Research Article Open Access
Cerebrospinal Fluid Neopterin and CXCL13 are Suitable Biomarkers for Staging and Detection of Treatment Failure in a Non-Human Primate Model of Human African Trypanosomiasis
Dawn N Maranga1*, Maina J Ngotho3, Victor M Mwadime2, Thomas A Adino2, John Kagira1,5, Maxwell Waema6, George A Omondi2, Sylvain Biéler4 and Joseph M Ndungu4
1Tropical and Infectious Diseases Department, Institute of Primate Research, Kenya
2Animal Sciences Department, Institute of Primate Research, Kenya
3Department of Animal Health and Production, Mount Kenya University, Kenya
4Foundation for Innovative New Diagnostics, Switzerland
5Department of Land Resources Planning Management, Jomo Kenyatta University of Agriculture and Technology, Kenya
6Department of Public Health, Jomo Kenyatta University of Agriculture and Technology, Kenya
- *Corresponding Author:
- Dawn N Maranga
Tropical and Infectious Diseases Department, Institute of Primate Research
P.O. Box 24481-00502 Karen, Nairobi, Kenya
Tel: +25420884308
E-mail: dawn.maranga@gmail.com
Received Date: March 17, 2017; Accepted Date: May 09, 2017; Published Date: May 12, 2017
Citation: Maranga DN, Ngotho MJ, Mwadime VM, Adino TA, Kagira J, et al. (2017) Cerebrospinal Fluid Neopterin and CXCL13 are Suitable Biomarkers for Staging and Detection of Treatment Failure in a Non-Human Primate Model of Human African Trypanosomiasis. J Neuroinfect Dis 8:246. doi: 10.4172/2314-7326.1000246
Copyright: © 2017 Maranga DN, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited
Visit for more related articles at Journal of Neuroinfectious Diseases
Abstract
Background: Follow-up of treated human African trypanosomiasis (HAT) patients has been a routine practice for confirming cure and early detection of treatment failure.
Objective: The aim of this study was to verify whether the neopterin and CXCL13 biomarkers are suitable for determining the stage of disease, and for monitoring the efficacy of treatment in a vervet monkey model of HAT.
Methods: Six monkeys were infected with Trypanosoma brucei. Late stage disease was induced by sub-curative treatment with diminazene aceturate (DA) from 28 days’ post-infection (dpi). When relapses occurred, the animals were treated curatively with melarsoprol (Mel B) from 82 dpi. Blood and cerebrospinal fluid (CSF) samples were collected at weekly intervals and assessed for parasitosis, as well as tested for neopterin and CXCL13 by ELISA, for a period of 39 weeks.
Results: The concentration of neopterin in serum increased rapidly after infection in early disease (stage 1), peaking at 14 dpi. Levels then dropped rapidly to pre-infection levels by 35 dpi. In contrast, there was a marginal increase in CSF neopterin during early infection, with a minor peak at 21 dpi. Serum CXCL13 increased rapidly from 7 dpi, peaking at 28 dpi, after which the levels dropped upon sub-curative treatment with DA. The concentration of CXCL13 in CSF also increased gradually in early stage disease and continued to rise after sub-curative treatment with DA. Curative treatment with Mel B resulted in its gradual decline, reaching pre-infection levels 105 days later.
Conclusions: The changes in CSF neopterin and CXCL13 correspond to important time-points and stages of the disease. That peak levels coincided with time of relapses demonstrates the potential of these biomarkers in staging and detecting treatment failure. Moreover, the rapid fall in CSF neopterin after curative treatment confirms its great potential for use in development of a test of cure.
Keywords
Trypanosomiasis; Neopterin; CXCL13; Parasitaemia; Test of cure; Late stage
Introduction
Human African trypanosomiasis (HAT), also known as sleeping sickness, is a tsetse-transmitted neglected tropical disease (NTD) caused by protozoan parasites of the genus Trypanosoma . The disease is endemic in resource limited settings in 36 sub-Saharan African countries, with more than 70 million people estimated to live at varying risks of infection [1]. There are two forms of HAT; an acute one caused by T. rhodesiense is endemic in eastern and southern Africa, and a chronic form by T. gambiense occurs in central and western Africa.
Human African trypanosomiasis progresses from an early haemolymphatic or stage one (S1) disease, to a late neurologic or stage two (S2) disease after parasites invade and cause damage to the central nervous system (CNS). Disease stage determination is critical in order to guide the choice of treatment in confirmed cases [2,3]. The distinction between S1 and S2 disease is based on examination of the CSF by microscopy. Patients are considered to be in S2 if parasites are demonstrated in the CSF, or the number of CSF white blood cells (WBC) exceed 5 cells/μl. These parameters are however insensitive and non-specific, and have attracted considerable debate [2-8].
A number of studies have identified biomarkers with potential for staging HAT and detecting treatment failure [4-6]. A good test of cure biomarker is one that would decrease rapidly after successful treatment, in order to reduce the risk of patients being lost to followup. Neopterin, a low molecular weight pteridine synthesized by activated macrophages and dendritic cells after interferon-γ (IFN- γ) stimulation, has been reported as a good S2 marker for both T. rhodesiense [7] and T. gambiense HAT [6]. It has also been shown to have strong predictive value for treatment outcome in T. gambiense HAT.
In an investigation of 512 T. gambiense HAT patients, CSF IgM and neopterin were the best in discriminating between the two stages (S1 and S2) of disease, with 86.4% and 84.1% specificity, respectively, at 100% sensitivity [6]. When a validation cohort (412 patients) was tested, neopterin correctly classified 88% of S1 and S2 patients, confirming its high staging power [6]. Subsequent studies also showed that CSF neopterin decreased rapidly in cured HAT patients and remained elevated in those who later relapsed [8].
CXCL13 is a chemokine ligand that is known to be expressed by stromal cells within B cell follicles in secondary lymphoid tissues [9]. Cerebrospinal fluid CXCL13 is correlated with inflammatory disease activity in various CNS diseases, such as multiple sclerosis and Lyme neuroborreliosis (LNB) [10] where it plays a critical role as a suitable marker of B cell recruitment [11].
The present study was carried out to assess the suitability of CSF neopterin and CXCL13 in determining the stage of disease and monitoring treatment efficacy in a vervet monkey model of HAT. As HAT is associated with massive immunological reactions and invasion of the CNS by parasites, this study was also used to improve our understanding of the changes in neopterin and CXCL13 in serum and CSF in different phases of the disease, and after sub-curative and curative treatment.
Materials and Methods
Trypanosomes
The trypanosome isolate used in this study was T. brucei GUTat 1 initially obtained from the biobank of the international livestock research institute (ILRI) in Nairobi and maintained in the institute of primate research (IPR) biobank. The isolate was passaged three times in irradiated (500 rad) mice, then cryopreserved in liquid nitrogen. For this study, the cryopreserved isolate was thawed and 10 Swiss white mice infected by intraperitoneal injection with approximately 104 parasites. Six to 7 days after infection, when parasitaemia in the mice was at its peak, they were euthanized by CO2 inhalation, and approximately 0.5 ml of blood drawn by cardiac puncture. The blood was then diluted serially in phosphate saline glucose (PSG), to a final concentration of 104 trypanosomes/ml, and used to infect each of the experimental animals.
Experimental animals
Eight vervet monkeys (Chlorocebus aethiops), including 4 males and 4 females, weighing between 2.0 and 6.0 kg, were used in the study. The monkeys were housed individually in 90 cm × 60 cm × 60 cm stainless steel cages at ambient temperatures of 18°C to 25°C, under biosafety level II animal holding conditions, and were allowed visual contact with conspecifics. The animals were first kept for a period of 90 days before infection, during which they were adapted to handling by technicians, screened for zoonotic diseases and treated for ecto- and endoparasites. Only disease-free animals were used in the study. They were maintained on commercial food (chow-Goldstar Feeds ® Ltd., Nairobi, Kenya), supplemented with fresh fruits and vegetables. Drinking water was provided ad libitum.
Study design
Six monkeys were infected by intravenous (iv) injection with approximately 104 trypanosomes in 1 ml PSG, while the other two were kept as uninfected controls. Each animal was evaluated daily for clinical signs. The animals were monitored for parasitaemia by daily examination of ear-prick blood by microscopy, and the parasitaemia scored using a rapid matching method [12].
Twenty-eight days’ post-infection (dpi), the animals were treated by intramuscular injection with diminazene aceturate (DA) (Veriben®, Sanofi, France) at a dose of 5 mg/kg body weight (bwt) for 3 consecutive days. When relapse parasitaemia was observed in half of the animals, they were all treated with melarsoprol (Mel B) (Arsobal® Specia, France) at a dose of 3.6 mg/kg iv, for 4 consecutive days from 82 dpi. This treatment has been shown to cure animals in late stage disease [13]. The animals were monitored up to 267 dpi, a six-month follow-up period for confirming cure, then they were euthanized by iv injection with 20% sodium pentobarbitone (Euthatal®, Lundbeck, Denmark) at a dose of 100 mg/kg bwt.
Sample collection and initial analysis
Every week starting two weeks before infection, the monkeys were anaesthetized using ketamine hydrochloride (Agrar® Agrar, Holland) at dose of 10 mg/kg bwt iv. Four ml of blood were drawn from the femoral vein, and 2 ml placed in a plain vacutainer and allowed to stand for 1-2 hours, after which serum was separated and stored at -20°C, whereas the remaining two ml of blood were placed in an ethylene diamine tetra acetic acid (EDTA) coated vacutainer for hematological analysis. The animals’ level of anaemia was determined by assessing the packed cell volume (PCV). A lumbar puncture was performed and the CSF examined immediately for the presence of trypanosomes, and the number of WBC were counted as previously described [13]. One ml of CSF was collected into an Eppendorf tube and stored at -20°C for laboratory analysis.
Neopterin assay
The concentration of neopterin in serum and CSF was determined using a competitive sandwich ELISA, according to manufacturer’s instructions (Neopterin ELISA, DRG Instruments, Marburg, Germany). Briefly, a volume of 25 μL of serum or CSF, standards and controls were added in duplicate to pre-coated anti-neopterin plates and incubated with 1/100 dilution of enzyme conjugate for two hours at room temperature. After washing, enzyme substrate was added and plates incubated for 30 minutes. The reaction was stopped using 1 M sulphuric acid and the absorbance read at 450 nm on an ELISA microplate reader (DYNEX MRX, Germany).
CXCL13 assay
The concentration of CXCL13 in serum and CSF was determined using an indirect ELISA, according to the manufacturer’s instructions (Quantikine ELISA human CXCL13, R&D Systems, MN, USA). Briefly, a volume of 100 μL of assay diluent was added to each well to which was then added 50 μL of serum or CSF, standards and controls in duplicate. The plate was then covered and incubated for two hours at room temperature. After washing, 200 μL of conjugate was added to each well and incubated for two hours at room temperature. After the second wash, enzyme substrate solution was added and the plate incubated for 30 minutes in the dark. The reaction was stopped using 1 M sulphuric acid, and the absorbance read at 450 nm in an ELISA microplate reader (DYNEX MRX, Germany).
Data analysis
Data was managed in spreadsheets which were then analyzed using GraphPad Prism version 5.0 for Windows (GraphPad software, USA). The mean ± SEM was established for all the tests at each sampling point. Differences between means were compared using Student’s t-test and ANOVA. The differences in mean values were considered to be statistically significant when P<0.05.
Ethical approval
All protocols and procedures used in this study were reviewed and approved by the institutional review committee (IRC) of the institute of primate research (IPR), Kenya.
Results
Clinical signs
The monkeys exhibited clinical signs of disease from between 2 and 4 dpi, coinciding with appearance of parasites in the blood, and were most pronounced between 7 and 21 dpi. These included a starry hair coat, loss of appetite, high mean body temperature of 39.1°C (range=38.9°C to 40.1°C), inactivity and hunching, enlargement of the spleen and peripheral lymph nodes, pallor of mucous membranes, with all animals experiencing a weight loss ranging between 0.4 kg and 1 kg, and oedema of the face and genitalia.
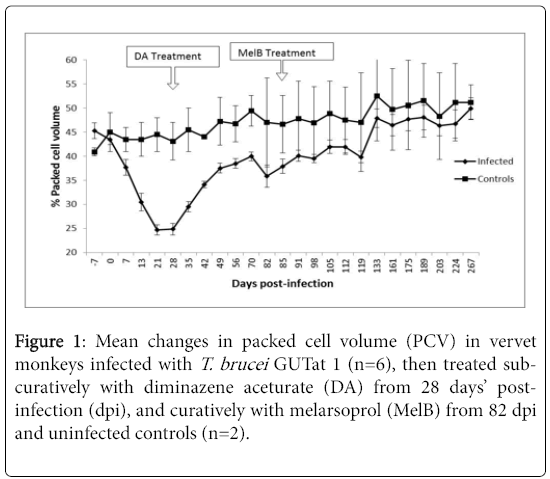
The animals’ clinical signs improved for a short period between 12 and 21 dpi, but thereafter deteriorated with progress of disease. Infected animals rapidly developed anaemia, demonstrated by a decrease (P<0.05) in percentage PCV [14], from a pre-infection mean of 45.3% to a mean of 24.9% by 28 dpi (Figure 1).
Sub-curative treatment with DA from 28 dpi resulted in a rapid improvement in the animals’ health condition. They regained their appetite, the body temperature normalized, the level of oedema decreased; they became more active, and there was an improvement in the PCV.
Starting 60 dpi however, the level of activity decreased again, and neurological signs appeared, including staring, hunching, loss of mobility, partial blindness and sleepiness. The animals recovered quickly after curative treatment with MelB, and all clinical signs disappeared within 4 to 6 weeks after treatment.
Parasitaemia and CSF parasitosis
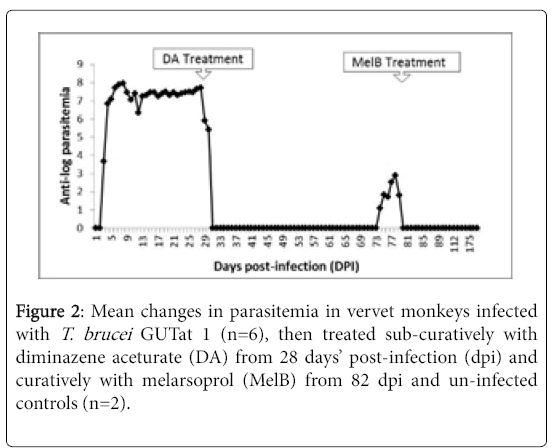
The animals developed patent parasitemia between 2 and 4 dpi, with a mean parasitemia of 105 trypanosomes/ml which increased rapidly to a first peak of approximately 109 trypanosomes/ml at 9 dpi, and remained elevated, with only minor fluctuations, up to 28 dpi when they were treated with DA (Figure 2). Trypanosomes disappeared from the blood after treatment with DA from 28 dpi, then relapses occurred from 70 dpi, and by 82 dpi, two animals had parasites in blood, and a third animal that displayed mild clinical aggression succumbed suddenly (76 dpi), despite the absence of relapse parasitaemia.
Trypanosomes were observed in the CSF of five monkeys from 7 dpi, and in all the animals from 14 dpi. Subsequently, trypanosomes persisted in the CSF until the animals were treated with Mel B from 82 dpi. No parasites were observed in the CSF of any animal after treatment with Mel B.
Cerebrospinal fluid white blood cells
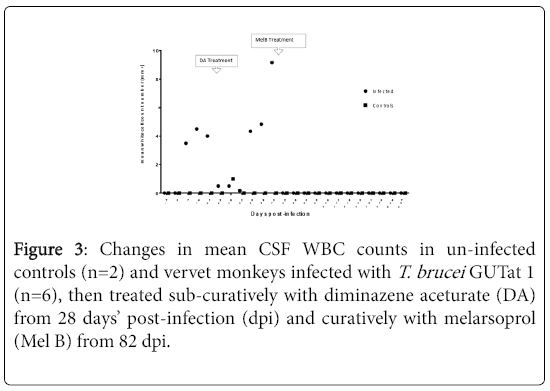
The mean changes in CSF WBC in monkeys before and after infection, and following sub-curative and curative treatment. While no WBC were found before infection, all infected animals showed an elevation of WBC before sub-curative treatment with DA at 28 dpi, coinciding with the first observation of parasites in the CSF. White blood cell count in CSF remained elevated in all infected animals until treatment with MelB was administered at 82 dpi when levels returned to normalcy. Thereafter, the levels remained within pre-infection ranges throughout the rest of the monitoring period. The were no changes in CSF WBC in uninfected controls.
Serum neopterin
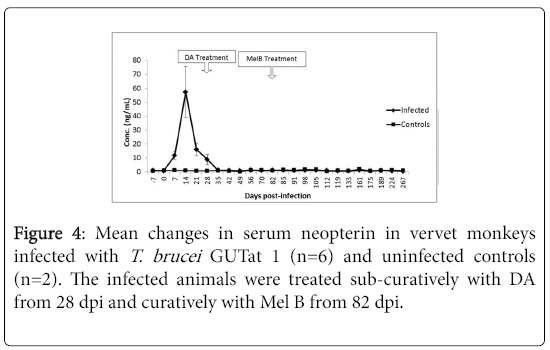
The changes in concentration of neopterin in the serum of monkeys infected with T. brucei , then treated with DA and later with MelB are shown in Figure 3. The mean serum concentration of neopterin in uninfected animals was 0.98 ng/ml ± 0.08 ng/ml (range=0.206 ng/ml to 1.516 ng/ml). After infection, the concentration increased rapidly from 7 dpi, coinciding with the first wave of parasitaemia, rising significantly above pre-infection levels (p<0.05) to reach a mean peak of 57.3 ng/ml ± 18.29 ng/ml at 14 dpi. This was followed by a progressive decline, reaching pre-infection levels by 35 dpi, which was sustained throughout the rest of the study period. These changes were not affected by treatment with either DA or MelB.
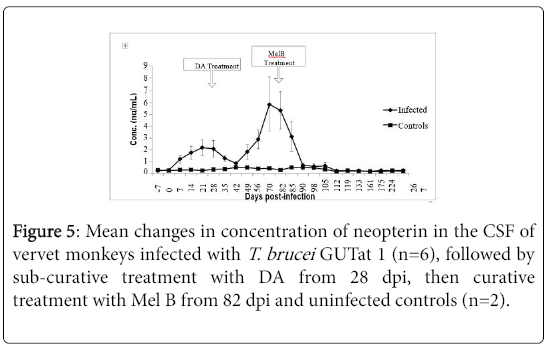
Cerebrospinal fluid neopterin
The changes in CSF neopterin in infected monkeys and following treatment with DA and Mel B are shown in Figure 4. The mean CSF concentration of neopterin in uninfected animals was 0.32 ng/ml ± 0.02 ng/ml (range=0.15 ng/ml to 0.75 ng/ml). In infected animals, a marginal increase in CSF neopterin was observed from 7 dpi, reaching a peak of 2.11 ng/ml ± 0.66 ng/ml at 21 dpi. This peak was observed 7 days after the peak in serum neopterin, and was followed by a decline, to pre-infection levels 42 dpi, 7 days after serum neopterin reached pre-infection levels. When the concentrations of neopterin in serum and CSF at each sampling point were compared before treatment, the levels in CSF were more than 10 times lower than in serum. After subcurative treatment with DA, a second increase in CSF neopterin was observed in the infected animals, starting at 42 dpi, rising significantly above pre-infection levels (p<0.05), and peaking at 70 dpi with mean levels of 5.85 ng/ml ± 2.23 ng/ml. After curative treatment with Mel B, CSF neopterin declined rapidly, to pre-infection levels within the next 7 days.
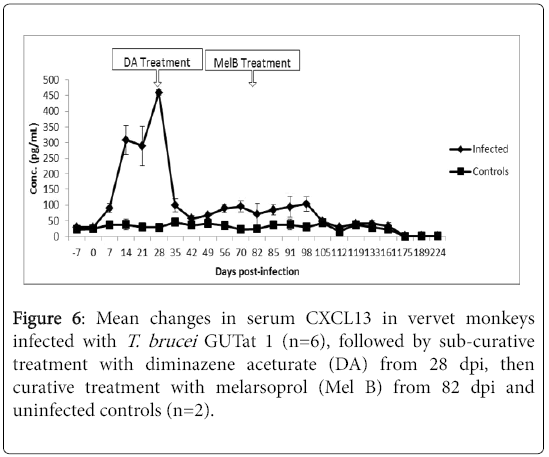
Serum CXCL13
The changes in serum CXCL13 in infected and uninfected control animals are shown in Figure 5. The mean concentration of CXCL13 in uninfected animals was 28.27 pg/ml ± 2.7 pg/ml. There was a rapid increase in serum CXCL13 from 7 dpi, coinciding with the first wave of parasitaemia. Levels increased significantly above pre-infection levels (p<0.05), to a peak mean concentration of 458.91 pg/ml ± 8.01 pg/ml at 28 dpi, when the animals were sub-curatively treated with DA. Following treatment with DA from 28 dpi, levels decreased rapidly, to slightly above pre-infection levels over the next 14 days. After the decrease, serum CXCL13 remained higher than pre-infection levels, up to 112 dpi, when the levels normalized.
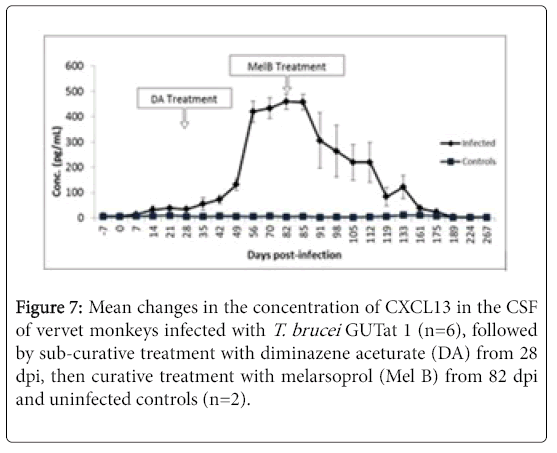
Cerebrospinal fluid CXCL13
The changes in concentration of CXCL13 in the CSF of monkeys after infection with T. brucei , followed by treatment with DA, then Mel B, are shown in Figure 6. Levels of CSF CXCL13 showed a slow, gradual increase rising significantly above pre-infection levels (p<0.05) starting from 14 dpi up to 28 dpi. Unlike neopterin, CSF CXCL13 levels continued to rise after treatment with DA, to a mean peak of 460.13 pg/ml ± 30.63 pg/ml at 82 dpi when curative treatment with Mel B was initiated. Treatment with Mel B from 82 dpi was followed by a slow decline in CSF CXCL13, reaching pre-infection levels 9 weeks later (Figure 7).
Discussion
The animals used in this study developed acute disease after infection with T. brucei GUTat 1, and progressed to the advanced or S2 disease after sub-curative treatment with DA, which was cured after treatment with MelB. The same behavior was reported in other studies using vervet monkeys infected with T. rhodesiense [13]. When animals infected for more than 21 days are treated with the dosage regime of DA described here, all parasites in the blood and organs die, except the ones in the brain. The parasites in the CNS are protected from this treatment because DA does not cross the blood-brain barrier [15]. The parasites continue multiplying and causing damage in the CNS, leading to the neurological signs that are characteristic of S2 HAT. This is consistent with the present study where both biomarkers and WBC increased progressively in the CSF, while remaining at pre-infection levels in the blood.
This study has demonstrated that serum and CSF neopterin and CXCL13 are good markers of the progression of disease and detection of treatment failure in vervet monkeys infected with T. brucei , while in addition, neopterin is a good marker for cure. An increase in the concentration of both biomarkers in both serum and CSF was observed 7 dpi, coinciding with the onset of clinical signs, parasitaemia and demonstration of parasites in the CSF. The peaks of neopterin in serum and CSF at 14 dpi and 21 dpi respectively appeared not to be influenced by treatment with DA or presence of trypanosomes. The concentration of neopterin in serum at each sampling point before treatment was several times higher than in the CSF. These changes are consistent with an acute phase response (APR) that occurs in T. brucei infections, and were correlated with the early onset of parasitaemia and rapid deterioration in clinical signs. The most pronounced clinical signs were observed during the second and third weeks of infection, followed by slight improvement during the fourth week before the animals were treated with DA. In previous studies, dogs infected with T. brucei developed a similar APR, demonstrated by a rapid increase in C-reactive protein (CRP), which coincided with the first wave of parasitaemia [16]. A significant positive association between neopterin and CRP has also been reported in women with transient hypertension of pregnancy and women with preeclampsia [17]. In the present study, the concentration of serum neopterin started to fall more than one week before sub-curative treatment with DA, in spite of a persistently high parasitaemia. That the decline coincided with improvement in clinical signs is further indication that the initial increase was part of an APR. In other studies, serum neopterin has been shown to increase early in infection before CRP, ahead of antibody production [18].
CSF neopterin is a useful marker of inflammation in a broad range of acute and chronic CNS disorders, and is a significantly more sensitive marker of inflammation than CSF pleocytosis [19]. Production of relevant amounts of neopterin occurs only in the monocytes/macrophages and astrocytes of humans and primates, but not in other animal species [20]. An increase in neopterin precedes the appearance of specific antibodies in serum, on average by one week. It has been investigated as an informative biomarker of the CNS immune activation of viral infections such as HIV associated dementia [20], cardiovascular risk assessment [21], and as a prognostic marker in cancer treatment [22].
Neopterin is produced by monocytes and macrophages after stimulation by IFN-γ produced by Th1 lymphocytes [23], while African trypanosomiasis is associated with early activation of the reticuloendotherial system (RES), comprising of the monocyte/ macrophage system [6]. The early increase in serum neopterin observed in this study is consistent with activation of the RES by the trypanosome infection. Neopterin was detected in the CSF from 7 dpi, and it increased up to 21 dpi, then declined to pre-infection levels by 35 dpi. The CSF level of neopterin during this period was always several times lower than the serum levels, and changes in CSF occurred one week after serum. It is therefore likely that the neopterin observed in CSF in early disease was of blood origin and not produced intrathecally. Previous studies have shown that there is a gradient between serum and CSF neopterin of a ratio of 1:40 [24].
Despite the persistence of parasites in the CSF after treatment with DA 28 dpi, CSF neopterin decreased transiently, and only increased again from 49 dpi, to peak around 70 dpi, coinciding with relapse parasitaemia. This would support the view that the observed increase in neopterin in the CSF during the first three weeks after infection was caused by massive inflammatory processes in the whole body as part of an APR, and that the neopterin detected in CSF at that time was of blood origin. The subsequent increase in CSF neopterin with advancing disease could have resulted from intrathecal production after invasion of the CNS by parasites. Trypanosomes in the CNS would have stimulated T lymphocytes to produce IFN-Y, which then stimulated invading monocytes and macrophages to produce neopterin within the CNS [6]. Continued inflammatory activities in the CNS, caused by invading parasites, present only in the CNS and not in the rest of the body, would explain the difference in neopterin levels between the CSF and serum. Unlike during the APR when inflammatory activities outside the CNS contributed to the observed high levels of serum neopterin, no increase in serum neopterin occurred during late stage disease and after treatment. This could be because inflammatory activities were confined to the CNS, and the neopterin draining from the CNS would not accumulate in the blood since it is cleared rapidly through the kidneys [21].
The CSF levels of neopterin decreased rapidly after curative treatment with Mel B, to pre-infection levels within 7 days. Neopterin is a good marker of ongoing inflammation [19]. In this study, curative treatment may have stopped inflammatory processes in the entire body, resulting in the rapid decrease in CSF neopterin. This rapid reduction in CSF neopterin after parasites are killed confirms that it would be an excellent biomarker for use as a test of cure for HAT.
Unlike neopterin, the concentration of CXCL13 in both serum and CSF continued to rise until the animals were treated with DA 28 dpi. During this period, CXCL13 was more than 3 times higher in serum than in CSF. Treatment with DA was followed by a decline in serum CXCL13, to slightly above pre-infection levels within 14 days. After the decrease, serum CXCL13 remained elevated above pre-infection levels up to 112 dpi, when the levels normalized. Conversely in CSF, CXCL13 was not affected by treatment with DA, and continued to rise, to a peak 82 dpi when the animals were treated curatively with MelB.
There was a slow decrease in CSF CXCL13 after curative treatment with MelB, to pre-infection levels 105 days after treatment possibly due to trypanosome free-floating antigen persisting even after treatment causing a sustained activation of B-cells and subsequent stimulation of CXCL13. This has been reported in previous vervet models where CSF IgM and IgG levels were detectable even after curative treatment throughout duration of experiment [5]. A similar slow decrease in CSF CXCL13 after treatment has been observed in patients with gambiense HAT [25] and neurosyphilis [26]. The slow and gradual decrease in CSF CXCL13 means that this biomarker would not be suitable for a test of cure.
The continued increase in levels of neopterin and CXCL13 in the CSF after sub-curative treatment with DA indicates that both biomarkers can be used to detect treatment failure in a T. brucei vervet monkey model of HAT. Neopterin could in addition be used for a test of cure, as the CSF levels of this biomarker decrease rapidly after curative treatment. The monkey model of HAT used in this study also provides an excellent opportunity to improve our understanding of the pathogenesis of the disease.
References
- Simarro P, Cecchi G, Franco J, Paone M, Diarra A, et al. (2012) Estimating and mapping the population at risk of sleeping sickness. PLoS Negl Trop Dis 6: e1859.
- Wastling SL, Welburn SC (2011) Diagnosis of human sleeping sickness: sense and sensitivity. Trends Parasitol 27: 394-402.
- Burri C, Yeramian P, Allen J, Merolle A, Serge K, et al. (2016) Efficacy, safety, and dose of Pafuramidine, a new oral drug for treatment of first stage sleeping sickness, in a Phase 2a clinical study and Phase 2b randomized clinical studies. PLoS Negl Trop Dis 10: e0004362.
- Maranga D, Kagira J, Kariuki C, Karanja S, Ngotho M, et al. (2013) IL-6 is up-regulated in late stage in vervet monkeys experimentally infected with Trypanosoma brucei rhodesiense. Clin Dev Immunol 2013: 320509.
- Ngotho M, Kagira J, Jensen H, Karanja S, Farah I, et al. (2009) Immuno-specific immunoglobulins and IL-10 markers for Trypanosoma brucei rhodesiense late stage disease in experimentally infected vervet monkeys. Trop Med Int Health 14: 736.
- Tiberti N, Hainard A, Lejon V, Courtioux B, Matovu E, et al. (2012) Cerebrospinal fluid neopterin as marker of the meningo-encephalitic stage of Trypanosoma brucei gambiense sleeping sickness. PLoS one 7: e40909.
- Maclean L, Odiit M, Sternberg J (2006) Intrathecal cytokine responses in Trypanosoma brucei rhodesiense sleeping sickness patients. Trans R Soc Trop Med Hyg 100: 270-275.
- Tiberti N, Matovu E, Hainard A, Enyaru JC, Lejon V, et al. (2013b) New biomarkers for stage determination in Trypanosoma brucei rhodesiense sleeping sickness patients. Clinical and Translational Medicine 2:1.
- Gunn M, Ngo V, Ansel K, Ekland , Cyster J, et al. (1998) A B-cell-homing chemokine made in lymphoid follicles activates Burkitt’s lymphoma receptor-1. Nature 391: 799–803.
- Rupprecht T, Pfister H, Angele B, Kastenbauer S, Wilske B, Koedel U (2005) The chemokine CXCL13 (BLC): A putative diagnostic marker for neuroborreliosis. Neurology 65: 448-450.
- Rupprecht T, Plate A, Adam M, Wick M, Kastenbauer S, et al. (2009) The chemokine CXCL13 is a key regulator of B cell recruitment to the cerebrospinal fluid in acute Lyme neuroborreliosis. J Neuroinflammation 6:42.
- Herbert WJ, Lumsden WHR (1976) Trypanosoma brucei: A rapid “matching” method for estimating the host’s parasitaemia. Exper Parasitol 237: 123-133.
- Ndung'u J, Ngure R, Ngotho J, Sayer P, Omuse J (1994) Total protein and white cell changes in the cerebrospinal fluid of vervet monkeys infected with Trypanosomarhodesiense and the post-treatment reaction. J Protozool Res 4:124-135.
- Quintó L, Aponte JJ, Menéndez C, Sacarlal J, Aide P, et al. (2006) Relationship between haemoglobin and haematocrit in the definition of anaemia. Trop Med Int Health 11 (8):1295-302.
- Jennings F, Gray GD (1983) Relapsed parasitaemia following chemotherapy of chronic T. brucei infections in mice and its relation to cerebral trypanosomes. Contr Microbiol Immunol 7: 147–154.
- Ndung'u J, Eckersall P, Jennings F (1991) Elevation of the concentration of acute phase proteins in dogs infected with Trypanosoma brucei. Acta Tropica 49:77-85.
- Von Versen-Hoeynck F, Hubel C, Gallaher M, Gammill H, Powers RW (2009) Plasma levels of inflammatory markers neopterin, sialic acid and C-reactive protein in pregnancy and preeclampsia. Am J Hypertens 22: 687-692.
- Wirleitner B, Schroecksnadel K, Winkler C, Fuchs D (2005) Neopterin in HIV-1 infection. Mol Immunol 42: 183.
- Dale RC, Brilot F, Fagan E, Earl J (2009) Cerebrospinal fluid neopterin in paediatric neurology: A marker of active central nervous system inflammation. Dev Med Child Neurol 51: 317-323.
- Hagberg L, Cinque P, Gisslen M, Brew B, Spudich S, et al.(2010) Cerebrospinal fluid neopterin: An informative biomarker of central nervous system immune activation in HIV-1 infection. AIDS Res Ther 7:15.
- Fuchs D, Stahl-Hennig C, Gruber A, Murr C, Hunsmann G, et al. (1994) Neopterin: Its clinical use in urinalysis. Kidney Internat 47: S8-S11.
- Sucher R, Schroecksnadel K, Weiss G, Margreiter R, Fuchs D, et al. (2010) Neopterin, a prognostic marker in human malignancies. Cancer Lett 287:13-22.
- Hoffmann G, Wirleitner B, Fuchs D (2003) Potential role of immune system activation-associated production of neopterin derivatives in humans. Inflamm Res 52: 313–321.
- Eisenhut M (2013) Neopterin in diagnosis and monitoring of infectious diseases. J Biomarkers 2013: 196432.
- Courtioux B, Boda C, Vatunga G, Pervieux L, Josenando T, et al. (2009) A link between chemokine levels and disease severity in human African trypanosomiasis. PLoS Negl Trop Dis3:e459.
- Marra C, Tantalo L, Sahi S, Maxwell C, Lukehart S (2010) CXCL13 as a cerebrospinal fluid marker for neurosyphilis in HIV-infected patients with syphilis. J Sex Transm Dis 37: 283-287.
Relevant Topics
- Bacteria Induced Neuropathies
- Blood-brain barrier
- Brain Infection
- Cerebral Spinal Fluid
- Encephalitis
- Fungal Infection
- Infectious Disease in Children
- Neuro-HIV and Bacterial Infection
- Neuro-Infections Induced Autoimmune Disorders
- Neurocystercercosis
- Neurocysticercosis
- Neuroepidemiology
- Neuroinfectious Agents
- Neuroinflammation
- Neurosyphilis
- Neurotropic viruses
- Neurovirology
- Rare Infectious Disease
- Toxoplasmosis
- Viral Infection
Recommended Journals
Article Tools
Article Usage
- Total views: 3322
- [From(publication date):
June-2017 - Apr 01, 2025] - Breakdown by view type
- HTML page views : 2500
- PDF downloads : 822