Changing Trophic Pattern in a Highly Urbanized Tropical Estuary (Cochin estuary), India
Received: 14-Oct-2019 / Manuscript No. jmsrd-22-001 / Editor assigned: 03-Aug-2022 / PreQC No. jmsrd-22-001 / Reviewed: 17-Aug-2022 / QC No. jmsrd-22-001 / Revised: 22-Aug-2022 / Manuscript No. jmsrd-22-001 / Accepted Date: 22-Aug-2022 / Published Date: 29-Aug-2022 DOI: 10.4172/2155-9910.1000356
Introduction
Coastal zones are bright spots for the current and future population distribution and growth in the world. The coasts of estuaries are one of the most heavily populated areas in the world. Estuaries are very rich in biodiversity and play a key role in nutrient recycling, oxygen production, microclimate regulation and world climate. But high population pressure in and around the coastal systems threaten the biodiversity within the ecosystem. It is estimated those Asian countries mainly: China, India, Bangladesh, Indonesia and Vietnam accounted for 70% of the global coastal zone population by 2060. India will contribute more to the overall population between the years 2000 to 2060 by the three fold increase in coastal zone population. If the existing trend of urban population growth tend to continue, Kochi urban agglomeration will be the first order urban center of the state, both area wise and population wise in the future decades (state urbanization report, 2011).
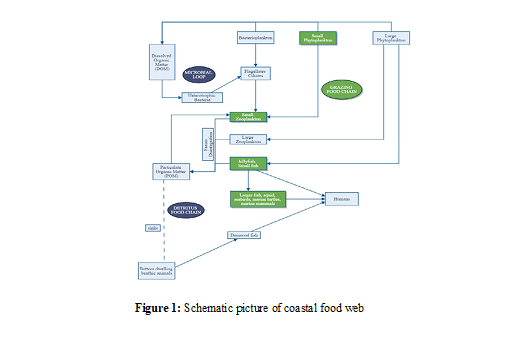
The energy flow in the estuarine system is regulated by three major path- ways: grazing food chain, microbial food chain and detritus chain. A schematic picture of coastal food web is represented in the Fig.1. In phytoplankton-generated grazing food webs, Zooplanktons and are fed upon by the secondary consumers (anchovies, silversides, and jelly fishes) which are then consumed by larger predatory and marine animals such as large fishes, marine mammals, marine turtles and seabirds. The microbial food chain is a trophic pathway which incorporates dissolved organic carbon into the grazing food chain. In the detritus based food chain, the role of phytoplankton and herbivorous zooplankton is taken over by the detritus and transfer energy directly to the fishes (Figure 1).
The photoautotrophs and chemoautotrophs acquire energy and heterotrophs consume the autotrophs thereby allowing the energy to flow in an ecosystem. The autotrophic production (primary production) determines the energy transfer in each trophic level of a food web. Primary production in estuaries is guided by two principles where it is regulated through both biotic mechanisms (trophic interactions including competition and predation) and abiotic mechanisms (nutrient fluxes, physical variability). Temporal shifts can occur in the relative importance of biotic and abiotic mechanisms, so that “top down” and “bottom up” forces work in concert but at time-varying rates, any changes in the population level by abiotic forcing at one trophic level, cascades to the other trophic levels. Many studies assume the food web importance of phytoplankton- zooplankton trophic interactions in estuarine systems, but rarely this has been measured at the community level. Phytoplankton community structure determines the efficiency of energy transfer between trophic levels [1].
A major portion of the Cochin estuary has already been diminished due to uncontrolled reclamation activities for various projects (International Container Transhipment Terminal (ICTT), Vembanad Railway bridge, Expansion of marine drive etc.) as well as due to illegal encroachment. According to the study conducted by Central Pollution Control Board (CPCB) and Indian Institute of Technology, New Delhi, in 2012, Cochin was ranked at the 24th position among the 88 industrial areas of India, where the comprehensive environmental pollution index (CEPI) was calculated for the selected cities. Under these circumstances, the study was conducted to study the trophic status, visavis the primary and secondary productivity of the Kochi estuary and its relation to fishery production.
Materials and Methods
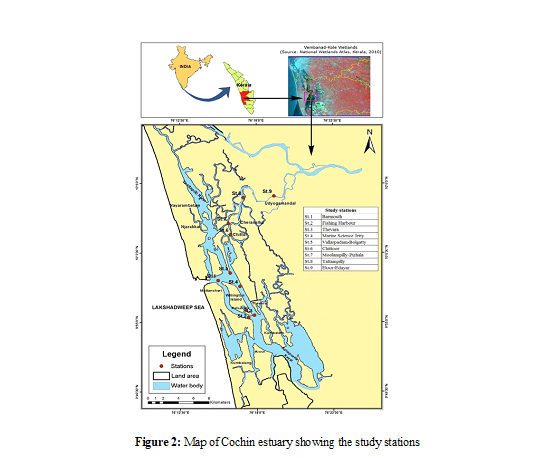
The Cochin estuary is part of the Vembanad wetland system, an impor- tant Ramsar site of Kerala. The Cochin estuary is characterized by an ox- bow shape running almost parallel to the Arabian Sea, with its northern boundary at Azhikode and southern boundary at Thanneermukkam bund. It is located between 76o9’25” E- 76 o 24’28” E longitudes and 9 o 47’31” N - 10 o 12’N latitudes (Fig.2). It is a micro tidal and positive estuary. The Cochin estuary is separated from the Arabian Sea by barrier spits interrupted by tidal inlets, one at Fort Cochin and other at Munambam.
The width of the estuary varies from 0.05 km to 4 km, while the depth varies from 0.05 m to 12 m. Cochin estuary is a monsoonal estuary in which the river discharge exhibits large seasonal variation. The tides in the estuary are of mixed semi diurnal tides. From the total area of the Cochin estuary (23100 ha), 6785 ha was demarcated as the study region. From this area, nine sampling stations were fixed, based on the various environmental disturbances (Figure 2).
Monthly field sampling was conducted for 24 months, from June 2009 to May 2011, in nine selected stations of the Cochin estuary. The monthly sampling data were aggregated together based on the rainfall and river runoff of the region to arrive at seasonal trends [2]. Based on the southwest monsoon and northeast monsoon pattern along the west coast of India, there are three well defined seasons, high runoff months characterized by southwest monsoon as monsoon period (June-September), moderate runoff months characterized by northeast monsoon as post-monsoon period (October-January) and low runoff months or dry period as pre-monsoon period (February- May). For the purpose of elaborating and discussing water quality and productivity parameters the nine study locations were demarcated into three zones. The northern zone of the estuary was represented by stations 6, 7, 8 and 9; stations 2 and 3 were clustered into southern zone while stations 1, 4 and 5 were demarcated as central zone of the estuary. The salinity regime based on the location was the major factor that was followed for demarcating three zones, where the southern zone and central zones were mesohaline (5-18 ppt) and the northern zone was oligohaline (0.5-5 ppt) according to the Venice System of classification (1959) (Figure 2).
Sampling and Methods
The rainfall data of the study area during the study period was obtained from the resources of the Hydrometeorology division of the Indian Meteorological Department (www.imd.gov.in). Daily river discharge during the study period has been obtained from Central Water Commission, Govt. of India (www.india-wris.nrsc.gov.in). Salinity was measured by Mohr-Knudsen method. Dissolved oxygen was estimated by the modified Winkler method .Ammonia-nitrogen was analyzed using the phenate method. Nitrite-nitrogen was measured using the diazotized method. Nitrate-nitrogen was estimated using the resorcinol method. Dissolved inorganic phosphate-phosphorus was measured using the ascorbic acid method. The dissolved silicate-silicon in the water was estimated using the molybdosilicate method [3].
The primary productivity (gross, net production and community respiration) was estimated by using the in situ incubation method, employing the Light and Dark bottle method). For the estimation of water column production, the average euphotic zone depth of the estuary was calculated and the photoperiod was taken as 12 hours. The shallowness of the estuary allows the light to penetrate up to the bottom, resulting in high benthic productivity. So, we consider the euphotic zone depth as 80% of the Secchi disc depth. The gross primary productivity (in terms of area) was converted to column productivity (in terms of volume) by multiplying it with the euphotic depth [4]. The photosynthetic efficiency of the estuary was calculated from the column production as well as the solar radiation data (source: European Centre for median range weather forecasts). The nitrate interference in the community respiration was effectively removed by the azide modified oxygen method. The chlorophyll a, b, c and the accessory pigments like phaeophytin, carotenoids and active chlorophyll a were estimated by the Vacuum filtration –acetone ex- traction method. Total chlorophyll (chl a + chl b + chl c), total pigments (chlorophylls+ carotenoids+ pheophytin) and ratios of chlorophyll b/a, chlorophyll c/a and total phytoplankton chlorophyll a to total phytoplankton chlorophyll a + phaeopigments concentration was calculated. These ratios indicate the state of phytoplankton population and for a healthy state of phytoplankton assemblages it is less than unity.
The secondary productivity of the Cochin estuary was calculated in terms zooplankton carbon. The estimation of zooplankton carbon was done as follows. The basic value of zooplankton biomass in ml m-3 has to be first converted into organic carbon, so as to find the energy available through zooplankton. The settling volume of zooplankton was converted into dry weight using a factor of 0.075 g dry wt.ml-1. Carbon biomass is calculated as 38% of this value, as per the estimate from the biochemical studies done by Cushing. The column secondary productivity was calculated from the zooplankton carbon values and the generation time of zooplankton as suggested by Cushing. It was calculated from the relationship D = 71.72 t -1.22, where ‘t′ is the temperature. The euphotic zone depth was considered in the calculation so as to derive the secondary productivity in terms of area. The transfer efficiency from phytoplankton to zooplankton can be estimated from the ratio between phytoplankton carbon and zooplankton carbon (P: Z).
Tertiary production in terms fishery potential was calculated based on the primary production and secondary production. Fish production varies between 0.3 to 0.6% of the primary production. Generally 25-40% of the total fish production can be taken as the sustainable catch. Fishery potential was estimated from the secondary trophic level, assuming that tertiary production is equal to 10% of the secondary production. The mean trophic level of individual fish was obtained from Fish Base 2015, www.fishbase.org.
Statistical analysis two ways ANOVA (Analysis of Variance), standard deviation and correlation was done based on SPSS.22 software packages for Windows for testing the presence of significant differences and correlation among the parameters between stations and between seasons. The PRIMER v 6 (Plymouth Routines in Multivariate Ecological Research, version 6.1.9), was used for Cluster analysis of data. SURFER.11 and ORIGIN 8.5 were used for data analyses and graphical representation of data [5].
Results
River discharge and rainfall showed a positive correlation significant at 1% level (p=0.01). The highly significant correlation between rainfall and river discharge might be due to the similar patterns of annual rainfall and the river runoff. The inter-annual comparison of rainfall data recorded the peak during 2010-11 period compared to 2009-10 period. The salinity of the Cochin estuary was oligomesohaline in nature during the study period. Salinity of the central and southern zones of the estuary was mesohaline (5-18 ppt) in nature and the northern zone showed oligohaline conditions (0.5-5 ppt). The dissolved oxygen values were higher in the surface waters of the estuary than the bottom waters. The dissolved oxygen concentration was higher in the northern zone (6.42 ± 0.29 mg L-1) of the estuary and lower in the southern zone (5.87 ± 0.29 mg L-1) of the estuary. The ANOVA result of dissolved oxygen showed that variations between seasons (p≤0.001) and between stations (p≤0.001) were significant at 1% level.
The average value of ammonia-nitrogen in the Cochin estuary was 11.48 ± 8.02 µmol L-1. The ammonia-nitrogen values were higher in the northern zone (13.85 ± 2.46 µmol L-1) compared to the southern zone (10.99 µmol L-1) and central zone (10.20 µmol L-1) of the estuary. It was higher during the 2010-11 periods (12.21 ± 10.16 µmol L-1) and the maximum was recorded during the pre-monsoon period (13.45 ± 2.36 µmol L-1). The average nitrite-nitrogen of the estuary was 0.72 ± 0.43 µmol L-1. The nitrite-nitrogen content of the bottom waters was higher in most of the stations. It was higher during the pre-monsoon period (1.01 ± 0.28 µmol L-1) and lower during the post-monsoon period (0.45 ± 0.28 µmol L-1). Mean station-wise values of nitrate-nitrogen ranged from 9.84 ± 2.24 µmol L-1 at station 1 to 16.16 ± 2.24 µmol L-1 at station 9. The average phosphate-phosphorus of the estuary was 7.38 ± 8.18 µmol L-1. Mean monthly variation ranged from 44.27µmol L-1 in February 2010 to 0.13 µmol L-1 in April 2010. Mean station wise values ranged from 41.83 µmol L-1 (station 8) to 22.71 µmol L-1 (station 1). Silicate-silicon values were found to be higher during the monsoon period (37.50 ± 4.32 µmol L-1), which may be due to the heavy land runoff (Thasneem et al., 2016, under press).
Primary production
The average gross primary production of the Cochin estuary was 2.27 ± 0.46 gC m-3 d-1 during the study period. Highest GPP was observed during the post-monsoon period (2.57 ± 0.26 gC m-3d-1) followed by the monsoon period (2.12 ± 0.26 gC m-3d-1) and pre-monsoon period (2.11± 0.26 gC m-3d-1). Station wise mean values of GPP were highest at station 8 (2.51± 0.23 gC m-3 d-1) and lowest at station 1 (1.87 ± 0.23 gC m-3 d-1). The recorded GPP was maximum in the northern zone (2.40± 0.15 gC m-3 d-1) and minimum in the central zone (2.11± 0.15 gC m-3 d-1). The surface waters were found to be more productive than the bottom waters. Mean monthly values of recorded GPP were maximum in April 2011 at station 8 (4.28 gC m-3 d-1) and minimum in February 2010 at station 3 (0.77 gC m-3 d-1). The annual GPP was higher during the 2010-11 period (2.40 ± 0.49 gC m-3 d -1) compared to the 2009-10 period (2.14± 0.41gC m-3 d-1). ANOVA result of gross primary production was significant at 1% level between seasons (p≤0.001) and between stations (p≤0.001).
The average net primary production of Cochin estuary was 1.26 ± 0.30 gC m-3 d -1. The NPP was higher in the southern zone (1.37 ± 0.14 gC m-3 d-1) and low in the central zone of the estuary (1.11± 0.14 gC m-3 d-1). The highest NPP values were observed in the post-monsoon period (1.44 ± 0.17 gC m-3 d-1) followed by the pre-monsoon period (1.41 ± 0.17 gC m-3 d-1) and monsoon period (1.10 ± 0.17 gC m-3 d-1). The mean annual values of NPP did not show any variation. The value was 1.26 gC m-3 d-1 for both the years. ANOVA of net primary production was significant at 1% level between seasons (p≤0.001), between stations (p≤0.001), between the stations and surface and bottom waters (p≤0.01).
For the estimation of annual column production, the euphotic zone depth is taken as 2.94 meters and the photoperiod as 12 hours. In the present study, the annual column production of the estuary was 2437.52 gC m-2 y-1. The total area of the study region was around 67.85 km2. The total annual gross production was 165386 t C y-1. Based on the present observations, it is seen that the annual column production has decreased by one order of magnitude. It can be inferred that this decrease is caused by anthropogenic influences of the ecosystem [6].
The efficiency of energy transformation is known as photosynthetic efficiency and it is equal to the energy fixed by producers during photosynthesis per unit light energy available in the system at any given time and space. It depends on the water quality and phytoplankton population in an ecosystem. Based on this observation, the efficiency of the present day estuary was calculated. It ranged from 0.05% to 0.2% with an average efficiency of 0.1%. It clearly depicts that the stress on the ecosystem has decreased the efficiency of production.
The average respiration in the Cochin estuary is 0.28 ± 0.10 gC m-3 d-1. Mean station wise values of respiration ranged from 0.31 ± 0.02 gC m-3 d-1at stations6 and 9 to 0.25 ± 0.02 gC m-3 d-1at station 1. The highest respiration values were recorded in the northern zone of the estuary (0.30 ± 0.02 gC m-3 d-1) while the southern and central zone (0.27 ± 0.02 gC m-3 d-1) exhibited the same rate of respiration. Mean monthly values of respiration were maximum in August 2009 at station 2 (1.33 gC m-3 d-1) and minimum in February 2010 at station 3 (0.05 gC m-3 d-1). The respiration of the estuary was higher during the 2010-11 periods (0.30 ± 0.08 gC m-3 d-1) as compared to the 2009-10 period (0.27 ± 0.12 gC m-3 d-1). During the post-monsoon period, respiration was the maximum (0.33 ± 0.05 gC m-3 d-1) while it was minimum in the pre-monsoon period (0.23 ± 0.05 gC m-3 d-1). ANOVA of community respiration was significant at 1% level between seasons (p≤0.001).
The mean chlorophyll a value for all the nine stations in the estuary was 11.17 ± 8.01 mg m-3. The variation was from 6.30 ± 3.11 mg m-3 at station 1 to 14.66 ± 3.11 mg m-3 at station 6. Chlorophyll a was found to be higher in the bottom waters of the estuary in most of the stations. The highest chlorophyll a value (12.43 ± 1.31mg m-3) was recorded in the northern zone of the estuary and the lowest (9.90 ± 1.31mg m-3) in the central zone of the estuary. Relatively higher value of chlorophyll a was observed during the post-monsoon period (15.08 ± 5.58 mg m-3) which decreased to an average of 4.78 ± 5.58 mg m-3during the monsoon period. Mean monthly values of chlorophyll a were maximum in January 2010 (88.86 mg m-3) at station 7 and minimum in August 2009 (0.18 mg m-3) at station 9. The mean annual value of chlorophyll a was higher during the 2009-10 periods (11.74± 9.35 mg m-3) as compared to the 2010-11 periods (10.60± 6.78mg m-3). The ANOVA analysis of chlorophyll a variations were significant at 1% level between seasons (p≤0.001), between stations (p≤0.01) and between seasons and stations ( p≤0.001).
The average value of chlorophyll b in Cochin estuary was 2.28±3.14 mg m-3. Mean station wise mean values were maximum at station 4 (3.49 ± 0.78 mg m-3) and minimum at station 1(1.07 ± 0.78 mg m-3). The southern zone of the estuary had the lowest value of chlorophyll b (1.61± 0.50 mg m-3) and the highest value was observed from central zone (2.51 ± 0.50 mg m-3). The mean monthly values of chlorophyll b varied from 0.04 mg m-3 in January 2011 at station 1 to 26.46 mg m-3 in September 2009 at station 4. The mean annual value of chlorophyll b was (3.03 mg m-3) two times higher than the second year (1.52 mg m-3). The post-monsoon period values of chlorophyll b (4.12 ±1.61mg m-3) were four times higher than the values in the monsoon period (1.53± 1.61mg m-3) and pre-monsoon period (1.17± 1.61mg m-3). The ANOVA result of chlorophyll b suggested that the variations were significant at 1% level between seasons (p≤0.001) and significant at 5% level between seasons and stations (p≤0.05).
The mean value of chlorophyll c in the estuary was 4.18 mg m-3 during the present study. Mean station wise values of chlorophyll c varied from 5.89 ± 1.10 mg m-3 at station 5 to 2.69 ± 1.10 mg m-3 at station 1. The chlorophyll c value was maximum in the central zone of the estuary (4.35 ± 0.22 mg m-3) and minimum in the southern zone (3.92 ± 0.22mg m-3). The post-monsoon period showed the highest values of chlorophyll c (7.15 ±2.57 mg m-3) while in all the other seasons, the chlorophyll c values were more or less the same. The variations were significant at 1% level between seasons (p≤0.001) and between seasons and stations (p≤0.01).
The average carotenoid values of Cochin estuary was 9.36 ± 7.13 mg m-3. Mean station -wise values of carotenoid recorded the highest at station 5(12.45 ± 1.88 mg m-3) and lowest at station 1 (6.55 ± 1.88 mg m-3). The carotenoid values were high in the southern zone (9.60 ± 0.23 mg m-3) and low in the central zone (9.13 ± 0.23 mg m-3) of the estuary. The maximum value of carotenoid in the central zone was recorded at station 5 (12.45 mg m-3) and the minimum value was recorded at station 1(6.55 mg m-3). The carotenoid values were high during the post-monsoon period (14.63 ± 5.18 mg m-3) and low during the monsoon period (4.28 ± 5.18 mg m-3). Mean monthly values of carotenoid were maximum in September 2009 (51.53 mg m-3) at station 5 and minimum in September 2010 (0.26 mg m-3). The annual variation of carotenoid showed the peak during the 2009-10 periods (10.73±8.32mg m-3). ANOVA results of carotenoid showed that the variations were significant at 1% level between seasons (p≤0.001) and between seasons and stations (p≤0.001). It was significant at 5% level between stations (p≤0.05).
The values of phaeophytin pigments revealed the dominance of dead photosynthetic pigments in the estuary. Station wise mean values of phaeophytin showed the highest value at station 3 (24.76 ± 5.45 mg m-3) and lowest at station 8 (11.55 ± 5.45 mg m-3). The phaeophytin pigments were found to be very high in the southern zone of the estuary (20.58 ± 4.09 mg m-3) and low in the central zone (13.11 ± 4.09 mg m-3). The phaeophytin values peaked during the post-monsoon period (26.39 ± 9.23 mg m-3). Mean monthly values of phaeophytin were maximum in May 2011 at sta- tion 3 (152.06 mg m-3) and minimum in August 2009 at station 8 as well as in September 2010 at station 9 (0.45 mg m-3). It was high during the 2009-10 periods (22.10 mg m-3). The variations were significant at 1% level between seasons (p≤0.001) and 5% level of significance between stations (p≤0.05) and between seasons and stations (p≤0.05).
The average value of active chlorophyll in the Cochin estuary was 10.68 ± 9.85 mg m-3. Mean station wise values of active chlorophyll ranged from 7.03 ± 3.32 mg m-3 at station 8 to 15.17 ± 3.32 mg m-3 at station 3. It was high (12.51 ± 2.49 mg m-3) in the southern zone of the estuary and low (7.91 ± 2.49 mg m-3) in the central zone of the estuary. Mean monthly variations of active chlorophyll were maximum in May 2011(89.45 mg m-3) at station 3 and minimum in July 2009 (0.12 mg m-3) at station 9. It was higher during the 2009-10 periods (12.99mg m-3). The active chlorophyll values were maximum during the post- monsoon period (15.96 mg m-3) and minimum during the monsoon period (4.70 mg m-3). The variations were significant at 1% level between seasons (p≤0.001) and significant at 5% level between stations (p≤0.05) and between seasons and stations (p≤0.05).
Secondary Productivity
The mean zooplankton carbon of the Cochin estuary was 254.55 ± 89.98 mgC m-3 during the 2009-11period. The zooplankton carbon was slightly higher during the 2010-11 period (286.20 ± 111.23 mgC m-3) compared to the 2009-10 period (229.90 ± 48.69 mgC m-3). The seasonal fluctuations were not pronounced. The maximum value was recorded during the pre-monsoon period (287.96 mgC m-3) and the minimum during the postmonsoon period (218.13 mgC m-3). Lowest zooplankton carbon in the monsoon period was observed at station 6 in August 2009 (57 mgC m-3) and the same value was recorded from station 8 in June 2010 (57 mgC m-3). In the post-monsoon period, the zooplankton carbon ranged from 57.00 mgC m-3 at station 5 to 684 mgC m-3 at station 7, both the minimum and maximum was recorded in October 2009. In the pre-monsoon period, the zooplankton carbon was lowest in February 2010 at station 8 (28.50 mgC m-3) and highest in April 2011 at station 5 (2280 mgC m-3). The zooplankton carbon was higher in the southern zone (289.59 ± 39.37 mgC m-3) of the estuary and lower in the northern zone (217.40 ± 39.37 mgC m-3) of the estuary. Mean monthly values of zooplankton carbon ranged from 126.67 mgC m-3 in July 2009 to 532 mgC m-3 in April 2011. The mean monthly variation of zooplankton in Cochin estuary during 2009-11. ANOVA of zooplankton carbon was significant at 1% level between stations (p≤0.01)
The secondary productivity was found to be higher during the pre-monsoon period (7.03±0.80 mgC m-2 d-1 and low during the post-monsoon period (5.04±0.80 mgC m-2 d-1). During the monsoon period, the secondary productivity ranged from 3.19±1.35 mgC m-2 d-1 in July 2009 to 9.91±2.27 mgC m-2 d-1 in August 2009. During the post-monsoon period, it varied between 4.10±0.77 mgC m-2 d-1 in January 2011 to 6.85±0.78 mgC m-2 d-1 in December 2009. During the pre-monsoon period, the lowest secondary production was recorded in February 2010 (4.68± 1.12 mgC m-2 d-1) and highest in April 2011 (12.96±3.57 mgC m-2 d-1). The zooplankton secondary production was higher during the 2010-11 period (84.73 mgC m-2 d-1) compared to the 2009-10 period (66.06 mgC m-2 d-1). The secondary production by the zooplankton of the Cochin estuary was75.40 mgC m-2 d-1 and the annual secondary production was 2287 mgC m-2y -1. The secondary production of our study area was 155.17 tCy-1.
Tertiary production
Based on the primary production, the wet weight of the fish production comes to be about 1225510 t by using a conversion factor of 7.41 (Vinogradov), which was applied in the Vembanad Lake by Madhupratap et al., 1977. According to Ware (2000), fish production varies between 0.3 to 0.6% of the primary production. Based on this, the actual tertiary production was estimated at 7353.06 t in Cochin estuary. Generally, 25- 40% of the fish potential can be taken as the sustainable catch (Qasim et al., Ware). So the sustainable catch from the study area was 2941.22 t y-1.
Fishery estimates of the study region was calculated from the literature of Kurup et al., 1989 and Renjith et al., 2004.The fish catch from our study area was worked out to be about 1997.24t. Since the catchment from the area is also sold through alternate ways, an additional 12 t is also considered, making the total annual catch to be about 2009.24 t.
During the study period, the total fish landing of the zone was taken as2009.24 t y-1. Assuming the average protein content of fish to be 20%, of which 50% could be reckoned as carbon, i.e. 10% of the wet weight of the fish. So the realized tertiary production in the Cochin estuary was 294.12 tC y-1, but the actual tertiary production was estimated as 200.94 tC y-1. The total annual primary production in the estuary was 165386 t of C y- 1and the fish production was 200.94 tC y-1.
Estimation of fishery potential is based on the assumption that ecological efficiency from the secondary trophic level to the tertiary level is about 10% (Pauly and Christensen, 1995). Tertiary production was calculated as 1% of the primary production. Based on the secondary production estimated from the estuary, the actual fish production potential was 15.52 tC y-1.
The trophic level of analysis of fishes from Cochin estuary showed that mid-level carnivory (3.5-3.99) and carnivory (3-3.49) dominates in the estuary. The top-level carnivory (4.0-4.5) constitute about 10.34%. The herbivorous fishes feeding on phytoplankton and plant matter formed the lowest percentage of 8.34%. The removal of top level predators in the ecosystem indicates the prevalence of overfishing in the ecosystem. The trophic level of fishes ranged from 2.2 to 4.2. The lowest trophic value of 2.2 was observed for two species of fishes, Valamugil speigleri and Oreochromis mossambicus. The highest trophic value of 4.2 was recorded for two species of fishes, Lutjhanus johnii and Pseudorhombus arsius (Table 1).
| Feeding | Trophic level | % Composition |
|---|---|---|
| Herbivory | 2-2.49 | 8.62 |
| Omnivory | 2.5-2.99 | 12.07 |
| Carnivory | 3-3.49 | 31.03 |
| Mid level carnivory | 3.5-3.99 | 37.93 |
| Top level carnivory | 4.0-4.5 | 10.34 |
Table 1: Mean percentage composition of fishes in Cochin estuary at different trophic levels.
In the Vembanad Lake, only 45% of total fishes constituted by herbivorous fishes and 7% are carnivorous fishes so the total catch of the plank- ton feeding fishes is 898.76t. In the present study, 8.62% is constituted by herbivoruous fishes and 80% of fishes were carnivorous [7]. Elimination of predatory fish communities has been reported from the oceans around the globe due to overfishing activities and it has a greater impact on the ecosystems. Fishing down marine food webs explained the dominance of carnivorous fishes in the ecosystem might be due to the overfishing of top level predators in the ecosystem.
Discussion
The primary production was more or less uniform throughout the study period. In the present study, the primary production (GPP and NPP) was highest during the post-monsoon period in the Cochin estuary. The nitrate and silicate concentrations were found to be high during the monsoon period. But, ammonia, phosphate and nitrite were high during the pre-monsoon period. This indicated that high concentration of nutrients brought by monsoon period runoff and ample supply of sunlight helped to increase the phytoplankton production. Subsequently, during the pre- monsoon period, the gross primary production in the Cochin estuary was decreased. It may be due to high salinity, low amount of nutrients and decrease in runoff. The net primary production in the Cochin estuary recorded the lowest during monsoon period. Our result support the findings of that the light penetration in the water column is considerably reduced due to high turbidity in the monsoon months, resulting in low primary production in the Cochin estuary. In contrast to this, reported peaks of production during monsoon period and early post-monsoon period in the Cochin estuary. The Light: Nutrient Hypothesis developed in the context of ecological stoichiometry theory states that an increase in nutrients will increase both primary production and nutrient content of primary producers, especially when light is the limiting factor [8]. According to our results, the nutrient concentration was high during the monsoon and pre monsoon period but the primary production was high during the post monsoon period. It may be due to the fact that light penetration in the water column acts as a limiting factor for the primary production. The net and gross primary production were strongly correlated, significant at 1% level (r2=0.743) and showed a weak negative correlation significant at 5% level with attenuation coefficient (r2=-0.521). In the present study, the correlation analysis of primary production did not show any correlation with the water temperature, salinity and PH.
The calculated net primary production accounts about 55% of the gross primary production which means that about 45% would be utilized for respiration. But practically the community respiration comes to about 12% of gross primary production. The difference in percentage of respiration with GPP may be due to the large export potential of organic matter by the ecosystem as reported by Cermeno et al., 2006 from Ria de vigo, a coastal ecosystem in Spain. Reported that net primary production is 70% of gross production and 30% for respiration in the Cochin estuary.
Community respiration showed a strong positive correlation with gross and net production (r2=0.867, r2=0.603) in the estuary. The same results were observed from the Lore estuary on the French Atlantic coast. Observed a strong coupling between respiration and primary production (r>0.8) in the northeast Gulf of Mexico estuaries. The relationship between primary production and respiration is an important factor in shaping the functioning of ecosystems. It determines the cycling of carbon, oxygen and energy in ecosystems. The closeness of the ratio of primary production to respiration has been identified as indicators of ecosystem maturity and system proximity to a well-developed, climactic status [9].
In the present study, the average gross primary production of the estuary was eight-fold higher than the respiration. Qasim et al., 1969 reported that the gross primary production (767±304 mgC m-2 d-1) in the Cochin estuary was three times higher than the respiration (261±154 mgC m-2 d-1). They also reported high GPP/R ratios during monsoon period and low during the pre-monsoon period, indicating the system was net autotrophic. Also reported that the plankton community respiration exceeds primary production except during spring season in the Gulf of Riga is a relatively autonomous subsystem of found that the productivity to respiration ratio was high during high tide in the Darwin harbour, Australia.
Reported that high amount of dissolved oxygen in the surface waters might be due to the increased organic inputs from the urban area as well as the industrial effluents into the Ashtamudi estuary. Similar observations were observed in the present study, supported by the findings in the Cochin estuary and Patil and Anil, 2011 in the Zuari estuary. The high amount of organic matter and high bacterial population of the sediment lowers the amount of dissolved oxygen in the water column. Mean station wise variation and low dissolved oxygen at the bottom clearly support the above theory. The organic matter of the sediment was very high in Station 9 and low in Station 3, upon which the resulting decomposition of organic matter lowers oxygen in the deeper layers of the water body (Department of Environment and Climate change- project report, 2013). In aquatic systems, oxygenation is the result of an imbalance between the processes of photosynthesis, degradation of organic matter, reaeration (Granier et al.), and the physicochemical properties of water (Aston). In the present study, approximately16% of the samples exhibited a dissolved oxygen concentration ≤5mg L-1, indicating a biologically stressed condition in the estuary.
The mean monthly values of NPP: GPP ratio of the estuary ranged from 0.40 to 0.79. About half of the values lie below 0.5, indicating the unhealthy status of the Cochin estuary. Ketchum et al., 1958 have pointed out that the ratio of NPP: GPP photosynthesis in a healthy population should approach unity if respiration is 5-10% of the total photosynthesis. It also indicates the physiological state of phytoplankton organisms arising due to nutrient deficiency. In such cases the ratio would be nearer to zero [10].
The increased respiration in the Cochin estuary indicates that heterotrophic organisms dominated during the study period. So the Cochin estuarine system consumes more organic carbon than they produce. The decadal changes suggest that increased human interference transformed the autotrophic system to a highly heterotrophic system. Our present findings were supported by the works in the Cochin estuary.
The heterotrophy of the Cochin estuary in the present study may be due to the input of large allochthonous organic matter to the system particularly from adjacent marshes and mangroves. The total inorganic carbon of Cochin estuary was high, by which it acts as a source of carbon rather than a sink (Department of Environment and Climate change Report, 2014). Heterotrophy is common in turbid estuaries, because heterotrophy or autotrophy in the ecosystems is controlled by factors such as light that limit primary production (Little, 2000).
Chlorophyll a concentration was higher in the northern zone. This suggests that the upper estuary is more productive than the lower estuary, as a result of the higher nitrogen concentrations from riverine origins. Chlorophyll a in pre-monsoon period and post-monsoon period showed a threefold increase as compared to the monsoon period. The low values of chlorophyll during the monsoon period may be due to the increased flushing of phytoplankton biomass in the estuary. The relationship among river discharge, salinity and chlorophyll a did not show a definite trend. The maximum chlorophyll concentrations were observed during the months of least river runoff, when the surface salinity was high in the Cochin estuary. In the present study, the above relationship was worked out only in some months. The correlation analysis of chlorophyll a showed a weak negative correlation with river discharge and weak positive correlation with salinity. It may be due to the high tolerance of phytoplankton to withstand salinity changes irrespective of seasons. A similar observation was made by Jyothibabu et al., 2006 from the same region. In the present study the seasonal cycles of gross primary production and chlorophyll a were synchronous.
The mean monthly values of total pigments (Chlorophyll a +b + c) ranged from 4.14 to 47.44 mg m-3. The higher ratio of carotenoids to chlorophyll a indicates an unhealthy and chlorotic phytoplankton population (Ketchum et al., 1958). The high ratio may be due to the stirred up sediments and degradation products of chlorophyll a, which increases the carotenoid values as suggested by Qasim and Reddy, 1967. Seventy percent of the samples exhibited a ratio less than one. The carotenoid pigment showed a strong positive correlation with chlorophyll a, b and c (r2= 0.718, r2= 0.809, r2=0.840); 5% level of significance with net primary production (r2=0.488) and a weak negative correlation with attenuation coefficient significant at 5% level (r2= -0.434).
The phaeophytin values indicated the dominance of dead photosynthetic pigments. The ratio of chlorophyll b/a ratio ranged from 0.003 to 4.14 while the ratio of phaeopigments to chlorophyll a was higher, ranging from 0.08 to 47.57. The ratios of phaeophytin to chlorophyll a were greater than one, suggesting the abundance of detritus and predominance of degraded chlorophyll in the water column. About 65% of the mean monthly ratios were greater than one. These ratios of different components of chlorophyll give an insight into the photo-adaptability as well as physiological and degradation state of the communities. The health states of the phytoplankton assemblage of the estuaries were assessed by analyzing the ratio of total phytoplankton chlorophyll a: total phytoplankton chlorophyll a+ phaeopigments concentration. In the present study, about 62.5% of the mean monthly values were less than 0.5, indicating the unhealthy status of the phytoplankton community. Thomas et al., 2005 used this ratio to study the phytoplankton community structure of two South African estuaries and showed that most of the values greater than 0.5 suggested the actively growing and physiologically healthy status of the phytoplankton community structure. The high concentration of phaeopigments compared to chlorophyll a was observed in the most of the stations. This suggests the mass mortality of phytoplankton due to osmotic stress and grazing by zooplankton (O’Boyle and Silke, 2010). The phaeophytin pigments showed a positive correlation significant at 1% level with net primary production (r2=0.523), chlorophyll a b and c (r2=0.761, r2=0.0664, r2=0.701) and carotenoids (r2=0.871).
Cluster and MDS analysis
The station wise MDS ordination of primary production in the estuary showed a stress value below 0.05, it is an excellent similarity between the surface and bottom water of nine stations. The bottom waters of station 5 and 6 showed very high similarity. The surface and bottom strata of the stations 1, 3, 6 and 8 showed a close similarity in the primary production. The surface water of station 4 was dissimilar with all the other stations. The surface waters of station 5 showed a very distant similarity with other stations.
The dendrogram of mean monthly values of primary production (GPP, NPP) and chlorophyll (a, b, c) showed six clusters. July and November 2009 formed the first and fifth clusters. The second cluster is formed by months from June 2010, July 2010, August 2009 and 2010, May 2010 and December 2010. The third cluster is formed by the months February to April 2009, June 2009, September 2009 and December 2010. The fourth cluster formed by January 2010 and May 2011. October 2009 and 2010 formed the sixth cluster. The dendrogram of the primary production of the estuary showed that the southwest and northeast monsoon period significantly influence the spatiotemporal variations of production.
In the present study the annual column production was 2437.52 gC m-2 y-1and the secondary production by zooplankton was 2287 mgC m-2 y -1. Clearly, net zooplankton is not utilizing a very large proportion of the phytoplankton production. The wide gap in the primary and secondary production indicates a large amount of primary production left in the estuary. It sinks to the bottom and produces anaerobic conditions (Qasim et al., 1969). According to Mann, 2000 the gap in the phytoplankton production and zooplankton production in the estuaries may be due to various reasons:
1. Net zooplankton feed on only a subset of the various sizes of phytoplankton,
2. Estuarine zooplankton consume a lot of detritus, and
3. There is a mismatch between the time scales of events in the phytoplankton and zooplankton. Phytoplankton which under favorable conditions divides about once a day can make use of the short bursts of nutrient availability.
The micro zooplankton populations, made up of individuals that require a month or so to complete life cycle, are not able to enough to take the full advantage of short-term fluctuations phytoplankton availability. As a result much of the phytoplankton production sinks to the bottom and is utilized by benthic communities.
Zooplankton of the estuaries may be a better indicator of climate change than marine zooplankton because the estuarine environment is more affected by changes in air temperature and precipitation than the open sea (Intxausti et al.). During our present study 2009-10 period was hotter than 2010-11 period with low rainfall and river discharge. The major nutrients in the estuary like phosphate, nitrite, nitrate and silicate) was high during 2009-10 periods. The zooplankton biomass and column secondary production was low during 2009-10 periods. It may have implications on the climatic fluctuations in the estuary. The water column contains high amount of nutrients, but the increased water temperature makes the inefficient utilization of it by the plankton communities, resulting in low biomass and secondary production at the secondary level. Increased heating can enhance existing stratification, reducing the availability of nutrients in the surface (Richardson and Schoeman). Under such conditions plankton community is dominated by picoplankton and flagellates, which are mostly grazed by heterotrophic protists, small crustaceans and gelatinous zooplankton (Ryther, Iverson, Pauly and Christensen, Richardson). This long and inefficient food web has lower nutritional quality, supporting less production at higher trophic levels. Summarizing, nutrient concentration in the marine environment is the main factor defining the local food web, and water temperature is a valuable proxy for nutrient enrichment.
The ratio of carbon transfer from phytoplankton to zooplankton was calculated. In the present study, it was high during the post-monsoon period, but very low during the pre-monsoon period. Madhu et al., 2007 reported that the transfer efficiency was quite high during monsoon period and post-monsoon period, but became low during pre-monsoon period. During the period of fresh water dominance, a substantial amount of carbon remains unconsumed due to the lack of grazers. Qasim et al., 1969 stated that the average rate of consumption from the daily net production works out as 10% of the production by the plants during the monsoon period months, 20% during the post-monsoon period months and 46% during the pre-monsoon period months. Our present study revealed that the transfer efficiency was high during the monsoon period. The coefficient of energy transfer was 7.4% for Cochin backwaters. In the present study, the transfer ratio was approximately 24%. During the last decades, the efficiency has increased significantly, which may be due to the reason that zooplankton communities exhibiting herbivory have shifted to omnivory or carnivory.
Trophic level production and energy transfer in Cochin estuary
A conceptualized planktonic food web of Cochin estuary is showed in. The fate of primary production depends on the path carbon takes within the planktonic food webs. The two type’s food webs are the grazing and microbial food chain depends on the size of the primary producers. The bio- genic carbon is transported most effectively in the grazing food chain. So it supports a good fishery compared to the microbial food web systems. The planktonic food web dominated by small autotrophs channeled the biological carbon to higher trophic levels (Marquis et al.). In the Cochin estuary, the nano and picoplankton contribute a major share in the primary production compared to micro phytoplankton. Primary producers and detritus are assigned trophic level one, because they produce energy for the entire ecosystem. The detailed study of nano and pico phytoplankton will give more insight to the food web structure of the estuary. In the present study they were not taken in to account. The total annual gross production of the Cochin estuary was estimated to be 165386 tonnes of Carbon per year.
In the grazing food chain primary production by the micro phytoplankton is transferred to secondary producers. Grazers such as (microcrustaceans, such as copepods, cladocerans and jelly fishes, etc), herbivorous fishes, filter feeding benthos consuming microalgae and other bottom in- vertebrates (sea-urchins, which mainly consume algae). The herbivorous organisms which feed on primary producers occupy trophic level two. Assuming that about 10% of the primary production is available to the herbivores. In the present study the actual secondary production much lower than the realized, the secondary production of all the grazers was not taken into account. Feeding behavior of dominant organisms gives a good idea about the food web operating in the estuarine system. Omnivorous feeding mechanisms of secondary producers make the food web more complex to derive. Based on the available data, the predator-prey interactions (Phytoplankton-zooplankton) relationship showed that top- down mechanism operates in the estuarine system. The top level predators control population of lower group of animals.
Predators feeding on herbivores occupy trophic level three. The tertiary production derived from secondary production showed that fishery resources in the estuary were over exploited. The trophic level analysis of fishery catch showed the dominance of carnivory rather than herbivory. A trophic cascade takes place when the effect of a change in predation pressure propagates across consecutive trophic levels in the food web. If the predatory fishes were overexploited and decreased in abundance, thus the zooplankton abundance was reduced but with an increase of phytoplankton abundance.
These findings showed the changing trophodynamic structure of the estuary due to various anthropogenic activities. The industrial pollution can be mitigated by effective and transparent monitoring of industrial activities in the Eloor-Edayar region. Urbanisation and developmental activities in the Thevara, Vallarpadam-Bolgatty, and Moolampilly-Pizhala resulted in the encroachment of the estuary by violating and regulating the coastal regulation zone (CRZ) norms. National Green Tribunal can enforce laws to prevent the reclamation in the estuaries. Several patches of mangroves have been denuded for the construction and developmental activity in the Vallarpadam-Bolgatty area, so urgent afforestation measures have to be implemented in the region to maintain the green lung cover of the city. The hydrology and regular flows from rivers into the estuary has been seriously affected unplanned developmental activities in the Cochin city. So detailed studies on the hydrobiology of the backwater, salinity, inorganic nutrient regime in different zones, river flow patterns are to be undertaken before any future developmental programs are implemented. Since tourism activity is on the rise in Cochin estuary, urgent action is required to restrict various pressure associated with this on the wetland and its resources. A carrying capacity based model needs to be developed to protect the ecosystems from any modification. Over exploitation of fishery resources was widely observed in the Cochin estuary. Unscientific method of fishing practices and use of destructive gears should be prohibited. The change in environmental and biotic production potential as is evident from the present study is to be seriously considered. So that it could be integrated with the management measures adopted by Ramsar convention for the region under the Vembanad wetland.
Acknowledgements
Authors are grateful to the Department of Environment and Climate Change (DoECC) for financial assistance and to the Dept. of Marine Biology, Microbiology and Biochemistry, School of Marine Sciences, Cochin University of Science and Technology for providing necessary facilities.
References
- Arias AH, Menendez MC (2014) Marine Ecology in a changing world. CRC press, Taylor & Francis Group, Boca Raton, London, New York.
- Bartell S M, Brenkert AL, Neill RVO, Gardner RH (1988) Temporal variation in regulation of production in a pelagic food web model. Complex Interactions in Lake Communities 101-118.
- Bendschneider K, Robinson R (1952) A new spectrophotometric method for the determination of nitrite in seawater. J Mar Res 11: 87- 96.
- Bhargava RMS, Dwivedi SN (1976) Seasonal distribution of phytoplankton pigments in the estuarine system of Goa. Indian J Mar Sci 5: 87-90.
- Bidigare RR, Frank TJ, Zastrow C, Brooks JM (1986) The distribution of algal chlorophylls and their degradation products in the Southern Ocean. Deep Sea Research 33: 923-937.
- Carpenter SR, Kitchell JF, Hodgson JR, Cochran PA, Elser JJ, et al. (1987) Regulation of lake primary productivity by food web structure. Ecology 68: 1863-1876.
- Clarke KR, Gorley RN (2006) PRIMER-E. Primer E Ltd Plymouth Cloern.
- Cushing DH (1971) Upwelling and the production of fish. Adv Mar Biol 9: 255-335.
- Cushing DH (1973) Production in the Indian Ocean and the transfer from primary to the secondary level. The biology of the Indian Ocean 3: 475-486.
- Gopinathan CP, Rodrigo JX, Kasim HM, Rajagopalan MS (1994) Phytoplankton pigments in relation to primary production and nutrients in the inshore waters of Tuticorin, southeast coast of India. Indian Journal of Marine sciences 23: 209-212.
Indexed at, Google Scholar, Crossref
Citation: Thasneem TA, Bijoy Nandan S and Geetha PN (2022) Changing trophic pattern in a highly urbanized tropical estuary (Cochin estuary), India. J Marine Sci Res Dev.12:356. DOI: 10.4172/2155-9910.1000356
Copyright: © 2022 Thasneem TA. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 1836
- [From(publication date): 0-2022 - Apr 03, 2025]
- Breakdown by view type
- HTML page views: 1488
- PDF downloads: 348