Challenges in the Management of Glaucoma in a Patient with Severe Ocular Surface Disease: A Case Report
Received: 26-Feb-2016 / Accepted Date: 13-May-2016 / Published Date: 20-May-2016 DOI: 10.4172/2476-2075.1000111
6455Introduction
Ocular surface disease (OSD) is a significant problem for glaucoma patients worldwide, who will require long-term topical treatment. The risk of OSD increases with age, with a reported prevalence of up to 60%. The severity and burden of OSD also increase with the number of topical anti-glaucoma medications used in treatment due to multiple, daily exposures of the ocular surface (OS) to toxic active compounds in the drug itself, other components of the formulation or preservatives [1,2]. The presentation of OSD varies depending on its severity, but usually includes symptoms of dry eyes, allergy and distorted vision, which can be debilitating and affect quality of life. The clinical signs of the disease include tear-film instability, toxic keratoconjunctivitis, eyelid abnormalities and allergic manifestations. However, the lack of concordance between symptoms and signs often makes the diagnosis of OSD and assessment of its severity challenging.
Management becomes even more difficult when a patient with preexisting OSD develops glaucoma, with serious complication affecting patients with severe OSD in terms of high prevalence, severity and difficulty in diagnosis and treatment. Reducing intraocular pressure (IOP), limiting optic nerve damage and maintaining the visual field is critical. The cessation of topical anti-glaucoma medication often results in the disappearance of allergic inflammation, but might allow the glaucoma to progress to irreversible blindness. If inflammation continues, severe chronic OS inflammation might be difficult to treat from a glaucoma perspective. Evidence of the best surgical management in patients with glaucoma and severe OSD is scarce. Currently, no widely accepted, precise clinical guidelines exist for this very common and relevant condition, which has significant clinical impact.
We present a challenging case of advanced glaucoma with severe OSD and benzalkonium chloride (BAK) allergy. The induced OS changes from topical anti-glaucoma therapy in addition to an already compromised OS led to poor tolerance and compliance with IOPlowering eye drops. To decrease dependence on topical medications and reduce exposure to toxic compounds exacerbating OSD, laser and surgical treatments were attempted. The exacerbated damage to the OS not only made the patient’s glaucoma surgery difficult and complicated, but also significantly increased the risk of the surgery’s failure. In our paper, we aim to provide guidelines for ophthalmologists treating glaucoma patients with severe OSD. We hope to contribute to existing ophthalmological knowledge by sharing our experience and the challenges encountered in managing this case and looking for the best possible treatment.
Case Presentation
A 57-year-old woman was being treated with latanoprost and timolol eye drops for ocular hypertension in our eye department. She had pre-existing dry eyes, which she was managing with ocular lubrication (Carbomer gel and lacrilube ointment). She was otherwise healthy and not on any systemic medications. She had no known allergies and no family history of glaucoma. Her visual acuity was 6/4 in the right and left eyes. Over an eight-year follow-up period, her IOPs fluctuated between 18 mmHg and 26 mmHg. She had no significant glaucomatous optic neuropathy. Her corneal thickness averaged 545 μm, and the Humphrey visual field was full in both eyes. She maintained a yearly follow-up schedule.
Two years later, between her scheduled follow-up appointments, she presented to our department with blurred vision, red eyes and eye pain. Her visual acuity was 6/36 in the right eye and 6/24 in the left. Her IOPs were 41 mmHg in the right eye and 43 mmHg in the left. Gonioscopy showed open angles. She had 0.9 disc cupping bilaterally with visual field defects in the Humphrey visual field testing. She also had severe OSD, as evidenced by increased tear film break-up time (TBUT), superficial punctuate keratopathy and “cellophane-like conjunctiva”. Further questioning revealed that she had stopped all eye drops on her own volition due to drug intolerance, which was affecting her quality of life.
The patient was promptly started on oral acetazolamide, in addition to various topical anti-glaucoma medications, to lower her IOP, but she was intolerant of numerous eye drops, including dorzolamide, travoprost, brinzolamide, timolol and latanoprost. She was started on preservative-free eye drops (tafluprost) together with treatment for dry eyes [Carmellose (Celluvisc) eye drops]. Short-term use of mild corticosteroids, being watchful for steroid-induced elevation in IOP, failed to satisfactorily lower her IOPs, which were 30 mmHg in the right eye and 36 mmHg in the left.
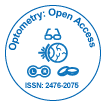
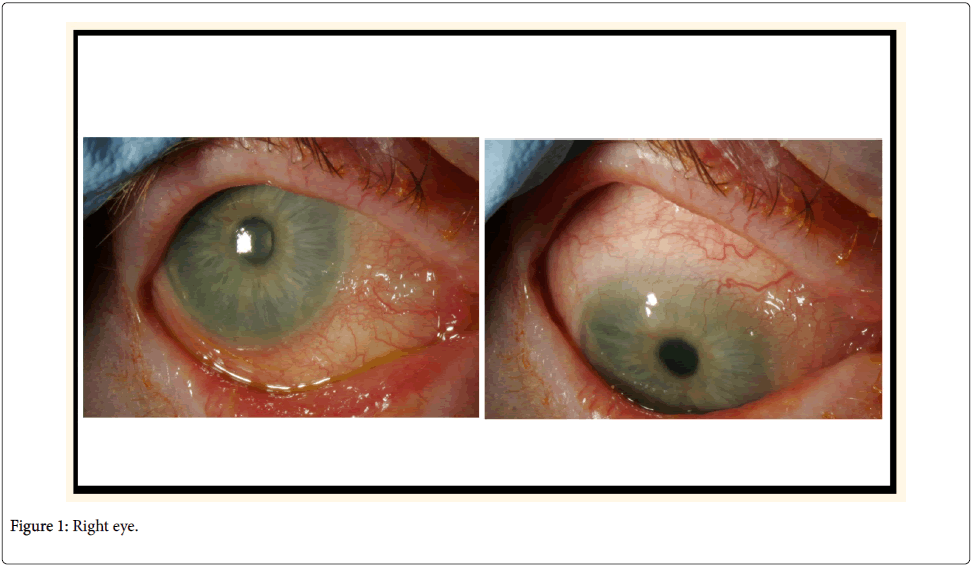
In an effort to reduce her IOPs and decrease dependency on oral acetazolamide and long-term topical treatment, the patient had bilateral 360-degree selective laser trabeculoplasty (SLT), with no significant response. She then had mitomycin C augmented trabeculectomy to her left eye, which failed with scarring of bleb despite repeated post-operative bleb needling with 5-fluorouracil. Not surprisingly, the surgery was difficult mainly in terms of handling the conjunctiva. Next, the least interventionist approach-bilateral cyclodiode therapy-was attempted, followed by cataract surgery. After the final surgery, she could tolerate a medication combination of brinzolamide and timolol. At her most recent follow-up examination (Figures 1 and 2), her visual acuity was 6/6 in both eyes, with IOP of 20 mmHg in both eyes and 0.9 cupping.
Discussion
OSD refers to a group of disorders affecting various components of the OS, ultimately resulting in a dysfunction of the ocular tear film and a reduction in OS integrity. The ocular manifestations of OSD can include foreign body sensation, discomfort, fluctuating vision, infection, OS and eyelid scarring. Severe visual impairment from OSD can ensue from limbal stem cell deficiency or loss of corneal clarity. The early signs might be non-specific, subtle and easily overlooked unless the patient is symptomatic. No widely accepted criteria for diagnosing OSD exist. Chronic clinical effects include reduced tear secretion, basal Schirmers and TBUT, increased Rose-Bengal staining of the cornea and lid margin laxity. The OSD score indicates that up to 60% of glaucoma patients have OSD, and both conditions can independently affect quality of life and compliance [3].
Thirty-six per cent of patients with glaucoma have severe OSD [1]. Patients with concomitant glaucoma and OSD might have incomplete responses to anti-glaucoma medications, which could be due partly to the disease or inflammation of the OS and partly to poor compliance with topical medication treatment. If the OS has problems, topical anti-glaucoma drop absorption could be altered- decreasing their pharmacological effects and increasing their toxicological effects. Moreover, topical treatment using one or more preserved eye drops might exacerbate the pre-existing OSD [4]. The induced OS changes from topical anti-glaucoma therapy on top of an already compromised OS lead to poor tolerance and compliance with IOP-lowering eye drop treatment.
Measuring the TBUT is a quick, reliable method of monitoring ocular surface health. If the inter-blink interval is longer than TBUT, the OS is exposed to external insults. Up to 70% of glaucoma patients can show a decrease in TBUT, indicating the presence of inflammation [3]. A central corneal staining is indicative of an advanced dry eye condition, but more diffused staining could indicate a reaction to topical medications. Lissamine green stains cells earlier than fluorescein stain in the dysfunction state before they become devitalized, allowing for earlier detection of OSD. A tear osmolarity value greater than 308 mOsm/L is a sensitive indicator for dry eye.
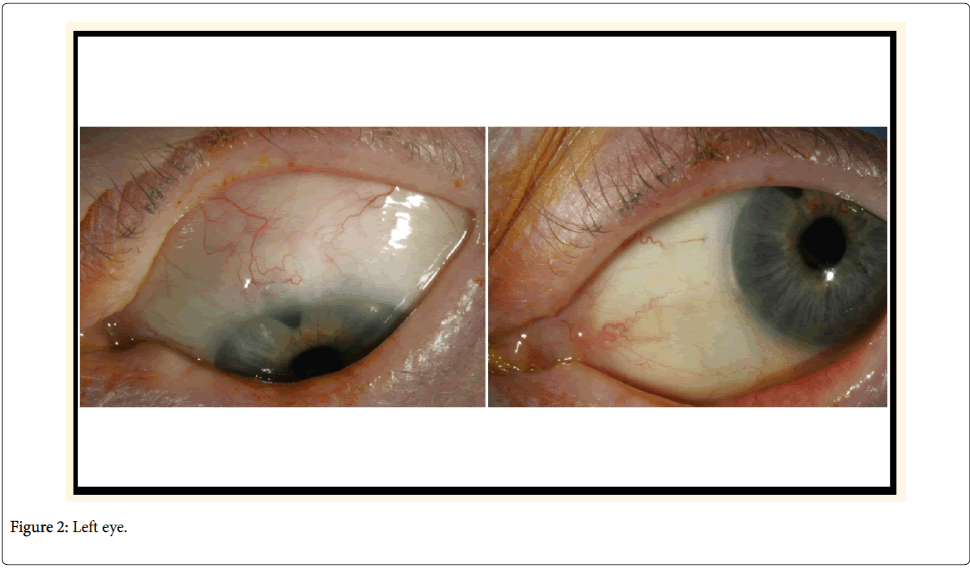
BAK, the most commonly used ocular preservative, is largely responsible for the ocular toxicities and inflammation. BAK is a quartenary ammonium compound with cationic surfactant properties (a detergent with direct effects on the lipid layer of cell membranes), ranging in concentration from 0.004 to 0.02%. It is associated with serious adverse reactions and dose-dependent toxicity, proportional to the duration of total exposure [5]. At low concentrations (0.0001%), BAK might arrest cellular growth on the OS (Figure 3A). At medium concentrations (0.01%), programmed cell death occurs (Figure 3B). High concentrations (0.05% to 0.1%) can result in cell necrosis.
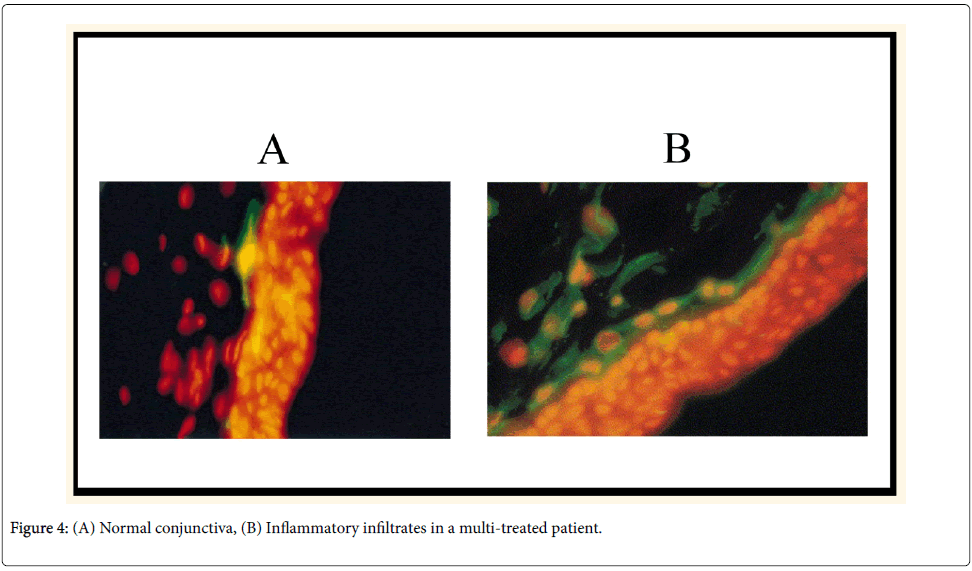
Ocular toxicity of BAK preservatives is thought to be mediated via T-cell activation and proliferation (Th1 and Th2 systems), simultaneous overexpression of CCR4 and CCR5, and overexpression of human leucocyte antigen (HLA)-DE on epithelial cells (Figure 4) [6]. These findings suggest that inflammatory mechanisms combining allergy with toxicity are present together, occurring simultaneously in glaucoma patients’ OS.
The presence of BAK on the OS can lead to a reduction in corneal epithelial integrity, a secondary increase in corneal and conjunctival inflammatory cells, loss of goblet cells and a reduction in tear film [7]. BAK might activate a cascade of pro-inflammatory mediators, including cytokines, chemotactic factors and metalloproteinases (MMP 9), leading to the activation of fibroblasts, which results in the deposition of excess collagen, and increased fibrosis. A fibrotic tissue response to the bleb can occur post-operatively due to collagen deposition that might encapsulate the bleb or block aqueous outflow, resulting in a flat, inefficient bleb. The induced inflammation and chronic damage to the ocular surface not only make any glaucoma surgery difficult and complicated, but also significantly increase the failure risk of any subsequent surgery due to long-term OS inflammation, which will rapidly increase healing and post-operative scarring [8]. Concomitant mitomycin use can exacerbate the already compromised OS during trabeculectomy.
Except for a few independent studies, no substantive evidence demonstrates the efficacy of minimally invasive glaucoma surgery devices, such as iStent, trabectome and canaloplasty [9,10]. Such devices show promise, but many questions remain. The glaucos trabecular microbypass iStent bypasses the juxtacanalicular trabecular meshwork and has a half pipe designed with a snorkel that rests in the anterior chamber. In one published study, the insertion of a single stent reduced the IOP from 21.4 mmHg to 12.4 mmHg, but the insertion of additional stents showed no clear benefit [9]. This titanium L-shaped stent is designed to be implanted ab interno, leaving the conjunctiva and sclera undisturbed. Avoiding a subconjunctival bleb in an already compromised conjunctiva decreases the likelihood of visionthreatening complications such as hypotony and bleb-related infections. Complications include stent dislocation, migration, transcient post-operative hyphaema and inadequate IOP control requiring glaucoma drainage devices (GDDs).
The ab interno trabectome microelectrocautery is a foot pedalactivated device that creates a direct communication for aqueous flow between the anterior chamber and Schlemm’s canal, along the length of the ablated arc. Published research showed the mean IOP fell from 25.7 mmHg to 16.6 mmHg at 2-year follow-up [11]. The trabectome preserves the conjunctiva, avoids the bleb and associated risks, and protects against hypotony with downstream episcleral venous resistance. However, its drawbacks include peripheral anterior synaechia, transceint corneal injury, Descemet’s detachment and inadvertent iris injury.
Non-penetrating Schlemm’s canaloplasty allows the microcatheterization of the entire Schlemmn’s canal via an ab externo approach. The tensioned suture attempts to reverse the process of canal collapse in glaucomatous eyes, thereby lowering IOP. In one study, the mean IOP in patients’ undergone canaloplasty decreased from 23.3 mmHg to 16.3 mmHg at 2-year follow-up [12]. However, it is a difficult technique to master.
GDDs offered a potentially effective treatment option in our patient. GDDs are designed to divert aqueous from the anterior chamber to an external reservoir, where a fibrous capsule forms about 4 to 6 weeks after surgery and regulates flow. A posterior sclerostomy ensures that the tube rests parallel to the iris surface and minimizes corneal neovascularization. These devices have been successful in controlling IOP in eyes with previously failed trabeculectomy and in eyes with insufficient conjunctiva from scarring or injury. The amount of conjunctival scarring can determine the size of the implant and available area for a single-plate or double-plate device. Valved devices provide more immediate IOP control and a lower rate of hypotony; non-valved devices are often occluded with a ligature suture, and the post-operative IOP remains unchanged until the formation of a fibrous capsule. The Ahmed Baerveldt Comparison Study demonstrated that, at one year, the mean IOP was 15.4 mmHg in the Ahmed group versus 13.2 mmHg in the Baerveldt group, but the Baerveldt group required more surgical interventions post-operatively [13]. Complications included tube-related problems, plate migration, valve malfunction and tube erosion.
A feasible treatment plan for patients with glaucoma and OSD should be individualized on a patient-by-patient basis, while continuing to apply therapy to reduce IOP, limit optic nerve damage and maintain visual field for the management of glaucoma. This includes a combination of treatments to address the underlying pathological process, curtail the progression of glaucoma and provide symptom relief. It is important to minimize the preservative load on the OS. Concomitant factors such as blepharitis, meibomian gland dysfunction and underlying dry eyes should be treated aggressively to minimize the symptoms and signs of OSD. Next, aggressive ocular lubrication should occur using preservative-free artificial tears, lubricating gel and ointment, as needed.
A BAK allergy should be suspected when OSD treatment does not show clinical improvement and the intolerance of multiple medication classes is reported, as in our case. In such cases, a BAK-free or preservative-free medication should be prescribed. The newer preservative classes, including unique molecules (e.g., sofZia and Purite), have been shown to decrease toxicity in in vitro and in animal studies [4]. Clinicians should allow sufficient time (e.g., four to six weeks) for the BAK-induced ocular surface effects to resolve. Reducing the amount and number of topical treatments can decrease the inflammation present in glaucoma patients and improve outcomes related to glaucoma surgery. As in our case, some patients with severe OSD might not be able to tolerate any topical medication regardless of whether they use preservatives or not.
Thus, the benefits of laser and/or surgical treatments should be weighed to decrease dependency on medical therapy and exposure to toxic compounds that exacerbate OSD in these patients. If this fails, implanting GDDs or a minimally invasive glaucoma surgery device, such as an iStent, trabectome or canaloplasty, might be necessary to decrease toxicity from topical medications and prevent the progression of visual field loss.
Conclusion
OSD can be a major cause of intolerance to glaucoma medication. Patients with severe OSD often have glaucoma refractory to medical therapy often due to subacute or chronic inflammation. If the OS has problems, topical anti-glaucoma drop absorption might be affected, increasing or decreasing pharmacological and toxicological effects. Managing patients with glaucoma and OSD must involve reducing eye drop-induced toxicity and developing a specific treatment for OSD, in addition to controlling the glaucoma. The clinical impact of the interplay between lowering IOP and maintaining the ocular surface is relevant, and treatment of one ocular condition—whether glaucoma or OSD—should not come at a price. Patients with glaucoma need a careful ocular surface examination at the beginning and during treatment as many will develop OSD during their lives, which can lead to worse topical treatment compliance and hence IOP control and worse surgical outcomes. Both pathologies glaucoma and OSD, if not appropriately managed, can lead to blindness. In an effort to maximize patient outcomes, an in-depth understanding of the consequences of medical and surgical intervention on an unhealthy OS is critical. Various problems could arise, and both short and long-term complications should be monitored. We have presented one original case report, which is not easily generalizable, but it serves as a stepping-stone for further research to facilitate the turning of these little studied surgical options into the best evidence-based medical practice. Our review of current clinical and experimental literature while sharing our experience and challenges in managing and treating this case will contribute to the general ophthalmological knowledge by proposing new insights to guide the development of guidelines and future research in this important area.
Acknowledgements
The authors thank Prof C Baudouin (Paris, France) for the use of images 3 and 4.
References
- Stewart WC, Stewart JA, Nelson LA (2011) Ocular surface disease in patients with ocular hypertension and glaucoma. Curr Eye Res 36:391-398.
- Rossi GC, Pasinetti GM, Scudeller L, Raimondi M, Lanteri S, et al. (2013) Risk factors to develop ocular surface disease in treated glaucoma or ocular hypertension patients. Eur J Ophthalmol23:296-302.
- Anwar Z, Wellik SR, Galor A (2013) Glaucoma therapy and ocular surface disease: current literature and recommendations. CurrOpinOphthalmol24:136-143.
- Noecker R, Miller KV (2011)Benzalkonium chloride in glaucoma medications Ocul Surf 9:159-162.
- Ammar DA, Noecker RJ, Kahook MY (2011) Effects of benzalkonium chloride- and polyquad-preserved combination glaucoma medications on cultured human ocular surface cells. AdvTher28:501-510.
- Baudouin C, Liang H, Hamard P, Riancho L, Creuzot-Garcher C, et al. (2007) The ocular surface of glaucoma patients treated over the long term expresses inflammatory markers related to both T-helper 1 and T-helper 2 pathways. Ophthalmology 115:109-115.
- Broadway D, Hitchings R (1996) Conjunctival damage induced by long-term topical anti-glaucoma therapy. ActaOphthalmolScand 74:97.
- Broadway DC, Chang LP (2001)Trabeculectomy, risk factors for failure and the preoperative state of the conjunctiva. J Glaucoma10:237-249.
- Saheb H, Ahmed, II (2012) Micro-invasive glaucoma surgery: current perspectives and future directions. CurrOpinOphthalmol 23:96-104.
- Schmidt W, Kastner C, Sternberg K, Allemann R, Lobler M, et al. (2013) New concepts for glaucoma implants--controlled aqueous humor drainage, encapsulation prevention and local drug delivery. CurrPharm Biotechnol14:98-111.
- Vold SD (2011) Ab internotrabeculotomy with the trabectome system: what does the data tell us? IntOphthalmolClin 51:65-81.
- Grieshaber MC (2012) Ab externoSchlemm's canal surgery: viscocanalostomy and canaloplasty. Dev Ophthalmol 50:109-124.
- Barton K, Gedde SJ, Budenz DL, Feuer WJ, Schiffman J (2011) The Ahmed Baerveldt Comparison Study methodology, baseline patient characteristics, and intraoperative complications. Ophthalmology118:435-442.
Citation: Jeganathan VSE, Agarwal PK (2016) Challenges in the Management of Glaucoma in a Patient with Severe Ocular Surface Disease: A Case Report. Optom open access 1: 111. DOI: 10.4172/2476-2075.1000111
Copyright: © 2016 Jeganathan VSE. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 12657
- [From(publication date): 6-2016 - Apr 11, 2025]
- Breakdown by view type
- HTML page views: 11730
- PDF downloads: 927