Changes in the Vascular Extracellular Matrix as a Potential Cause of Myocyte Loss via Anoikis in Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy
Received: 11-Dec-2017 / Accepted Date: 20-Dec-2017 / Published Date: 22-Dec-2017 DOI: 10.4172/2161-0681.1000332
Abstract
Objective: Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) is a generalized vasculopathy caused by mutations in the NOTCH3 gene, leading to degeneration and loss of vascular smooth muscle cells (VSMC). The relationship between NOTCH 3 mutations and VSMC death is unknown. One possible cause of myocyte deficit may be anoikis, a special type of apoptosis due to loss or inappropriate cell adhesion to extracellular matrix.
Method: To verify this hypothesis we examined immune expression of main compounds of vascular extracellular matrix (laminin, fibronectin and collagen IV) and selected metallo proteinases (MMP-2, MMP-3, and MMP-9) in cerebral, skin and skeletal muscle arterial vessels in autopsy material and biopsy specimens of CADASIL patients.
Results: The immmune reactions revealed decreased expression of laminin and increased expression of collagen IV and fibronectin in arterial vessels. Moderately intense immune reactivity to MMP-9 and MMP-2 was present while immune reactivity to MMP-3 was absent.
Conclusion: Quantitative and qualitative changes in vascular extracellular matrix found in CADASIL vessels may lead to VSMC loss via anoikis pathway.
Keywords: Anoikis; CADASIL; Extracellular matrix; Matrix metallo proteinases; vascular smooth muscle cell
Introduction
Anoikis, a term originating from the Greek word for ‘homelessness’, is a type of apoptotic cell death due to loss or inappropriate cell adhesion to extracellular matrix (ECM). Anoikis acts as a defense mechanism preventing detached cells from re-adhesion to incorrect locations. The process can occur via three main mechanisms: (1) abnormal expression of integrins connecting cells with ECM; (2) changes in ECM compounds leading to ECM shrinkage or loss of adhesive proteins; and (3) paracellular proteolysis of molecules involved in cell-matrix interactions due to increased activity of tissue metallo proteinases. In vessels, ECM surrounds every myocyte forming pericellular matrix and creates basal lamina separating endothelial cells from vascular smooth muscle cells (VSMC). Main compounds of the vascular ECM are elastic fibers and different types of collagens building up matrix skeleton, proteoglicans filling space between skeleton fibers, and various adhesive proteins, including integrins, fibronectin, laminins, vimentin, and others. ECM proteins are synthesized by VSMC, pericytes and endothelial cells, and decomposed by enzymes known as metallo proteinases (MMPs). Important roles in vascular pathology are played by three metallo proteinases: MMP-2, MMP-3, and MMP-9. They are able to degrade collagens, fibronectin, laminins and other proteins acting as cell surface receptors. They are also thought to play a major role in cell behaviors, such as cell proliferation, migration, differentiation and apoptosis [1].
Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) is caused by mutations in the NOTCH 3 gene and characterized by degeneration and loss of VSMC and pericytes, however, the relationship between NOTCH 3 mutations and VSMC/pericytes death is unknown. A hypothesis concerning anoikis involvement in VSMC death in CADASIL was drawn from experimental study on a mouse model of the disease in which loss of myocyte connections with adjacent structural elements of vessel wall preceded the development of characteristic for the disease changes: accumulation of granular osmiophilic material (GOM) and the ectodomain of the NOTCH 3 receptor in vessel wall [2]. In CADASIL, preserved VSMC often show rounded instead of longitudinal shape on transverse sections through small arterial vessels. Such rounded appearance is characteristic of detached cells which are devoid of physical influences from ECM. Moreover, our previous study revealed disturbed expression of integrin subunit β-1 in CADASIL vessels that my lead to myocytes detachment from ECM [3]. This finding as well as the results reported by other authors suggests that degeneration and loss of VSMC can be secondary to pro-life or protecting signals originating from ECM. To verify the hypothesis that anoikis may be involved in VSMC degeneration and loss in CADASIL, we examined immune expression of proteins other than integrins, involved in VSMC anchorage: main compounds of vascular ECM and selected MMPs in arterial vessels of patients with CADASIL.
Material And Methods
Material was composed of 10 autopsy brains and 10 skin-muscle biopsies of patients aged 32-73 years with CADASIL diagnosis, and age and gender matched control material containing 10 normal human brains and 10 normal skin-muscle biopsies. The diagnosis of CADASIL was established on the basis of ultra-structural criteria, i.e., the presence of pathognomonic for the disease GOM deposits in vessel walls [4], and in the majority of cases was additionally confirmed by genetic studies. Clinical details of the examined CADASIL material are presented in our previous paper [3].
Immuno histopathological studies were performed on formalinfixed and paraffin embedded sections, employing the avidin-biotinperoxidase method with DAKO LSAB 2 SYSTEM (DAKO, K0675). On dehydrated tissue slides immune reactions with antibodies against ECM components: collagen IV (DAKO, M0785, 1: 50), fibronectin (DAKO, A245; 1:200) and laminin (DAKO, M638, 1:40), as well as against metallo proteinases: MMP-2 (Santa Cruz Biotechnology, sc-53630, 1:50), MMP-3 (Santa Cruz Biotechnology, sc-21732; 1:50) and MMP-9 (Santa Cruz Biotechnology, sc-21733, 1:50) were applied at room temperature. The final immune complexes were developed with diaminobenzidine, counterstained with hematoxylin, and assessed by light microscopy. Specificity of the immune staining was confirmed for all studied proteins by running a negative control with the absence of respective primary antibody. Intensity of the immune reactions was measured using Image J software and gray levels method. In digital color photos of slides, colors were converted into the gray scale and then intensities of the gray values were measured across the entire vessel wall. The results of the measurements were statistically analyzed with the use of t-Student test for independent variables. A p-value of less than 0.05 was considered significant. All results were expressed as the mean ± standard error of the mean.
Results
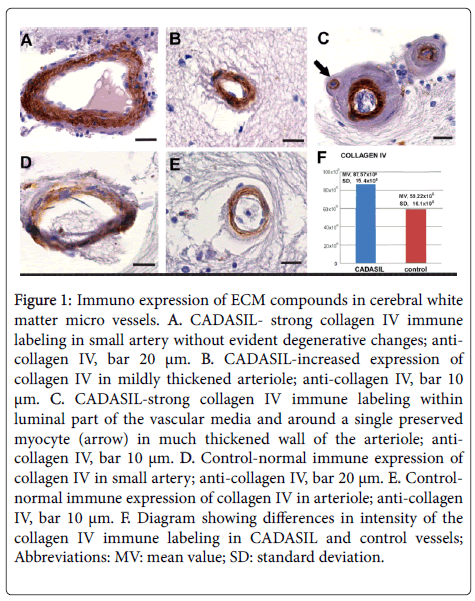
Immuno expression of ECM compounds: collagen IV, fibronectin, and laminin in CADASIL, arterial vessels revealed increased expression of collagen IV (Figures 1A-1C) compared to control (Figures 1D-1F) (p<0.05). Overexpression of collagen IV was noted in all vessels independently on their stage of degeneration. Even in arteries devoid of VSMC strong collagen IV immune labeling was observed in “paraendothelial” part of the media while the immune reactivity in its peripheral part was poor or absent (Figure 1C).
Figure 1: Immuno expression of ECM compounds in cerebral white matter micro vessels. A. CADASIL- strong collagen IV immune labeling in small artery without evident degenerative changes; anti-collagen IV, bar 20 μm. B. CADASIL-increased expression of collagen IV in mildly thickened arteriole; anti-collagen IV, bar 10 μm. C. CADASIL-strong collagen IV immune labeling within luminal part of the vascular media and around a single preserved myocyte (arrow) in much thickened wall of the arteriole; anti-collagen IV, bar 10 μm. D. Control-normal immune expression of collagen IV in small artery; anti-collagen IV, bar 20 μm. E. Control-normal immune expression of collagen IV in arteriole; anti-collagen IV, bar 10 μm. F. Diagram showing differences in intensity of the collagen IV immune labeling in CADASIL and control vessels; Abbreviations: MV: mean value; SD: standard deviation.
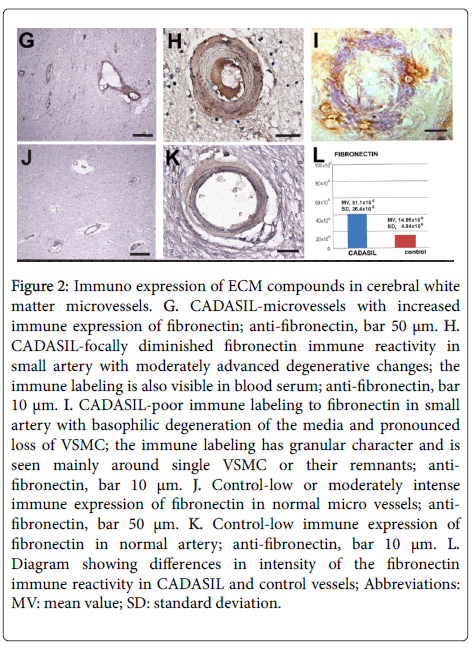
The immune reaction to fibronectin was more intense compared to control and statistically significant (p>0.05) (Figures 2G-2L) but the difference was not so evident like in the case of immune reactivity to collagen IV. Fibronectin immune expression revealed pronounced individual differences and it was frequently various even in the same vessel (Figure 2H). Increased immune labeling to fibronectin was not only seen in micro vessels with a relatively normal appearance (Figure 2G) but also in vessels with advanced degenerative changes (Figures 2H and 2I). In the last vessels fibronectin immune labeling was mainly observed in the pericellular ECM (Figure 2I).
Figure 2: Immuno expression of ECM compounds in cerebral white matter microvessels. G. CADASIL-microvessels with increased immune expression of fibronectin; anti-fibronectin, bar 50 μm. H. CADASIL-focally diminished fibronectin immune reactivity in small artery with moderately advanced degenerative changes; the immune labeling is also visible in blood serum; anti-fibronectin, bar 10 μm. I. CADASIL-poor immune labeling to fibronectin in small artery with basophilic degeneration of the media and pronounced loss of VSMC; the immune labeling has granular character and is seen mainly around single VSMC or their remnants; anti-fibronectin, bar 10 μm. J. Control-low or moderately intense immune expression of fibronectin in normal micro vessels; anti-fibronectin, bar 50 μm. K. Control-low immune expression of fibronectin in normal artery; anti-fibronectin, bar 10 μm. L. Diagram showing differences in intensity of the fibronectin immune reactivity in CADASIL and control vessels; Abbreviations: MV: mean value; SD: standard deviation.
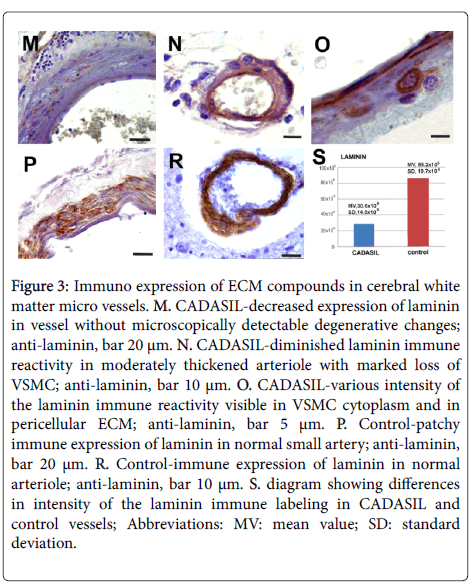
Fibronectin expression was also observed in serum (Figure 2H) and sometimes in perivascular parenchyma. In CADASIL vessels evidently diminished immune expression of laminin compared to controls was seen (Figures 3M-3S) (p<0.05). Decreased immune reactivity to laminin was observed even in vessels without microscopically visible degenerative changes (Figure 3M). Similarly to fibronectin, laminin immune expression of different intensity was often preserved only around VSMC (Figure 3O).
Figure 3: Immuno expression of ECM compounds in cerebral white matter micro vessels. M. CADASIL-decreased expression of laminin in vessel without microscopically detectable degenerative changes; anti-laminin, bar 20 μm. N. CADASIL-diminished laminin immune reactivity in moderately thickened arteriole with marked loss of VSMC; anti-laminin, bar 10 μm. O. CADASIL-various intensity of the laminin immune reactivity visible in VSMC cytoplasm and in pericellular ECM; anti-laminin, bar 5 μm. P. Control-patchy immune expression of laminin in normal small artery; anti-laminin, bar 20 μm. R. Control-immune expression of laminin in normal arteriole; anti-laminin, bar 10 μm. S. diagram showing differences in intensity of the laminin immune labeling in CADASIL and control vessels; Abbreviations: MV: mean value; SD: standard deviation.
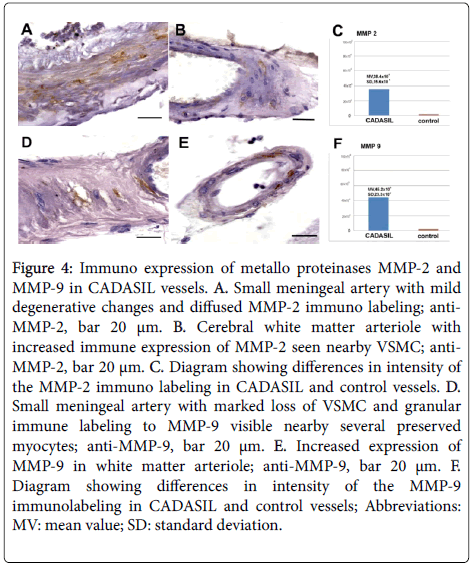
Immuno expression of metallo proteinases: MMP-2, MMP-3 and MMP-9 in control material immune reactions to MMPs in the vascular media were negative. Immuno expression of MMP-2 was observed in all CADASIL cases (Figures 4A-4C) and the intensity of the immune labelling was statistically significant (p<0.001). The immune reaction was seen in VSMC cytoplasm and ECM, and was more evident in cerebral than in skin or muscle vessels.
Figure 4: Immuno expression of metallo proteinases MMP-2 and MMP-9 in CADASIL vessels. A. Small meningeal artery with mild degenerative changes and diffused MMP-2 immuno labeling; anti- MMP-2, bar 20 μm. B. Cerebral white matter arteriole with increased immune expression of MMP-2 seen nearby VSMC; anti- MMP-2, bar 20 μm. C. Diagram showing differences in intensity of the MMP-2 immuno labeling in CADASIL and control vessels. D. Small meningeal artery with marked loss of VSMC and granular immune labeling to MMP-9 visible nearby several preserved myocytes; anti-MMP-9, bar 20 μm. E. Increased expression of MMP-9 in white matter arteriole; anti-MMP-9, bar 20 μm. F. Diagram showing differences in intensity of the MMP-9 immunolabeling in CADASIL and control vessels; Abbreviations: MV: mean value; SD: standard deviation.
Very poor immune labeling to MMP-3 was noted only in 2 CADASIL cases characterized by vascular changes resembling polyarteritis nodosa. MMP-3 expression revealed cells only in inflammatory infiltrates surrounding vessels with severe hyalinization. Since the immune labeling was not observed in the vascular media we assessed vessel immune reactivity to MMP-3 as negative.
Pronounced and statistically significant (p<0.001) immune expression of MMP-9 was noted in numerous arterial vessels in all CADASIL cases (Figures 4D-4F). Similarly to MMP-2 immuno reactivity, MMP-9 immuno expression was more evident in cerebral than in skin or muscle vessels, and it was shown in ECM and a part of VSMC. Considerable variability in intensity of the MMP-9 immuno staining of individual cases was seen and it was more pronounced than in immune staining for MMP-2.
Discussion
Our study on arterial vessels derived from patients with CADASIL diagnosis revealed changes in compounds of vascular ECM (increased immune expression of collagen IV and fibronectin, and decreased immune expression of laminin), as well as increased immune reactivity to metalloproteinases MMP-2 and MMP-9 [5]. In vessels exchange of ECM compounds is very dynamic and reflects their functional state. Interactions between ECM and vessel wall cells are mutual: ECM proteins are mainly synthesized by VSMC/pericytes and ECM compounds, in turn, influence the phenotype, biological properties and fate of VSMC.
In CADASIL vessels the most evident change was overexpression of collagen IV. Experimental studies revealed that in response to mechanical stress VSMC synthesizes abundant amount of matrix proteins, mostly collagen [6]. Since in CADASIL mechano reactivity of vessels is disturbed [7-9], it is possible that normal levels of vascular tension can be interpreted by VSMC as too high and, like in the case of arterial hypertension, it can initiate cascade of reactions leading to vessel fibrosis. It is known that collagen stimulates phenotypic changes in VSMC towards the synthetic phenotype [10] more vulnerable for apoptosis [11] especially when VSMC are submitted to mechanical stress [12].
Fibronectin also favors phenotypic transformation of VSMC towards the synthetic phenotype [13]. In spite of individual differences, immune reactivity to fibronectin was generally increased in vascular ECM, especially pericellularly. That observation suggests that some myocytes are better preserved and they may become synthetic cells producing fibronectin. In our study, the immune expresion of fibronectin in the vascular media was heterogenous and sometimes even focal lack of the immune reactivity was seen. In such areas lack of fibronectin can cause break of bounds between VSMC and ECM within focal adhesions and cell death via anoikis pathway [14]. The presence of fibronectin degradation products can also lead to apoptosis of VSMC [15] as demonstrated during the development of aneurysm [16] and vein varicoses [17].
In our study, the immune labeling to laminin in the arterial media was diminished. This phenomenon was observed even in vessels without degenerative changes which suggest that impaired expression of laminin may be an early phenomenon in CADASIL. It is known that laminin not only binds to integrin receptor on the VSMC but it also binds to elastin receptor and thus takes part in the control of vessel wall tension [18]. In addition, laminin inhibits growth of VSMC [13] therefore its decreased expression in CADASIL may favor myocyte transformation towards the synthetic phenotype.
Changes in ECM compounds demonstrated in our study, may lead not only to transformation of VSMC towards more vulnerable to apoptosis synthetic phenotype but also to their loss via anoikis pathway. It is known, that changes in ECM can cause alterations in mechanical forces affecting VSMC. Altered endogenic tension can modify, in turn, myocyte actin cytoskeleton leading to cell shrinkage, its detachment and death via anoikis. It is worth noting that abnormalities in myocyte cytoskeleton have been found in CADASIL [19].
In normal vessels some MMPs are constitutively active at a low level, however, due to strict control of their activation, physiologic exchange of ECM compounds are limited. The activity of MMPs is precisely controlled at three levels: gene expression, activation of precursor form (zymogens) and inhibition of their activity by different active molecules including tissue inhibitor metalloproteinase. In vessels, MMPs are produced by VSMC and their expression is increased in pathological conditions, such as stroke [20]. In CADASIL material we observed increased immune reactivity to MMP-9 and, to a lesser degree, to MMP-2. In such conditions equilibrium between MMPs and their inhibitors in vessel wall is changed towards proteolysis [21-23]. Since both MMPs are able to degrade laminin and fibronectin it may explain the diminished expression of the first protein and focal lack of the second one in vascular ECM observed in our study. It is worth stressing that not only compounds of vascular ECM and integrin receptors can undergo decomposition by MMPs but also TACE, an enzyme responsible for proteolysis of the extracellular domain of the NOTCH 3 receptor. One of the characteristic features of CADASIL is the accumulation of the NOTCH 3 ectodomain in vessel wall. Diminished expression/activity of TACE may favor this phenomenon aggravating vessels injury. Increased expression of MMP-2 and MMP-9 in the vascular media in CADASIL may result in reorganization of ECM. Both MMPs may facilitate vessel fibrosis [24], and are able to release fibrogenic TGF-β from ECM. MMP-2 activation via stimulation of TGF-β1 activity contributes to the increased VSMC production of collagen [25]. Vessel fibrosis may be additionally favored by MMP-9 which is able to polymerize collagen fibers [26]. The increased expression of MMPs in CADASIL vessels may be one of the factors leading to collagen IV overproduction in the course of the disease.
Increased expression of MMP-2 and MMP-9 in the vascular media in CADASIL may also result in loss of VSMC. Research studies have shown that MMP-2 can cause anoikis of endothelial cells [21], and apoptosis of VSMC [22]. Its activation also results in N-cadherin discharge from the surface of VSMC and loss of its protective influence on VSMC against apoptosis [23].
Conclusion
In summary, although anoikis as a phenomenon in CADASIL is still a matter of speculation, there is a growing evidence of its possible impact on VSMC degeneration and loss. Modifications of vascular ECM compounds and increased expression of metallo proteinases may lead to VSMC detachment and death via anoikis pathway. But anoikis seems to rather be a secondary phenomenon in CADASIL and suggests a presence of multiplicity of pathogenic paths triggered by NOTCH3 mutations in the disease.
Acknowledgements
All procedures were in line with the ethical principles for medical research involving human subjects as stipulated in the Helsinki Declaration. The study protocol has been approved by the local Bioethics Committee. Authors report no conflict of interest.
References
- De Luca C, Papa M (2017) Matrix Metalloproteinases, Neural Extracellular Matrix, and Central Nervous System Pathology. Prog Mol Biol Transl Sci 148: 167-202.
- Ruchoux MM, Domenga V, Brulin P, Maciazek J, Limol S, et al. (2003) Transgenic mice expressing mutant Notch 3 develop vascular alterations characteristic of cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Am J Pathol 162: 329-342.
- Dziewulska D, Nycz E (2016) Disturbed integrin expression in the vascular media in CADASIL. Folia Neuropathol 54: 375-381.
- Davous P (1998) CADASIL: a review with proposed diagnostic criteria. Eur J Neurol 5: 219-233.
- Hu X, De Silva TM, Chen J, Faraci FM (2017) Cerebral Vascular Disease and Neurovascular Injury in Ischemic Stroke. Circ Res 120: 449-471.
- Williams B (1998) Mechanical influences on vascular smooth muscle cell function. J Hypertension 16: 1921-1929.
- Belin de Chantemèle EJ, Retailleau K, Pinaud F, Vessières E, Bocquet A, et al. (2008) Notch3 is a major regulator of vascular tone in cerebral and tail resistance arteries. Arterioscler Thromb Vasc Biol 28: 2216-2224.
- Dubroca C, Lacombe P, Domenga V, Maciazek J, Levy B, et al. (2005) Impaired vascular mechanotransduction in a transgenic mouse model of CADASIL arteriopathy. Stroke 36: 113-117.
- Gobron C, Vahedi K, Vicaut E, Stucker O, Laemmel E, et al. (2007) Characteristic features of in vivo skin microvascular reactivity in CADASIL. J Cereb Blood Flow Metabol 27: 250-257.
- Halka AT, Turner NJ, Carter A, Ghosh J, Murphy MO, et al. (2008) The effects of stretch on vascular smooth muscle cell phenotype in vitro. Cardiovasc Pathol 17: 98-102.
- Owens GK, Kumar MS, Wamhoff BR (2004) Molecular regulation of VSMC differentiation in development and disease. Pysiol Rev 84: 767-780.
- Katsumi A, Orr AW, Tzima E, Schwartz MA (2004) Integrins in mechanotransduction. J Biol Chem 279: 12001-12004.
- Berk BC (2001) VSMC autocrine growth mechanisms. Physiol Rev 81: 999-1022.
- Michel JB (2003) Anoikis in the cardiovascular system. Known and unknown extracellular mediators. Arterioscler Thromb Vasc Biol 23: 2146-2154.
- Leskinen ML, Lindstedt KA, Wang Y, Kovanen PT (2003) Mast cell chymase induces SMC apoptosis by mechanism involving fibronectin degradation and disruption of focal adhesions. Arterioscler Thromb Vasc Biol 23: 238-243.
- Fontaine V, Jacob MP, Houard X, Rossignol P, Plissonnier D, et al. (2002) Involvement of the mural thrombus as a site of protease release and activation in human aortic aneurysms. Am J Pathol 161: 1701-1710.
- Jacob MP, Cazaubon M, Scemama A, Prié D, Blanchet F, et al. (2002) Plasma matrix metalloproteinase-9 as a marker of blood stasis in varicose veins. Circulation 106: 535-538.
- Spofford ChM, Chilian WM (2001) The elastin-laminin receptor functions as a mechanotransducer in vascular smooth muscle. Am J Physiol Heart Circ Physiol 280: 1354-1360.
- Tikka S, Ng YP, Di Maio G, Mykkanen K, Siitonen M, et al. (2012) CADASIL mutations and shRNA silencing of NOTCH3 affect actin organization in cultured vascular smooth muscle cells. J Cereb Blood Flow Metabol 32: 2171-2180.
- Levkau B, Kenagy RD, Karsan A, Weitkamp B, Clowes AW, et al. (2002) Activation of metalloproteinases and their association with integrins: an auxiliary apoptotic pathway in human endothelial cells. Cell Death Differ 9: 1360-1367.
- von Wnuck Lipinski K, Keul P, Ferri N, Lucke S, Heusch G, et al. (2006) Integrin-mediated transcriptional activation of inhibitor of apoptosis proteins protects smooth muscle cells against apoptosis induced by degraded collagen. Circ Res 98: 1490-1497.
- Koutsouki E, Beeching CA, Slater SC, Blaschuk OW, Sala-Newby GB, et al. (2005) N-Cadherin-dependent cell–cell contacts promote human saphenous vein smooth muscle cell survival. Arterioscler Thromb Vasc Biol 25: 982-988.
- Wang M, Kim SH, Monticone RE, Lakatta EG (2015) Matrix Metalloproteinases Promote Arterial Remodeling in Aging, Hypertension, and Atherosclerosis. Hypertension 65: 698-703.
- Jiang L, Zhang J, Monticone RE, Telljohann R, Wu J, et al. (2012) Calpain-1 regulation of matrix metalloproteinase 2 activity in vascular smooth muscle cells facilitates age-associated aortic wall calcification and fibrosis. Hypertension 60: 1192-1199.
- Wei J, Hemmings GP (2000) The NOTCH 4 locus is associated with susceptibility to schizophrenia. Nat Genet 25: 376-377.
- Williams B (1998) Mechanical influences on vascular smooth muscle cell function. J Hypertens 16: 1921-1929.
Citation: Dziewulska D, Nycz E, Oleszkiewicz CR (2017) Changes in the Vascular Extracellular Matrix as a Potential Cause of Myocyte Loss via Anoikis in Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy. J Clin Exp Pathol 7: 332. DOI: 10.4172/2161-0681.1000332
Copyright: ©2017 Dziewulska D, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 4663
- [From(publication date): 0-2017 - Apr 07, 2025]
- Breakdown by view type
- HTML page views: 3847
- PDF downloads: 816