Research Article Open Access
Change in Neurocognition in People with Co-occurring Alcohol Misuse and Depression: 12-Month Follow-up
Sally Ann Hunt1*, Amanda Louise Baker2, Patricia T. Michie3 and Frances J. Kay-Lambkin41Clinical Psychologist and Clinical Research Manager, Centre for Translational Neuroscience and Mental Health, University of Newcastle, University Drive, Callaghan, NSW, Australia
2Professor, Centre for Translational Neuroscience and Mental Health, University of Newcastle, University Drive, Callaghan, NSW, Australia
3Emeritus Conjoint Professor of Psychology, School of Psychology, University of Newcastle, University Drive, Callaghan, NSW, Australia
4NHMRC Research Fellow, Program Director (Translation), NHMRC CRE in Mental Health and Substance Use, National Drug and Alcohol Research Centre, University of New South Wales, Sydney, NSW, Australia and Conjoint Academic, Centre for Translational Neuroscience and Mental Health, University of Newcastle, University Drive, Callaghan, NSW, Australia
- Corresponding Author:
- Sally Hunt
Centre for Translational Neuroscience and Mental Health
University of Newcastle, University Drive, Callaghan NSW, Australia
Tel: +61 2 40335690
Fax: +61 2 40335692
E-mail: Sally.Hunt@newcastle.edu.au
Received date: February 02, 2014; Accepted date: April 28, 2014; Published date: April 30, 2014
Citation: Hunt SA, Baker AL, Michie PT, Kay-Lambkin FJ (2014) Change in Neurocognition in People with Co-occurring Alcohol Misuse and Depression: 12-Month Follow-up. J Addict Res Ther S10:004. doi:10.4172/2155-6105.S10-004
Copyright: © 2014 Hunt SA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Addiction Research & Therapy
Abstract
Objective: Co-occurring alcohol misuse and depression (CAD) are highly prevalent and are both with associated cognitive impairments. Few studies have assessed how cognitive functioning changes over time in association with improvements in alcohol use and/or depression. This is the first study to assess cognitive function and symptoms of depression and alcohol use in a CAD sample at baseline and again at 12-months. We explored whether reduction in alcohol use or improvement in symptoms of depression were associated with changes in cognitive functioning.
Methods: Neuropsychological assessment was administered at baseline and after 12-months in a CAD sample of 71 people who had participated in a psychological intervention in the interim. Changes in alcohol use and depression over 12 months were interpreted as potential factors in improvement in neuropsychological function.
Results: Depressive symptoms and alcohol use improved over 12-months while overall neuropsychological test performance was stable. Improvement in verbal memory, working memory and executive function was predicted by improvement in depressive symptoms measured by the BDI-II, but not by change in alcohol use quantity or frequency of heavy drinking.
Conclusion: Among people with CAD, alleviation of the symptoms of depression may have greater influence on cognitive functioning in areas such as memory and executive function, than a reduction in alcohol use. Further treatment outcome research in CAD samples should include neurocognitive assessments in order to elucidate our understanding of cognitive impairments and potential improvements with intervention.
Keywords
Depression; Alcohol-Induced Disorders; Comorbidity; Neuropsychology; Neurocognition; Addiction; Cognition
Introduction
Alcohol use disorders (AUD) and depression are highly prevalent conditions with Australian data reporting 12-month prevalence of 7% for affective disorders and 6% for alcohol use disorders [1]. These two conditions co-occur at rates greater than chance in community and clinical samples [2,3]. In clinical samples, prevalence of depression in people seeking treatment for AUD ranges from 25.7% [4] to 70% [5]. Another Australian study reported that people meeting DSM-IV alcohol dependence criteria were 4.5 times more likely to meet criteria for an affective disorder than non-drinkers [6].
Information from neuropsychological assessments is a powerful tool in the therapy room and research environments. Cognitive deficits can be shown to predate the onset of clinical symptoms in some conditions [7-9] and even predict course and prognosis. Hazardous use of alcohol has been associated with impairments in memory, attention, executive functions, impulsivity, verbal fluency and visuospatial functioning [10-16]. However, there are conflicting findings regarding the longitudinal pattern of cognitive deficits after entering a phase of abstinence. There is consensus that cognitive impairment is at its greatest in the period immediately after abstinence commences [16,17]. However, debate continues about the longer term pattern of change. Studies have demonstrated improvement in memory and executive function in the first months of abstinence [15] while others have found ongoing impairments in these domains [16,18], with deficits persisting for months or years after cessation of alcohol misuse [19].
The cognitive repercussions during episodes of acute depression are also well known. Major Depressive Disorder (MDD) has been linked to impaired executive function, memory, psycho-motor function, verbal fluency, speed of information processing and attention [20-26]. Mixed results have been found in studies of neuropsychological functioning of people who have recovered from an acute episode of depression. Some report evidence for persistent deficits in attention and executive functions after remission [27-30] and others show some reversible dysfunctions in people with remitted depression [31]. A review of 30 studies by Douglas and Porter concluded that verbal measures of learning, memory, and fluency were most likely to improve concurrently with mood, while executive function and attention deficits persisted as trait features of depression [32].
Because the co-occurrence of AUD and depression (CAD) is so frequent and both conditions in isolation are associated with cognitive impairments, it is important to examine what happens cognitively in the highly prevalent CAD presentation, and longitudinally when one or both of these conditions have improved. Despite the high prevalence of this comorbidity, only four published studies could be found directly comparing cognitive functioning in participants with a recent CAD presentation (in the absence of other substance use or mental health comorbidity) to those with alcohol use only [33], depression only [34], healthy controls [34-36] or published norms [33,37]. Only two of these examined non-abstinent participants [34,37]. The literature is divided about whether the addition of co-occurring depression exacerbates neurocognitive deficits in an alcohol abusing sample. Compared to people with alcohol misuse without co-occurring depression, CAD groups have demonstrated greater deficits in visuospatial learning and immediate visual memory [33,34]. However other results suggest that the deficits of AUD are not further worsened by depressive symptoms [36]. Comparison between a CAD sample and published norms or healthy controls has also failed to show a significant cognitive impairment in CAD groups, either with test scores in the normal range [37] or performance on neuropsychological tests below published norms but not significantly worse than healthy controls [33].
Establishing the stability of neurocognitive deficits in people with CAD is important, and has the potential to inform treatment planning for this group. For example, if deficits in memory and executive functioning remained despite effective remission of depressive symptoms and/or alcohol misuse, then specific training in living skills, quality of life or cognitive remediation may be necessary as an adjunct to standard cognitive behavior therapy treatment protocols.
To our knowledge the present study is the first to examine longerterm change in cognitive functioning in a CAD sample. Participants underwent assessment at baseline and again at 12-months and then participated in a motivational interviewing and cognitive behavior therapy (MICBT) intervention which addressed depression and alcohol use, without cognitive remediation [38]. We explored whether changes in symptoms of depression or in consumption of alcohol were associated with changes in cognitive functioning.
Extrapolating from studies of neurocognitive function in remitted depression, executive function and attention seem less likely to improve than other cognitive impairments [32]. Research on long term patterns of cognitive impairment after recovery from AUD is less clear. However memory and executive function also seem to be relevant cognitive domains to assess in this group. It was hypothesized that change in alcohol consumption and depression will have unique and significant contributions to change in memory and executive function over 12-months.
Materials and Method
Ethics and clinical trials registration
This project received approval from the relevant ethics committees and was registered with the Australian New Zealand Clinical Trials Registry (ANZCTR: www.anzctr.org.au; identifier: ACTRN12607000057482).
Sample
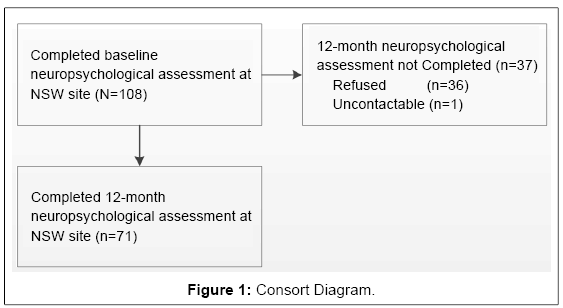
The baseline neuropsychological assessment was completed by 108 participants in a randomized controlled trial (RCT) of integrated versus single-focused Motivational Interviewing/Cognitive Behaviour Therapy (MI/CBT) for co-occurring hazardous alcohol use and depression known as the DAISI study [38,39]. DAISI participants were invited to complete baseline and 12-month neuropsychological assessment if their entry to DAISI fell within a neuropsychological data collection time period and they were located at the Newcastle, New South Wales, study site. This paper reports on 71 participants who completed both the baseline and 12-month follow-up symptom and neuropsychological assessments (recruitment and attrition shown in Figure 1).
Eligible participants were experiencing depressive symptoms evidenced by BDI–II total scores of ≥17 (14-19=mild; 20-28=moderate; 29-63=severe) and consuming alcohol at hazardous levels in the month before baseline assessment. The Australian NHMRC recommended alcohol use levels at the time of data collection (averaging ≥ 28 standard drinks per week for men and ≥ 14 per week for women) were used to define hazardous use of alcohol [40]. Exclusion criteria included current psychotic illness, history of brain injury, and nonfluency in English. Participants were not excluded on the basis of current pharmacotherapy, however entry to the study was delayed until one month after commencing any new medications or changing treatment regimens to allow stabilization. Polysubstance use was not an exclusionary criterion and was included as a predictor in regression analyses. Preliminary analyses also demonstrated that baseline neuropsychological test performance did not differ between those with and without polysubstance use.
Procedure
Participants were community members who responded to advertisements for the study in local media or were referred by health service providers, alcohol and other drug clinical services, government agencies and non-government organizations. Potential participants expressed interest in the study by giving written permission to be contacted or by calling the research team. During the initial contact participants were screened for eligibility using a Beck Depression Inventory FastScreen (BDI-FS) [41] and discussion of recent alcohol use. Informed consent to participate was obtained from those wishing to proceed with the study prior to the initial assessment. The initial symptom assessment was carried out at the research center by registered or provisionally registered psychologists who had been trained in administration and interpretation of the assessments. Approximately 1 week later participants completed the neuropsychological assessment administered by psychologists or clinical psychologists. Participants received AUD $20 reimbursement for their time.
After baseline assessment participants were offered one of four treatments (Supplementary Figure 1) [38]. Regardless of completion of the treatment phase, participants were invited to complete the 12-month follow-up assessment. Psychologists who were blind to baseline assessment and treatment allocation carried out the 12-month follow-up symptom and neuropsychological assessment, offering AUD $20 as reimbursement.
Measures
At baseline and 12-month follow-up, participants’ alcohol use, mental health, memory and executive functioning were assessed using the measures described below. Primary outcome measures were 12-month changes in neuropsychological tests known to be sensitive to the cognitive domains of memory and executive function. Change in depressive symptoms and alcohol use frequency and quantity were used as predictors in the main analysis.
Neuropsychological measures: A range of neuropsychological tests were carried out and are reported elsewhere [37]. Of relevance to the aims of the current study are the domains of memory and executive function and estimation of premorbid intelligence.
Memory: To assess auditory attention and working memory the Digit Span (DS) subtest of the Wechsler Adult Intelligence Scale (WAISIII) [42] was used. Verbal memory was assessed by the Rey Auditory Verbal Learning Test (RAVLT) [43] which tests immediate and delayed recall of a word list.
Executive Function: Two subtests from the Delis-Kaplan Executive Functioning System (D-KEFS) [44] were used: the Controlled Oral Word Association Test (COWAT) [45] and the Color-Word Interference task (CWI). The COWAT measures verbal fluency in an effortful, phonemic format (Letter Fluency: LF), from overlearned concepts (Category Fluency: CF), and while shifting between overlearned concepts (Category Switching: CS). The CWI is a modified STROOP test [46] in which the ability to inhibit an overlearned response in order to generate a conflicting verbal response of naming dissonant ink colours is measured. Two CWI subtests were analysed: the traditional STROOP inhibition task which requires the inhibition of reading in preference of naming dissonant ink colors (In), and switching between naming dissonant ink colors and reading the words (Inhibition/Switching: IS).
Premorbid Intelligence: The Wechsler Abbreviated Scale of Intelligence (WASI) [47] Vocabulary subscale was administered and combined with demographic variables such as age, gender, and education to estimate premorbid intelligence. This combination of demographic predictors and current performance on a “hold” measure such as Vocabulary has been shown to generate a reliable estimate of premorbid intelligence [48]. To approximate the variance accounted for by premorbid functioning, demographics and baseline Vocabulary were entered into the regression equation at step 1.
All neuropsychological measures were administered and scored in accordance with the published protocols. To minimize the impact of practice effects at 12-months, published alternate forms were used for retesting where possible for the COWAT and RAVLT. The 12-month delay was intended to offset the impact of practice effects associated with retesting where alternate forms were not available.
Alcohol and other substance use measures: Alcohol use was primarily measured in terms of average number of standard drinks consumed per week (quantity of use) and average number of heavy drinking days per week (frequency of use) derived from a Time Line Follow Back for the previous two weeks; TLFB) [49]. One standard drink contained 10g ethanol. A heavy drinking day was defined by drinking 6 or more standard drinks in one day for men and 4 or more standard drinks in one day for women. To validate participants’ selfreported alcohol consumption, collateral interviews asking about quantity and frequency of alcohol use were conducted at 12-months.
Additionally, Structured Clinical Interview for DSM IV-TR (SCID) [50] alcohol abuse and dependence items were used to derive DSMIV and DSM-5 AUD diagnoses with the exception of the new DSM- 5 craving criterion which had not been assessed [51,52]. Participants also completed the Alcohol Use Disorders Identification Test (AUDIT) [53] and an Opiate Treatment Index (OTI) [54] estimated average daily use occasions of 11 classes of substances (alcohol, heroin, other opiates, cannabis, amphetamines, cocaine, tranquillizers, barbiturates, hallucinogens, inhalants and tobacco) in the preceding month.
Depression measures: Severity of depressive symptoms was assessed using the BDI-II [44,55]. Cognitive (9 items, range 0-27), affective (4 items, range 0-12) and somatic (8 items, range 0-24) depressive symptom sub-scales were derived from the BDI-II [56,57]. The SCID depression items were also assessed and from these DSM-IV/ DSM-5 Major Depressive Disorder diagnoses were derived [50,51,58].
Statistical analysis
Data were analyzed using SPSS for Windows (version 21.0). All neuropsychological tests were scored and age adjusted according to the standard published procedures. Where available, the mutualized t-score, z-score or standard score was used. Change scores were coded so that improvement was reflected in a higher, positive score.
T-tests examined change over 12 months in memory (RAVLT immediate recall; RAVLT delayed recall; Digit Span), executive function (COWAT-LF; COWAT-CF; COWAT-CS; CWI-In; CWI-IS), alcohol use (mean drinks per week; heavy drinking days per week), and depression (BDI-II total score; BDI-II somatic sub-scale; BDI-II affective sub-scale; BDI-II cognitive sub-scale). Next we compared the predictive validity of change in depressive symptoms and alcohol use for change in neuropsychological tests sensitive to executive function and memory. To do this, hierarchical multiple regression analyses with change in neuropsychological test score as the dependent variable were carried out. Variables that have previously been shown to impact on outcome (age, gender, baseline use of antidepressant medication, baseline use of anxiolytics, hazardous use of alcohol during the 24-hours prior to neuropsychological assessment, age at leaving school, and treatment allocation) and baseline WASI Vocabulary score (to approximate premorbid IQ) were entered at Step 1. Baseline measure of the dependent variable and the baseline BDI-II, mean drinks per week and heavy use days per week were entered at Step 2. Change in mean drinks per week, change in days of heavy drinking and change in BDI-II scores were entered separately at steps 3, 4 and 5 respectively. To clarify the role of change in cognitive, affective and somatic depressive symptoms, sensitivity analyses were carried out as described above but with cognitive, affective and somatic sub-scales derived from BDI-II items entered in lieu of the BDI-II total score in separate steps at steps 5, 6 and 7 respectively.
A family wise error rate was applied, adjusting for the number of outcomes in the family (defined by the neuropsychological domain of interest, i.e. memory or executive function).
Results
Sample characteristics
Demographic and baseline symptom characteristics of participants are shown in Table 1. Participants were asked to abstain from alcohol and other substances for at least 24-hours before each neuropsychological assessment. Despite this instruction, at baseline 64.2% reported consuming alcohol in the previous 24-hours, and 37.7% of these did so at hazardous levels. Similarly, in the 24-hours prior to 12-month neuropsychological assessment 59.2% reported consuming alcohol, with 29.6% doing so at hazardous levels. The average number of days abstinent prior to each neuropsychological assessment was 3.6 days (range: 0-32days) prior to baseline and 20.5 days (range: 0-12 months) prior to 12-month assessment. Only 2.8% and 7.0% of participants reported use of other substances on the day prior to baseline and 12-month testing respectively. There were no significant differences in neuropsychological results between participants with recent hazardous alcohol or substance use and those who had not used in the past 24-hours, so use of alcohol or other substances in the preceding 24-hours was not included as a covariate in later analyses.
| Variable | M | SD | Range | n | % |
|---|---|---|---|---|---|
| Age, years | 46.99 | 10.77 | 23-70 | ||
| Sex, male/female | 44/27 | 62/38% | |||
| Australian born | 56 | 78.9% | |||
| Medication | |||||
| Antidepressant | 39 | 54.9% | |||
| Anxiolytic | 13 | 18.3% | |||
| Anti-craving | 4 | 5.6% | |||
| Antipsychotic | 5 | 7.0% | |||
| Work status | |||||
| Employed | 28 | 39.4% | |||
| Student | 1 | 1.4% | |||
| Retired | 9 | 12.7% | |||
| Unemployed | 29 | 40.8% | |||
| Other/no income | 4 | 5.6% | |||
| Primacy | |||||
| Depression | 25 | 35.2% | |||
| Alcohol misuse | 28 | 39.4% | |||
| Unclear primacy | 18 | 25.4% | |||
| Alcohol Measures | |||||
| AUDIT | 23.62 | 6.68 | 10-40 | ||
| Current SCID Alcohol Dependence | 59 | 85.5% | |||
| Emotional Functioning | |||||
| GAF | 57.63 | 8.49 | 30-70 | ||
| Current SCID MDD | 46 | 64.8% |
Table 1: Baseline sample characteristics (N=71).
At baseline, the diagnostic criteria for DSM-IV Alcohol Abuse and/ or Dependence were met by 88.6% currently and 92.9% lifetime. DSM-5 AUD criteria (excluding craving criterion) were met by 93.0% currently and 95.8% lifetime. DSM-IV/DSM-5 MDD criteria were met by 64.8% currently and 71.8% lifetime. Collateral interviews were completed at 12-months by a close friend or family member of 38% of participants, with 100% agreement on quantity consumed on a typical drinking day and frequency of heavy alcohol use.
Participants’ baseline use of substances other than alcohol is reported in Table 2. In the month prior to baseline assessment 51.4% had used tobacco, 21.4% used cannabis, 8.5% used amphetamines and 4.3% used tranquilizers. Lifetime SCID Substance Dependence was included as a covariate in the regression analyses.
| Substance | Lifetime use (%) (n=70) | Age at first use among users (SD) | Usage last month (OTI) | OTI mean among users in past month (SD) | |
|---|---|---|---|---|---|
| n | % | ||||
| Alcohol | 100.0 | 15.8 (4.2) | 70 | 100 | 8.6 (6.1) |
| Tobacco | 84.3 | 15.0 (4.9) | 36 | 51.4 | 15.9 (11.2) |
| Cannabis | 64.3 | 18.4 (6.3) | 15 | 21.4 | 6.7 (17.6) |
| Amphetamines | 36.6 | 24.1 (5.2) | 6 | 8.5 | 0.1 (0.1) |
| Tranquilisers | 12.9 | 29.0 (8.7) | 3 | 4.3 | 0.1 (0.1) |
| Hallucinogens | 32.9 | 20.8 (4.8) | 0 | 0.0 | NA |
| Other opiates | 7.1 | 20.0 (0.0) | 0 | 0.0 | NA |
| Heroin | 14.3 | 22.4 (8.3) | 0 | 0.0 | NA |
| Inhalants | 7.1 | 19.4 (3.4) | 0 | 0.0 | NA |
| Cocaine | 15.7 | 22.4 (3.6) | 0 | 0.0 | NA |
| Barbiturates | 5.7 | 22.3 (5.6) | 0 | 0.0 | NA |
Table 2: Patterns of substance use assessed at baseline.
Completion of follow-up assessment
Recruitment and attrition profiles are presented in Figure 1. Only participants who underwent baseline neuropsychological assessment were eligible for 12-month neuropsychological assessments. Some participants were unwilling or unable to complete each assessment, but were included if they had completed the majority of items. Details of the full sample in the DAISI trial are reported elsewhere as are comparisons of the results of the different treatments [38].
The average time between completion of baseline and 12-month follow-up assessment was 57 weeks (range: 52-75). Both baseline and follow-up assessments were completed by 71 participants (65.7% of the eligible baseline sample). Participants who completed the 12-month follow-up did not differ significantly from those who did not complete follow-up on any key baseline measures of cognitive functioning, depression, alcohol use, age or use of medication.
Change in neuropsychological functioning, depressive symptoms and alcohol use
As shown in Table 3, the mean scores for each neuropsychological subtest were in the average range compared to published norms at baseline and follow-up. After Bonferroni correction, none of the neuropsychological test variables differed significantly between baseline and 12-months.
| Baseline | 12-months | Change | |||||
|---|---|---|---|---|---|---|---|
| M | SD | M | SD | t | % improved | ||
| WAIS-R (standard score) | |||||||
| Digit span | 10.76 | 3.07 | 10.75 | 3.44 | 0.048 | 54.9% | |
| RAVLT (raw-score) | |||||||
| Total immediate recall (A1:A5) | 47.99 | 10.50 | 51.64 | 9.79 | -0.809 | 74.2% | |
| Delayed recall (A7) | 9.30 | 3.37 | 10.30 | 3.33 | -1.128 | 77.2% | |
| COWAT (standard score) | |||||||
| Letter fluency | 11.01 | 3.77 | 10.59 | 3.62 | 1.493 | 54.9% | |
| Category fluency | 11.27 | 3.46 | 10.46 | 3.57 | 2.282 | 43.7% | |
| Category switching | 11.27 | 3.46 | 11.48 | 4.19 | -0.447 | 64.8% | |
| Switching accuracy | 10.54 | 3.40 | 10.80 | 4.23 | -0.537 | 62.0% | |
| CWI (standard score) | |||||||
| Inhibition | 9.85 | 2.94 | 9.96 | 2.98 | -0.470 | 71.8% | |
| Inhibition / switching | 10.14 | 2.83 | 10.48 | 2.29 | -1.380 | 60.5% | |
***p<0.001; **p<0.01; **p<0.05 (Bonferroni adjusted)
Table 3: Baseline and 12-month means, standard deviations and change in agescaled neuropsychological variables (N=71).
Table 3 displays the baseline and 12-month severity of depressive symptoms on the BDI-II and alcohol use quantity and heavy use frequency, according to gender. Overall participants reduced their quantity and frequency of heavy alcohol consumption and experienced a reduction in depressive symptoms. At 12-months the study’s depression entry criteria was met by 51.4%, alcohol entry criteria by 76.8%, and both alcohol and depression entry criteria by 44.9%.
| Baseline | 12-months | Change | |||||
|---|---|---|---|---|---|---|---|
| n | M | SD | n | M | SD | t | |
| Overall | |||||||
| BDI | 71 | 30.39 | 9.14 | 70 | 18.10 | 11.61 | 9.243*** |
| BDI-II cognitive sub-scale† | 71 | 12.44 | 4.73 | 70 | 7.07 | 5.06 | 8.328*** |
| BDI-II affective sub-scale†† | 71 | 6.72 | 2.51 | 70 | 3.73 | 2.95 | 7.903*** |
| BDI-II somatic sub-scale††† | 71 | 11.31 | 3.98 | 70 | 7.30 | 4.74 | 6.920*** |
| Mean drinks / week | 60 | 48.82 | 29.77 | 60 | 29.62 | 27.84 | 5.713*** |
| Heavy drinking days / week | 61 | 4.32 | 2.27 | 61 | 2.60 | 2.55 | 5.295*** |
| Males | |||||||
| BDI | 44 | 29.63 | 9.48 | 43 | 16.26 | 10.82 | 8.443*** |
| BDI-II cognitive sub-scale† | 44 | 12.48 | 4.68 | 43 | 6.51 | 4.91 | 7.775*** |
| BDI-II affective sub-scale†† | 44 | 6.39 | 2.64 | 43 | 3.14 | 2.32 | 8.093*** |
| BDI-II somatic sub-scale††† | 44 | 10.91 | 4.14 | 43 | 6.60 | 4.80 | 5.632*** |
| Mean drinks / week | 35 | 55.88 | 31.50 | 35 | 34.44 | 30.49 | 4.703*** |
| Heavy drinking days / week | 36 | 4.49 | 2.32 | 36 | 2.85 | 2.81 | 4.128*** |
| Females | |||||||
| BDI | 27 | 31.59 | 8.59 | 27 | 21.04 | 12.40 | 4.494*** |
| BDI-II cognitive sub-scale† | 27 | 12.37 | 4.88 | 27 | 7.96 | 5.26 | 3.896*** |
| BDI-II affective sub-scale†† | 27 | 7.26 | 2.23 | 27 | 4.67 | 3.59 | 3.485** |
| BDI-II somatic sub-scale††† | 27 | 11.96 | 3.69 | 27 | 8.41 | 4.50 | 3.971*** |
| Mean drinks / week | 25 | 38.94 | 24.48 | 25 | 22.87 | 22.53 | 3.230** |
| Heavy drinking days / week | 25 | 4.09 | 2.23 | 25 | 2.24 | 2.15 | 3.291** |
***p<0.001; **p<0.01; **p<0.05
†9 items, range: 0-27; ††4 items, range: 0-12; †††8 items, range: 0-24
Table 4: Baseline and 12-month means, standard deviations and change in depression and alcohol use variables.
Prediction of change in executive function
Results are reported as significant if they met the Bonferroni corrected p-value and significant results are summarized in Table 5. Change in BDI-II total score significantly contributed to the prediction of change in COWAT-CF (p =0.009), however a sensitivity analysis showed that change in the individual BDI-II subscales did not. The model accounted for 54% of the variance in the change between baseline and 12-month COWAT-CF scores. A trend was noted for change in mean drinks per week to contribute to the prediction of change in CWI-IS however this was not significant after Bonferroni correction (p =0.037).
| Step | Variable | R2 | Δ R2 | Cohen’s f2‡ | F | p value | |
|---|---|---|---|---|---|---|---|
| Change in COWAT category fluency | 1 2 3 4 5 |
Premorbid IQ, treatment sessions†, treatment content††, treatment focus††† poly drug use, past 24 hours alcohol use, gender, current anxiolytic, current antidepressant, age, age at leaving school. Baseline BDI-II total score, baseline mean drinks/week, baseline heavy drinking days/week, baseline COWAT category fluency. Change in mean drinks/week baseline-12 months. Change in heavy drinking days/week baseline-12 months. Change in BDI-II total score baseline-12 months |
0.268 0.431 0.444 0.452 0.544 |
0.268 0.163 0.014 0.007 0.093 |
0.366 |
1.462 2.863 0.961 0.519 7.528 |
0.181 0.035 0.333 0.476 0.009** |
| Change in CWI inhibition switching |
1 2 3 4 5 |
Premorbid IQ, treatment sessions†, treatment content††, treatment focus††† poly drug use, past 24 hours alcohol use, gender, current anxiolytic, current antidepressant, age, age at leaving school. Baseline BDI-II total score, baseline mean drinks/week, baseline heavy drinking days/week, baseline CWI inhibition switching. Change in mean drinks/week baseline-12 months. Change in heavy drinking days/week baseline-12 months. Change in BDI-II total score baseline-12 months |
0.383 0.690 0.723 0.738 0.752 |
0.383 0.307 0.033 0.015 0.014 |
0.621 0.990 0.119 0.057 0.056 |
2.479 9.902 4.673 2.190 2.038 |
0.016 0.000*** 0.037 0.147 0.162 |
†1 session versus 10 sessions; ††Alcohol versus depression focus; †††Integrated versus single-focus
‡Effect size attributable to the addition of variables at each step
* Significant at the 0.05 level (Bonferroni corrected); ** Significant at the 0.01 level (Bonferroni corrected); *** Significant at the 0.001 level (Bonferroni corrected)
Table 5: Multiple regression analyses with change in depression and alcohol use as predictors of change in executive function.
Prediction of change in memory
Significant results are summarized in Table 6. Change in the BDI-II Cognitive sub-scale significantly contributed to the regression predicting change in RAVLT immediate recall, even when change in alcohol use variables was considered (p=0.001). The model accounted for 77% of the variance in the change between baseline and 12-month RAVLT immediate recall. There was also a trend for change in BDI-II total score to predict change in immediate recall, which was no longer significant after Bonferroni correction (p=0.037). Change in RAVLT delayed recall was also predicted by change in the BDI-II Cognitive sub-scale (p=0.001). This model accounted for 73% of the variance in the change between baseline and 12-month RAVLT delayed recall. Change in Digit Span (working memory) was predicted by change in BDI-II total score (p=0.001) and by change in BDI-II Affective subscale (p=0.002). The model accounted for 62% of the variance in the change between baseline and 12-month Digit Span. Change in alcohol use quantity and heavy use frequency did not predict change in any of the memory variables, regardless of whether change in depression was included in the model.
| Step | Variable | R2 | ΔR2 | Cohen’s f2‡ | F | p value | |
|---|---|---|---|---|---|---|---|
| RAVLT total immediate recall (model 1 – BDI-II total score) |
1 2 3 4 5 |
Premorbid IQ, treatment sessions†, treatment content††, treatment focus††† poly drug use, past 24 hours alcohol use, gender, current anxiolytic, current antidepressant, age, age at leaving school. Baseline BDI-II total score, baseline mean drinks/week, baseline heavy drinking days/week, baseline RAVLT total immediate recall. Change in mean drinks/week baseline-12 months. Change in heavy drinking days/week baseline-12 months. Change in BDI-II total score baseline-12 months |
0.157 0.672 0.672 0.672 0.709 |
0.157 0.514 0.000 0.000 0.037 |
.0186 1.570 0.000 0.000 0.127 |
0.746 15.665 0.038 0.008 4.696 |
0.690 0.000*** 0.847 0.931 0.037 |
| RAVLT total immediate recall (model 2 – BDI-II subscale scores) |
5 6 7 |
Change in BDI-II cognitive subscale Change in BDI-II affective subscale Change in BDI-II somatic subscale |
0.752 0.756 0.767 |
0.080 0.004 0.011 |
0.323 0.016 0.047 |
11.924 0.634 1.624 |
0.001** 0.431 0.211 |
| RAVLT delayed recall (model 1 – BDI-II total score) |
1 2 3 4 5 |
Premorbid IQ, treatment sessions†, treatment content††, treatment focus††† poly drug use, past 24 hours alcohol use, gender, current anxiolytic, current antidepressant, age, age at leaving school. Baseline BDI-II total score, baseline mean drinks/week, baseline heavy drinking days/week, baselineRAVLT delayed recall. Change in mean drinks/week baseline-12 months. Change in heavy drinking days/week baseline-12 months. Change in BDI-II total score baseline-12 months |
0.286 0.648 0.655 0.665 0.691 |
0.286 0.362 0.007 0.011 0.025 |
0.401 1.028 0.020 0.030 0.084 |
1.605 10.283 0.744 1.203 3.042 |
0.131 0.000*** 0.394 0.280 0.089 |
| RAVLT delayed recall (model 2 – BDI-II subscale scores) |
5 6 7 |
Change in BDI-II cognitive subscale Change in BDI-II affective subscale Change in BDI-II somatic subscale |
0.721 0.724 0.733 |
0.056 0.003 0.009 |
0.201 0.011 0.034 |
7.430 0.375 1.132 |
0.010* 0.544 0.295 |
| Digit Span (model 1 – BDI-II total score) |
1 2 3 4 5 |
Premorbid IQ, treatment sessions†, treatment content††, treatment focus††† poly drug use, past 24 hours alcohol use, gender, current anxiolytic, current antidepressant, age, age at leaving school. Baseline BDI-II total score, baseline mean drinks/week, baseline heavy drinking days/week, baseline digit span. Change in mean drinks/week baseline-12 months. Change in heavy drinking days/week baseline-12 months. Change in BDI-II total score baseline-12 months |
0.221 0.382 0.382 0.385 0.540 |
0.221 |
0.284 0.261 0.000 0.005 0.337 |
1.133 2.609 0.001 0.155 12.513 |
0.360 0.050 0.973 0.696 0.001** |
| Digit Span (model 2 – BDI-II subscale scores) |
5 6 7 |
Change in BDI-II cognitive subscale Change in BDI-II affective subscale Change in BDI-II somatic subscale |
0.436 0.574 0.615 |
0.052 0.138 0.041 |
0.090 0.324 0.106 |
3.412 11.661 3.686 |
0.073 0.002** 0.063 |
†1 session versus 10 sessions; ††Alcohol versus depression focus; †††Integrated versus single-focus
‡Effect size attributable to the addition of variables at each step
* Significant at the 0.05 level (Bonferroni corrected); ** Significant at the 0.01 level (Bonferroni corrected); *** Significant at the 0.001 level (Bonferroni corrected)
Table 6: Multiple regression analyses with change in depression and alcohol use as predictors of change in memory.
Discussion
Our key finding is that improvements in the domains of memory and executive functioning were predicted by improvement in depression in a CAD sample. This occurred after participation in treatments that did not focus on cognitive remediation. Improvement in depressive symptoms as a predictor of cognitive improvement is consistent with numerous studies of improved cognitive functioning after a depressive episode has abated in non-CAD samples [31]. Furthermore, studies of the relationship between baseline severity of depressive symptoms (not necessarily reaching threshold for diagnosis) and neuropsychological impairment in people with alcohol use disorder, have shown baseline depressive symptoms to be an important predictor of cognitive performance [37,59].
Reduction in alcohol use quantity and heavy drinking frequency did not predict cognitive improvement. Given the level of cognitive functioning at baseline of the CAD sample was in the normal range, it is likely that the alcohol consumption of this group had not contributed significantly to cognitive functioning. An exclusion criterion for the current study was brain injury sufficient to prohibit CBT, which may have excluded people for whom alcohol-related cognitive problems occurred. It is also possible that some other protective factor might have mediated the effects of alcohol on cognitive functioning. For example, Australia has a program of mandatory thiamine fortification of bread and wheat flour which is thought to protect against thiamine deficiency, a known problem in alcohol dependence and risk factor for Wernicke–Korsakoff syndrome [60,61]. This may be an important protective factor that should be considered in future research in this area, along with assessment of other elements of nutritional intake.
Alcohol recovery may nevertheless play an important role in neuropsychological change in the CAD group. This paper reports on a small sample size with average cognitive functioning at baseline. Examination of the relationship between change in drinking and cognitive impairment in a sample with more severe cognitive decline at baseline could show a greater impact of reduction or cessation of alcohol use than we were able to detect here.
The use of a sample of men and women currently engaging in hazardous alcohol use and experiencing depression is one of the strengths of the study. Despite instructions to the contrary, the majority of participants had consumed alcohol in the 24-hours prior to neuropsychological assessment, which raises concern about the potential for these results to be affected by intoxication. However it is also recognized that acute cognitive deficits are observed in the first two weeks of abstinence which can give rise to misleading levels of improvement on subsequent retesting when the acute withdrawal has abated [17]. Research has shown the weeks immediately following withdrawal from alcohol to be associated with higher cognitive impairment which may explain the higher functioning of this stilldrinking sample at baseline and the lack of significant cognitive improvement [16,17]. Examining these participants in the context of recent alcohol use gives a more naturalistic picture of how they present for treatment. This better informs our understanding of the hurdles to accessing and utilizing psychological treatment faced by alcohol abusing clients.
Interpretation of these results should be considered in light of some limitations to the current study. Evidence of alcohol and other substance use was obtained by participant self-report. However, the absence of confirmatory toxicology was tempered by strong collateral validation of alcohol use at 12-months. Participant history of brain injury and other conditions which may affect cognition was also obtained by self-report without medical examination. Absence of a healthy control group is a further limitation of the study which has been minimized by the use of well normed neuropsychological assessments. It is also notable that the 12-month follow-up rate was 65.7%, although examination of baseline neuropsychological test performance suggests that the completers and non-completers were not dissimilar in cognitive function.
The finding that changes in depression have such an impact on cognitive improvement has important implications for intervening in the clinical CAD population. These results suggest that addressing depressive symptoms early in people with co-occurring alcohol misuse and depression could result in earlier cognitive improvement than in those whose depressive symptoms are not directly targeted. Previous research has shown that an integrated depression and alcohol intervention produced more rapid improvement in depression than either a depression focused or alcohol focused intervention [39]. Larger studies of interventions for this comorbidity, with longitudinal neuropsychological data are needed to explore this connection and improve our understanding of the trajectory of neuropsychological changes across the lifespan in people with CAD. At least, this study provides evidence that a CAD sample do not experience cognitive deficits that might interfere with the effectiveness of cognitive behaviour therapy for depression and alcohol misuse problems, and that treatment can occur in the context of current (and heavy) use.
Acknowledgements
This project received approval from the relevant ethics committees and was registered with the Australian New Zealand Clinical Trials Registry (ANZCTR: www.anzctr.org.au; identifier: ACTRN12607000057482). Funding for this research was provided by the National Health and Medical Research Council (NHMRC – Application ID: 351115) and the NSW Health Drug and Alcohol Council Research Grants Program. Neuropsychological tests were purchased with a discount from Harcourt Australia. The authors would also like to acknowledge the contribution of Associate Professor Terry Lewin for his assistance with interpretation of results, all of the clinicians and research assistants who conducted the assessments and treatment, and of course the participants who generously gave their time.
Financial Support
Funding for this research was provided by the National Health and Medical Research Council (NHMRC – Application ID: 351115) and the NSW Health Drug and Alcohol Council Research Grants Program. Neuropsychological tests were purchased with a discount from Harcourt Australia.
References
- Teesson M, Hall W, Lynskey M, Degenhardt L (2000) Alcohol- and drug-use disorders in Australia: implications of the National Survey of Mental Health and Wellbeing. Aust N Z J Psychiatry 34: 206-213.
- Davis L, Uezato A, Newell JM, Frazier E (2008) Major depression and comorbid substance use disorders. CurrOpin Psychiatry 21: 14-18.
- Watts M (2008) Understanding the coexistence of alcohol misuse and depression. Br J Nurs 17: 696-699.
- Penick EC, Powell BJ, Liskow BI, Jackson JO, Nickel EJ (1988) The stability of coexisting psychiatric syndromes in alcoholic men after one year. J Stud Alcohol 49: 395-405.
- Conner KR, Pinquart M, Gamble SA (2009) Meta-analysis of depression and substance use among individuals with alcohol use disorders. J Subst Abuse Treat 37: 127-137.
- Degenhardt L, Hall W, Lynskey M (2001) Alcohol, cannabis and tobacco use among Australians: a comparison of their associations with other drug use and use disorders, affective and anxiety disorders, and psychosis. Addiction 96: 1603-1614.
- Murray RM, Sham P, Van Os J, Zanelli J, Cannon M, et al. (2004) A developmental model for similarities and dissimilarities between schizophrenia and bipolar disorder. Schizophr Res 71: 405-416.
- Airaksinen E, Wahlin A, Forsell Y, Larsson M (2007) Low episodic memory performance as a premorbid marker of depression: evidence from a 3-year follow-up. ActaPsychiatrScand 115: 458-465.
- Stavro K, Pelletier J, Potvin S (2013) Widespread and sustained cognitive deficits in alcoholism: a meta-analysis. Addict Biol 18: 203-213.
- Burt DB, Zembar MJ, Niederehe G (1995) Depression and memory impairment: a meta-analysis of the association, its pattern, and specificity. Psychol Bull 117: 285-305.
- Løberg T, Miller WR (1986) Personality, cognitive, and neuropsychological correlates of harmful alcohol consumption: a cross-national comparison of clinical samples. Ann N Y AcadSci 472: 75-97.
- Pitel AL, Beaunieux H, Witkowski T, Vabret F, Guillery-Girard B, et al. (2007) Genuine episodic memory deficits and executive dysfunctions in alcoholic subjects early in abstinence. Alcohol ClinExp Res 31: 1169-1178.
- Veiel HO (1997)A preliminary profile of neuropsychological deficits associated with major depression. J ClinExpNeuropsychol 19: 587-603.
- Saults JS, Cowan N, Sher KJ, Moreno MV (2007) Differential effects of alcohol on working memory: distinguishing multiple processes. ExpClinPsychopharmacol 15: 576-587.
- Pitel AL, Rivier J, Beaunieux H, Vabret F, Desgranges B, et al. (2009) Changes in the episodic memory and executive functions of abstinent and relapsed alcoholics over a 6-month period. Alcohol ClinExp Res 33: 490-498.
- Loeber S, Duka T, Welzel H, Nakovics H, Heinz A, et al. (2009) Impairment of cognitive abilities and decision making after chronic use of alcohol: the impact of multiple detoxifications. Alcohol Alcohol 44: 372-381.
- Lezak, M.D., D.B. Howieson, and D.W. Loring, Neuropsychological Assessment. Fourth ed2004, New York, NY: Oxford University Press.
- Fein G, Torres J, Price LJ, Di Sclafani V (2006) Cognitive performance in long-term abstinent alcoholic individuals. Alcohol ClinExp Res 30: 1538-1544.
- Rourke SB, Grant I (1999) The interactive effects of age and length of abstinence on the recovery of neuropsychological functioning in chronic male alcoholics: a 2-year follow-up study. J IntNeuropsycholSoc 5: 234-246.
- Smith DJ, Muir WJ, Blackwood DH (2006) Neurocognitive impairment in euthymic young adults with bipolar spectrum disorder and recurrent major depressive disorder. Bipolar Disord 8: 40-46.
- Austin MP, Ross M, Murray C, O'Carroll RE, Ebmeier KP, et al. (1992) Cognitive function in major depression. J Affect Disord 25: 21-29.
- Strömgren LS (1977) The influence of depression on memory. ActaPsychiatrScand 56: 109-128.
- Cohen RM, Weingartner H, Smallberg SA, Pickar D, Murphy DL (1982) Effort and cognition in depression. Arch Gen Psychiatry 39: 593-597.
- Beatty WW (1990) Cognitive functioning in bulimia: Comparison with depression. Bulletin of the Psychonomic Society 28: 289-292.
- Ravdin LD, Katzen HL, Agrawal P, Relkin NR (2003) Letter and semantic fluency in older adults: effects of mild depressive symptoms and age-stratified normative data. ClinNeuropsychol 17: 195-202.
- Elliott R(2002) The neuropsychological profile in primary depression, in Cognitive deficits in brain disorders. JJ Harrison, OwensA, Taylor and Francis: London, p: 273-293.
- Weiland-Fiedler P, Erickson K, Waldeck T, Luckenbaugh DA, Pike D, et al. (2004) Evidence for continuing neuropsychological impairments in depression. J Affect Disord 82: 253-258.
- Paelecke-Habermann Y, Pohl J, Leplow B (2005) Attention and executive functions in remitted major depression patients. J Affect Disord 89: 125-135.
- Preiss M (2010) Cognitive deficits in hospitalized and never hospitalized remitted unipolar depressive patients. The European Journal of Psychiatry 24: 129-135.
- Hasselbalch BJ, Knorr U, Hasselbalch SG, Gade A, Kessing LV (2012) Cognitive deficits in the remitted state of unipolar depressive disorder. Neuropsychology 26: 642-651.
- Biringer E, Lundervold A, Stordal K, Mykletun A, Egeland J, et al. (2005) Executive function improvement upon remission of recurrent unipolar depression. Eur Arch Psychiatry ClinNeurosci 255: 373-380.
- Douglas KM, Porter RJ (2009) Longitudinal assessment of neuropsychological function in major depression. Aust N Z J Psychiatry 43: 1105-1117.
- Liu IC, Chiu CH, Yang TT (2010) The effects of gender and a co-occurring depressive disorder on neurocognitive functioning in patients with alcohol dependence. Alcohol Alcohol 45: 231-236.
- Hermens DF, Lee RS, De Regt T, Lagopoulos J, Naismith SL, et al. (2013) Neuropsychological functioning is compromised in binge drinking young adults with depression. Psychiatry Res 210: 256-262.
- Sullivan LE, Fiellin DA, O'Connor PG (2005) The prevalence and impact of alcohol problems in major depression: a systematic review. Am J Med 118: 330-341.
- Uekermann J, Daum I, Schlebusch P, Wiebel B, Trenckmann U (2003) Depression and cognitive functioning in alcoholism. Addiction 98: 1521-1529.
- Hunt SA, Baker AL, Michie PT, Kavanagh DJ (2009) Neurocognitive profiles of people with comorbid depression and alcohol use: implications for psychological interventions. Addict Behav 34: 878-886.
- Baker AL, Kavanagh DJ, Kay-Lambkin FJ, Hunt SA, Lewin TJ, et al. (2010) Randomized controlled trial of cognitive-behavioural therapy for coexisting depression and alcohol problems: short-term outcome. Addiction 105: 87-99.
- Baker AL, Kavanagh DJ, Kay-Lambkin FJ, Hunt SA, Lewin TJ, et al. (2014) Randomized controlled trial of MICBT for co-existing alcohol misuse and depression: outcomes to 36-months. J Subst Abuse Treat 46: 281-290.
- Stockwell T(2001) Harm reduction, drinking patterns and the NHMRC Drinking Guidelines. Drug and Alcohol Review20: 121-129.
- Steer RA, Rissmiller DJ, Beck AT (2000) Use of the Beck Depression Inventory-II with depressed geriatric inpatients. Behav Res Ther 38: 311-318.
- Wechsler D(1997) Wechsler Adult Intelligence Scale.(3edn) San Antonio, TX: The Psychological Corporation.
- Balzano J, Chiaravalloti N, Lengenfelder J, Moore N, DeLuca J (2006) Does the scoring of late responses affect the outcome of the paced auditory serial addition task (PASAT)? Arch ClinNeuropsychol 21: 819-825.
- Delis DC, Kaplan E, Kramer J (2001) Delis-Kaplan Executive Function System. The Psychological Corporation,San Antonio, TX.
- Borkowski JG, Benton AL, Spreen O (1967) Word fluency and brain damage. Neuropsychologia 5: 135-140.
- Demakis GJ (2004) Frontal lobe damage and tests of executive processing: a meta-analysis of the category test, stroop test, and trail-making test. J ClinExpNeuropsychol 26: 441-450.
- Wechsler D (1999) Wechsler Abbreviated Scale of Intelligence (WASI) manual. Psychological Corporation, San Antonio, TX.
- Vanderploeg RD, Schinka JA (1995) Predicting WAIS-R IQ premorbid ability: combining subtest performance and demographic variable predictors. Arch ClinNeuropsychol 10: 225-239.
- Sobell LC, MBSobell (1992) Timeline follow-back: A technique for assessing self-reported alcohol consumption in Measuring alcohol consumption: Psychosocial and biochemical methods. RZLitten and JP Allen, Humana Press: Totowa, NJ. p. 41-72.
- First MB(2001) Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edn, New York: New York State Psychiatric Institute.
- APA (2013) Diagnostic and Statistical Manual of Mental Disorders.(5edn), American Psychiatric Publishing. Washington, DC.
- APA (1994) Diagnostic and statistical manual of mental disorders. (4thedn), Washington, DC: American Psychiatric Association.
- Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M (1993) Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption--II. Addiction 88: 791-804.
- Darke S, Hall W, Wodak A, Heather N, Ward J (1992) Development and validation of a multi-dimensional instrument for assessing outcome of treatment among opiate users: the Opiate Treatment Index. Br J Addict 87: 733-742.
- Steer RA, Rissmiller DJ, Beck AT (2000) Use of the Beck Depression Inventory-II with depressed geriatric inpatients. Behav Res Ther 38: 311-318.
- Beck AT, SteerRA,BrownGK (1996) Beck Depression Inventory - II manual, San Antonio, TX: Psychological Corporation.
- Buckley TC, Parker JD, Heggie J (2001) A psychometric evaluation of the BDI-II in treatment-seeking substance abusers. J Subst Abuse Treat 20: 197-204.
- Seignourel PJ1, Green C, Schmitz JM (2008) Factor structure and diagnostic efficiency of the BDI-II in treatment-seeking substance users. Drug Alcohol Depend 93: 271-278.
- O'Brien-Simpson L, Di Parsia P, Simmons JG, Allen NB (2009) Recurrence of major depressive disorder is predicted by inhibited startle magnitude while recovered. J Affect Disord 112: 243-249.
- Schafer K, Butters N, Smith T, Irwin M, Brown S, et al. (1991)Cognitive performance of alcoholics: a longitudinal evaluation of the role of drinking history, depression, liver function, nutrition, and family history. Alcohol ClinExp Res15: 653-660.
- Code ANZFS(2009) Standard 2.1.1-Cereals and Cereal Products.
- Rees E, Gowing LR (2013) Supplementary thiamine is still important in alcohol dependence. Alcohol Alcohol 48: 88-92.
Relevant Topics
- Addiction Recovery
- Alcohol Addiction Treatment
- Alcohol Rehabilitation
- Amphetamine Addiction
- Amphetamine-Related Disorders
- Cocaine Addiction
- Cocaine-Related Disorders
- Computer Addiction Research
- Drug Addiction Treatment
- Drug Rehabilitation
- Facts About Alcoholism
- Food Addiction Research
- Heroin Addiction Treatment
- Holistic Addiction Treatment
- Hospital-Addiction Syndrome
- Morphine Addiction
- Munchausen Syndrome
- Neonatal Abstinence Syndrome
- Nutritional Suitability
- Opioid-Related Disorders
- Relapse prevention
- Substance-Related Disorders
Recommended Journals
Article Tools
Article Usage
- Total views: 15419
- [From(publication date):
specialissue-2014 - Apr 11, 2025] - Breakdown by view type
- HTML page views : 10853
- PDF downloads : 4566