Nüñez O, Moyano E, Galceran MT (2011) Analysis of UV ink photoinitiators in packaged food by fast liquid chromatography at sub-ambient temperature coupled to tandem mass spectrometry. J Chromatogr A 1218: 459-466.
- US Department of Health and Human Services, Food and Drug Administration, Guidance for Industry Bioanalytical Method Validation, 2001.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 16541
- [From(publication date):
specialissue-2014 - Nov 23, 2024] - Breakdown by view type
- HTML page views : 12075
- PDF downloads : 4466